Cost-Effectiveness of Intranasal Live-Attenuated Influenza Vaccine for Children: A Systematic Review
Abstract
:1. Introduction
2. Methods
2.1. Inclusion and Exclusion Criteria
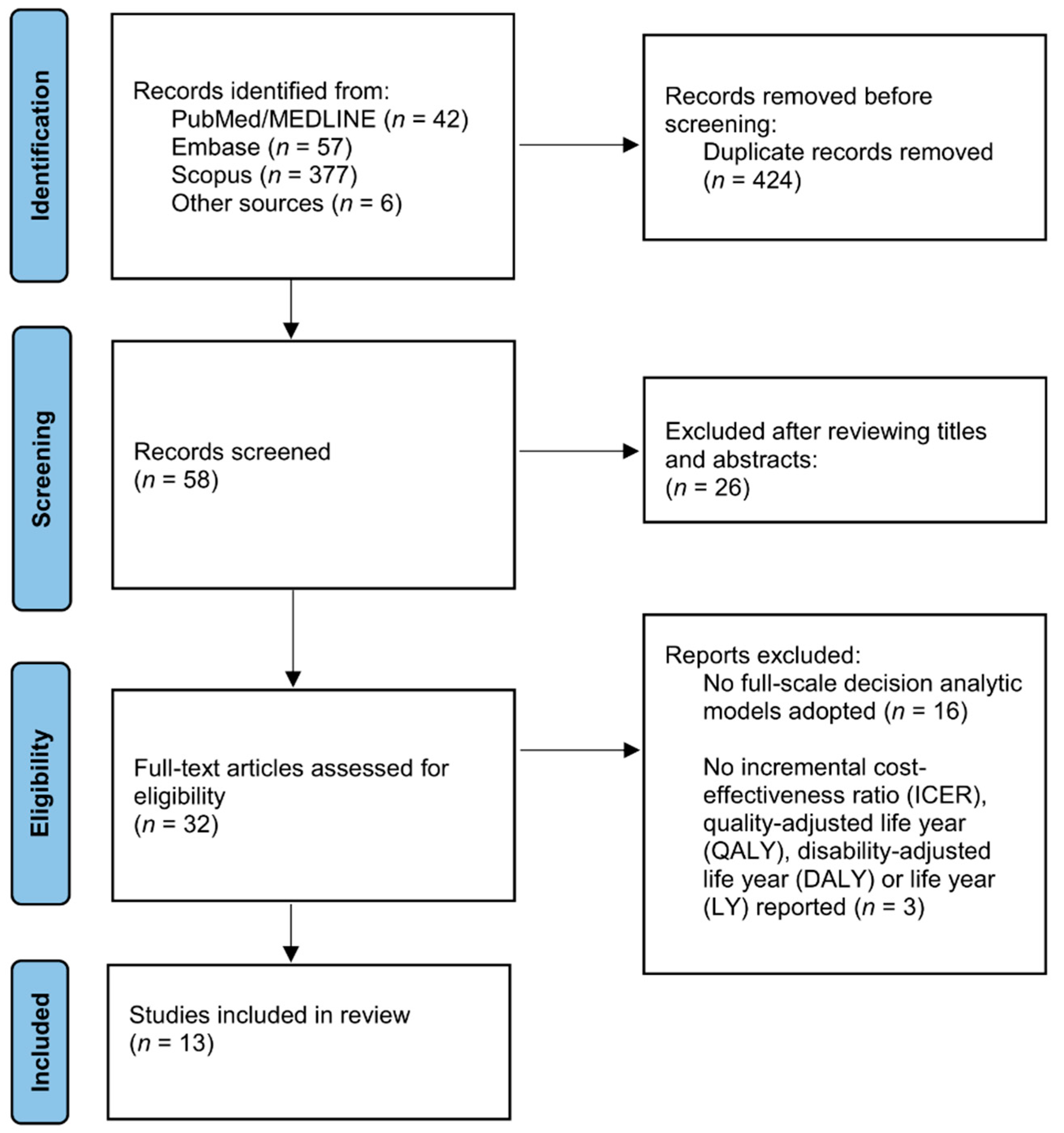
2.2. Literature Search and Study Selection
2.3. Data Extraction
2.4. Quality Assessment
3. Results
3.1. Study Characteristics
3.2. Economic Evaluations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J.; et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef]
- World Health Organization. Influenza. 2022. Available online: https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccines-quality/influenza (accessed on 20 July 2022).
- Wang, X.; Li, Y.; O’Brien, K.L.; Madhi, S.A.; Widdowson, M.-A.; Byass, P.; Omer, S.B.; Abbas, Q.; Ali, A.; Amu, A.; et al. Global burden of respiratory infections associated with seasonal influenza in children under 5 years in 2018: A systematic review and modelling study. Lancet Glob. Health 2020, 8, e497–e510. [Google Scholar] [CrossRef]
- Putri, W.C.; Muscatello, D.J.; Stockwell, M.S.; Newall, A.T. Economic burden of seasonal influenza in the United States. Vaccine 2018, 36, 3960–3966. [Google Scholar] [CrossRef] [PubMed]
- Antonova, E.N.; Rycroft, C.E.; Ambrose, C.S.; Heikkinen, T.; Principi, N. Burden of paediatric influenza in Western Europe: A systematic review. BMC Public Health 2012, 12, 968. [Google Scholar] [CrossRef]
- Ehlken, B.; Ihorst, G.; Lippert, B.; Rohwedder, A.; Petersen, G.; Schumacher, M.; Forster, J.; for the PRIDE Study Group. Economic impact of community-acquired and nosocomial lower respiratory tract infections in young children in Germany. Eur. J. Pediatr. 2005, 164, 607–615. [Google Scholar] [CrossRef]
- De Waure, C.; Veneziano, M.A.; Cadeddu, C.; Capizzi, S.; Specchia, M.L.; Capri, S.; Ricciardi, W. Economic value of influenza vaccination. Hum. Vaccines Immunother. 2012, 8, 119–129. [Google Scholar] [CrossRef]
- Bednarczyk, R.; Adjaye-Gbewonyo, D.; Omer, S.B. Safety of influenza immunization during pregnancy for the fetus and the neonate. Am. J. Obstet. Gynecol. 2012, 207, S38–S46. [Google Scholar] [CrossRef]
- Piedra, P.A.; Gaglani, M.J.; Kozinetz, C.A.; Herschler, G.; Riggs, M.; Griffith, M.; Fewlass, C.; Watts, M.; Hessel, C.; Cordova, J.; et al. Herd immunity in adults against influenza-related illnesses with use of the trivalent-live attenuated influenza vaccine (CAIV-T) in children. Vaccine 2005, 23, 1540–1548. [Google Scholar] [CrossRef]
- Rolfes, M.A.; Flannery, B.; Chung, J.R.; O’Halloran, A.; Garg, S.; Belongia, E.A.; Gaglani, M.; Zimmerman, R.K.; Jackson, M.L.; Monto, A.S.; et al. Effects of Influenza Vaccination in the United States during the 2017–2018 Influenza Season. Clin. Infect. Dis. 2019, 69, 1845–1853. [Google Scholar] [CrossRef]
- Glezen, W.P.; Couch, R.B.; MacLean, R.A.; Payne, A.; Baird, J.N.; Vallbona, C.; Tristan, M.; Byrd, N. Interpandemic Influenza in the Houston Area, 1974–1976. N. Engl. J. Med. 1978, 298, 587–592. [Google Scholar] [CrossRef]
- World Health Organization. WHO Launches New Global Influenza Strategy. 2019. Available online: https://www.who.int/news/item/11-03-2019-who-launches-new-global-influenza-strategy (accessed on 20 June 2022).
- European Centre for Disease Prevention and Control. Influenza: Recommended Vaccinations. Available online: https://vaccine-schedule.ecdc.europa.eu/Scheduler/ByDisease?SelectedDiseaseId=15&SelectedCountryIdByDisease=-1 (accessed on 20 June 2022).
- Boccalini, S.; Bechini, A.; Moscadelli, A.; Paoli, S.; Schirripa, A.; Bonanni, P. Cost-effectiveness of childhood influenza vac-cination in Europe: Results from a systematic review. Expert Rev. Pharm. Outcomes Res. 2021, 21, 911–922. [Google Scholar] [CrossRef]
- Ashkenazi, S.; Vertruyen, A.; Arístegui, J.; Esposito, S.; McKeith, D.D.; Klemola, T.; Biolek, J.; Kühr, J.; Bujnowski, T.; Desgrandchamps, D.; et al. Superior relative efficacy of live attenuated influenza vaccine compared with inactivated influenza vaccine in young children with recurrent respiratory tract infections. Pediatr. Infect. Dis. J. 2006, 25, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Baguelin, M.; Van Hoek, A.J.; Jit, M.; Flasche, S.; White, P.J.; Edmunds, W.J. Vaccination against pandemic influenza A/H1N1v in England: A real-time economic evaluation. Vaccine 2010, 28, 2370–2384. [Google Scholar] [CrossRef]
- Ambrose, C.S.; Wu, X.; Knuf, M.; Wutzler, P. The efficacy of intranasal live attenuated influenza vaccine in children 2 through 17 years of age: A meta-analysis of 8 randomized controlled studies. Vaccine 2012, 30, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Nichol, K.L. Cost-effectiveness and socio-economic aspects of childhood influenza vaccination. Vaccine 2011, 29, 7554–7558. [Google Scholar] [CrossRef] [PubMed]
- Tarride, J.E.; Burke, N.; Von Keyserlingk, C.; O’Reilly, D.; Xie, F.; Goeree, R. Cost-effectiveness analysis of intranasal live at-tenuated vaccine (LAIV) versus injectable inactivated influenza vaccine (TIV) for Canadian children and adolescents. Clin. Outcomes Res. 2012, 4, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Shim, E.; Brown, S.T.; DePasse, J.; Nowalk, M.P.; Raviotta, J.M.; Smith, K.J.; Zimmerman, R.K. Cost Effectiveness of Influenza Vaccine for, U.S. Children: Live Attenuated and Inactivated Influenza Vaccine. Am. J. Prev. Med. 2016, 51, 309–317. [Google Scholar] [CrossRef]
- Prosser, L.A.; Bridges, C.B.; Uyeki, T.M.; Hinrichsen, V.L.; Meltzer, M.I.; Molinari, N.-A.M.; Schwartz, B.; Thompson, W.W.; Fukuda, K.; Lieu, T.A. Health Benefits, Risks, and Cost-Effectiveness of Influenza Vaccination of Children. Emerg. Infect. Dis. 2006, 12, 1548–1558. [Google Scholar] [CrossRef]
- Hibbert, C.L.; Piedra, P.A.; McLaurin, K.K.; Vesikari, T.; Mauskopf, J.; Mahadevia, P.J. Cost-effectiveness of live-attenuated influenza vaccine, trivalent in preventing influenza in young children attending day-care centres. Vaccine 2007, 25, 8010–8020. [Google Scholar] [CrossRef]
- Prosser, L.A.; Meltzer, M.I.; Fiore, A.; Epperson, S.; Bridges, C.B.; Hinrichsen, V.; Lieu, T.A. Effects of adverse events on the projected population benefits and cost-effectiveness of using live attenuated influenza vaccine in children aged 6 months to 4 years. Arch. Pediatr. Adolesc. Med. 2011, 165, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Evers, S.; Goossens, M.; De Vet, H.; van Tulder, M.; Ament, A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int. J. Technol. Assess. Health Care 2005, 21, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, N.S.; Atkins, K.E.; van Leeuwen, E.; Halloran, M.E.; Baguelin, M. Cost-effectiveness of live-attenuated influenza vaccination among school-age children. Vaccine 2020, 39, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Thorrington, D.; Van Leeuwen, E.; Ramsay, M.; Pebody, R.; Baguelin, M. Cost-effectiveness analysis of quadrivalent seasonal influenza vaccines in England. BMC Med. 2017, 15, 166. [Google Scholar] [CrossRef]
- Gerlier, L.; LaMotte, M.; Grenèche, S.; Lenne, X.; Carrat, F.; Weil-Olivier, C.; Damm, O.; Schwehm, M.; Eichner, M. Assessment of Public Health and Economic Impact of Intranasal Live-Attenuated Influenza Vaccination of Children in France Using a Dynamic Transmission Model. Appl. Health Econ. Health Policy 2017, 15, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.; Begum, N.; Martinón-Torres, F.; Safadi, M.A.; Sackeyfio, A.; Hackett, J.; Rajaram, S. Cost-effectiveness analysis of the direct and indirect impact of intranasal live attenuated influenza vaccination strategies in children: Alternative country profiles. J. Mark. Access Health Policy 2016, 4, 36. [Google Scholar] [CrossRef]
- Nagy, L.; Heikkinen, T.; Sackeyfio, A.; Pitman, R. The Clinical Impact and Cost Effectiveness of Quadrivalent Versus Trivalent Influenza Vaccination in Finland. Pharmacoeconomics 2016, 34, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Baguelin, M.; Camacho, A.; Flasche, S.; Edmunds, W.J. Extending the elderly- and risk-group programme of vaccination against seasonal influenza in England and Wales: A cost-effectiveness study. BMC Med. 2015, 13, 236. [Google Scholar] [CrossRef]
- Damm, O.; Eichner, M.; Rose, M.A.; Knuf, M.; Wutzler, P.; Liese, J.G.; Krüger, H.; Greiner, W. Public health impact and cost-effectiveness of intranasal live attenuated influenza vaccination of children in Germany. Eur. J. Health Econ. 2015, 16, 471–488. [Google Scholar] [CrossRef]
- Pitman, R.J.; Nagy, L.D.; Sculpher, M.J. Cost-effectiveness of childhood influenza vaccination in England and Wales: Results from a dynamic transmission model. Vaccine 2013, 31, 927–942. [Google Scholar] [CrossRef]
- Taylor, S.; Lopez, P.; Weckx, L.; Borja-Tabora, C.; Ulloa-Gutierrez, R.; Lazcano-Ponce, E.; Kerdpanich, A.; Rodriguez-Weber, M.A.; Santos, A.M.D.L.; Tinoco, J.-C.; et al. Respiratory viruses and influenza-like illness: Epidemiology and outcomes in children aged 6 months to 10 years in a multi-country population sample. J. Infect. 2017, 74, 29–41. [Google Scholar] [CrossRef]
- Pellegrinelli, L.; Bubba, L.; Galli, C.; Anselmi, G.; Primache, V.; Binda, S.; Pariani, E. Epidemiology and molecular characterization of influenza viruses, human parechoviruses and enteroviruses in children up to 5 years with influenza-like illness in Northern Italy during seven consecutive winter seasons (2010–2017). J. Gen. Virol. 2017, 98, 2699–2711. [Google Scholar] [CrossRef] [PubMed]
- Grohskopf, L.A.; Sokolow, L.Z.; Broder, K.R.; Walter, E.B.; Bresee, J.S.; Fry, A.M.; Jernigan, D.B. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020–21 Influenza Season. MMWR Recomm. Rep. 2020, 69, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Young, K.; Gemmill, I.; Harrison, R. Summary of the NACI Seasonal Influenza Vaccine Statement for 2020–2021. Can. Commun. Dis. Rep. 2020, 46, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Rhorer, J.; Ambrose, C.S.; Dickinson, S.; Hamilton, H.; Oleka, N.A.; Malinoski, F.J.; Wittes, J. Efficacy of live attenuated influenza vaccine in children: A meta-analysis of nine randomized clinical trials. Vaccine 2009, 27, 1101–1110. [Google Scholar] [CrossRef]
- Negri, E.; Colombo, C.; Giordano, L.; Groth, N.; Apolone, G.; La Vecchia, C. Influenza vaccine in healthy children: A meta-analysis. Vaccine 2005, 23, 2851–2861. [Google Scholar] [CrossRef] [PubMed]
- Clements, M.L.; Betts, R.F.; Murphy, B.R. Advantage of live attenuated cold-adapted influenza A virus over inactivated vaccine for A/Washington/80 (H3N2) wild-type virus infection. Lancet 1984, 1, 705–708. [Google Scholar] [CrossRef]
- Gorse, G.J.; Campbell, M.J.; Otto, E.E.; Powers, D.C.; Chambers, G.W.; Newman, F.K. Increased anti-influenza A virus cytotoxic T cell activity following vaccination of the chronically ill elderly with live attenuated or inactivated influenza virus vaccine. J. Infect. Dis. 1995, 172, 1–10. [Google Scholar] [CrossRef]
- Ambrose, C.S.; Levin, M.J.; Belshe, R.B. The relative efficacy of trivalent live attenuated and inactivated influenza vaccines in children and adults. Influenza Other Respir. Viruses 2010, 5, 67–75. [Google Scholar] [CrossRef]
- Zhao, M.; Vandersluis, M.; Stout, J.; Haupts, U.; Sanders, M.; Jacquemart, R. Affinity chromatography for vaccines manufacturing: Finally ready for prime time? Vaccine 2019, 37, 5491–5503. [Google Scholar] [CrossRef]
- Andersohn, F.; Bornemann, R.; Damm, O.; Frank, M.; Mittendorf, T.; Theidel, U. Vaccination of children with a live-attenuated, intranasal influenza vaccine—Analysis and evaluation through a Health Technology Assessment. GMS Health Technol. Assess. 2014, 10, Doc03. [Google Scholar] [CrossRef]
- Zhou, L.; Su, Q.; Xu, Z.; Feng, A.; Jin, H.; Wang, S.; Feng, Z. Seasonal influenza vaccination coverage rate of target groups in selected cities and provinces in China by season (2009/10 to 2011/12). PLoS ONE 2013, 8, e73724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ren, X.; Tian, K.; Yu, J.; Zhu, A.; Zhang, L.; Gao, G.F.; Li, Z. The Impact and Vaccination Coverage of Seasonal Influenza among Children Aged 6–59 Months in China in 2017–2018: An Internet Panel Survey. Vaccines 2022, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, N. A review of the indirect protection of younger children and the elderly through a mass influenza vaccination program in Japan. Expert Rev. Vaccines 2014, 13, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Reichert, T.A.; Sugaya, N.; Fedson, D.S.; Glezen, W.P.; Simonsen, L.; Tashiro, M. The Japanese experience with vaccinating schoolchildren against influenza. N. Engl. J. Med. 2001, 344, 889–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Study | Setting | Year | Model Type | Population | Vaccination Strategy | Comparator | Perspective | Time Horizon | Discount Rate | Sensitivity Analysis | Funding Source |
|---|---|---|---|---|---|---|---|---|---|---|---|
| De Boer [24] | The Netherlands | 2021 | Dynamic transmission model | Children 2–16 Y | LAIV (in addition to the current strategy | Current strategy (people at risk and the elderly with TIV) | Societal; payer’s | 20 years | 4% | One way and probabilistic sensitivity analysis | Vaccine manufacturer |
| Wenzel [25] | England, Wales | 2021 | Dynamic transmission model | Pre-school (2–4 Y); Primary School (5–11 Y); Secondary school (12–16 Y) All children (2–16 Y) | LAIV (in addition to the current strategy) | Current strategy (people at risk and the elderly with TIV) | Public sector | 1 year | 3.5% | One way and probabilistic sensitivity analysis | NR |
| Thorrington [26] | England | 2017 | Dynamic transmission model | Children 2–11 Y; 2–16 Y | Quadrivalent LAIV for children and quadrivalent IIV for people at risk and the elderly | Current strategy (children with trivalent LAIV and people at risk and the elderly with TIV) | Public sector | 14 years | 3.5% | One way and probabilistic sensitivity analysis | NHS |
| Gerlier [27] | France | 2016 | Dynamic transmission model | Children 2–17 Y | LAIV (in addition to the current strategy) | Current strategy (people at risk and the elderly with TIV) | Societal; payer’s | 30 years | 4% | One way and probabilistic sensitivity analysis | Vaccine manufacturer |
| Gibson [28] | England, Wales | 2016 | Dynamic transmission model | Children 2–17 Y | LAIV (in addition to the current strategy) | Current strategy (people at risk and the elderly with TIV) | Public sector | 5 years | 3.5% | One way and probabilistic sensitivity analysis | Vaccine manufacturer |
| Gibson [28] | Brazil | 2016 | Dynamic transmission model | Children 2–17 Y | LAIV (in addition to the current strategy) | Current strategy (people at risk and the elderly with TIV) | Payer’s | 5 years | 3% | One way and probabilistic sensitivity analysis | Vaccine manufacturer |
| Gibson [28] | Spain | 2016 | Dynamic transmission model | Children 2–17 Y | LAIV (in addition to the current strategy) | Current strategy (people at risk and the elderly with TIV) | Payer’s | 5 years | 3% | One way and probabilistic sensitivity analysis | Vaccine manufacturer |
| Gibson [28] | Taiwan | 2016 | Dynamic transmission model | Children 2–17 Y | LAIV (in addition to the current strategy) | Current strategy (people at risk and the elderly with TIV) | Payer’s | 5 years | 3% | One way and probabilistic sensitivity analysis | Vaccine manufacturer |
| Nagy [29] | Finland | 2016 | Dynamic transmission model | Children 2–17 Y | LAIV | No vaccination among children | Societal; payer’s | 20 years | 3% | One way and probabilistic sensitivity analysis | Vaccine manufacturer |
| Shim [20] | USA | 2016 | Dynamic transmission model | Children 2–8 Y | LAIV | TIV among children | Public sector’s | 10 months | 3% | Two way and probabilistic sensitivity analysis | Vaccine manufacturer |
| Baguelin [30] | England, Wales | 2015 | Dynamic transmission model | Children 2–16 Y | LAIV (in addition to the current strategy) | Current strategy (the elderly with TIV) | Public sector | 14 years | 3.5% | One way and probabilistic sensitivity analysis | NIHR |
| Damm [31] | Germany | 2015 | Dynamic transmission model | Children 2–17 Y | LAIV (in addition to the current strategy) | Current strategy (people at risk with TIV) | Narrow third-party payer; board third-party payer | 14-year run-in phase; 10-year intervention | 3% | One way and probabilistic sensitivity analysis | Vaccine manufacturer |
| Pitman [32] | England, Wales | 2013 | Dynamic transmission model | Children 2–4 Y; 2–10 Y; 2–18 Y | LAIV (in addition to the current strategy) | Current strategy (the elderly with TIV) | Public sector | 200 years | 3.5% | One way and probabilistic sensitivity analysis | Vaccine manufacturer |
| Tarride [19] | Canada | 2012 | Decision-tree model | Children 2–17 Y | LAIV | Current use of TIV among children | Societal; payer’s | 1 year | NR | One way and probabilistic sensitivity analysis | Vaccine manufacturer |
| Prosser [23] | USA | 2011 | Decision-tree model | Children 6 M–23 M; 2 Y; 3–4 Y | LAIV | No vaccination among children | Societal | 5 years | 3% | One way and probabilistic sensitivity analysis | CDC |
| Prosser [21] | USA | 2006 | Decision-tree model | Children 6 M–23 M; 2 Y; 3–4 Y; 5–11 Y; 12–17 Y | LAIV | No vaccination among children | Payer’s | 1 year | NR | One way, two way and probabilistic sensitivity analysis | CDC |
| Study | Vaccine Price (per Dose) | Vaccine Coverage | LAIV Efficacy | WTP Threshold | Outcomes | Conclusions |
|---|---|---|---|---|---|---|
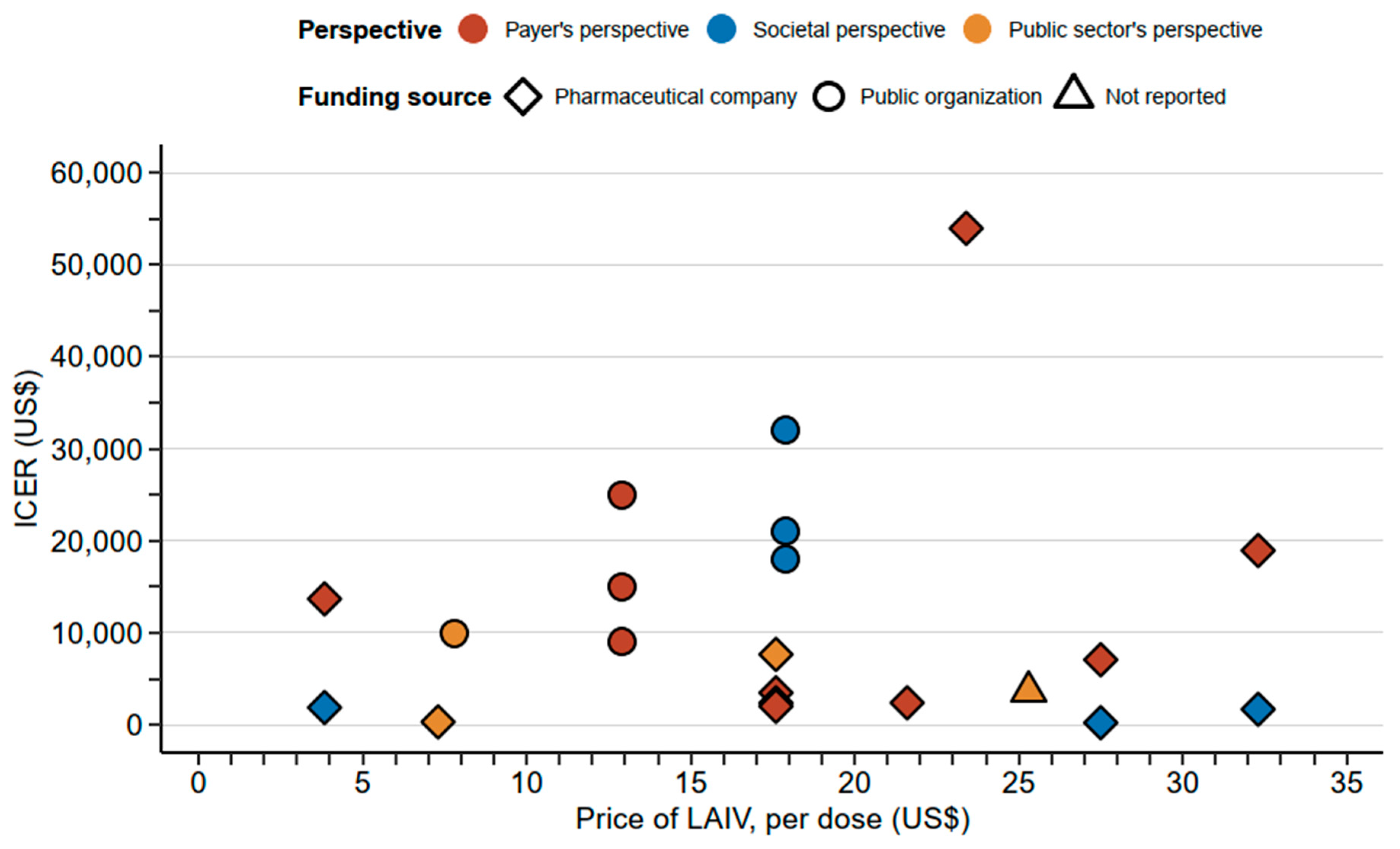
| De Boer [24] | LAIV: $3.84 TIV: $3.84 | 50% | 48% | $21,040 (€20,000) | Societal perspective: ICER = $1868/QALY Payer’s perspective: ICER = $13,680/QALY | Vaccinating 2–16 Y with LAIV in addition to the current strategy is cost-effective. |
| Wenzel [25] | LAIV: $25.3 | 55% | 70% | $24,632 (£20,000) | Pre-school (2–4 Y): ICER = $2530/QALY Primary School (5–11 Y): ICER = $787/QALY Secondary school (12–16 Y): ICER = $27,930/QALY All children (2–16 Y): ICER = $3583/QALY | Vaccinating 2–16 Y with LAIV in addition to the current strategy is cost-effective. Vaccinating primary school students (5–11 Y) is the most cost-effective. |
| Thorrington [26] | NR | <5 Y: 33.7% 5–16 Y: 54.9% High risk group: 45.1% Elderly: 71.0% | 70% | $24,632 (£20,000) | Maximum incremental cost of the quadrivalent vaccine = $0.25 | Quadrivalent influenza vaccines are cost-effective. |
| Gerlier [27] | LAIV: $32.3 TIV: $6.4 | 50% | 80% | $32,612 (€31,000) | Societal perspective: ICER = $1679/LY Payer’s perspective: ICER = $18,937/LY | Vaccinating 2–17 Y with LAIV in addition to the current strategy is cost-effective. |
| Gibson [28] | LAIV: $17.6 TIV: $7.9 | 50% | 80% | $31,560 (£30,000) | ICER = $6531/QALY | Vaccinating 2–17 Y with LAIV in addition to the current strategy is cost-effective in England and Wales |
| Gibson [28] | LAIV: $17.6 TIV: $5.0 | 50% | 80% | $31,560 (£30,000) | ICER = $2963/QALY | Vaccinating 2–17 Y with LAIV in addition to the current strategy is cost-effective in Brazil |
| Gibson [28] | LAIV: $17.6 TIV: $2.8 | 50% | 80% | $31,560 (£30,000) | ICER = $2022/QALY | Vaccinating 2–17 Y with LAIV in addition to the current strategy is cost-effective in Spain |
| Gibson [28] | LAIV: $17.6 TIV: $4.5 | 50% | 80% | $31,560 (£30,000) | ICER = $1708/QALY | Vaccinating 2–17 Y with LAIV in addition to the current strategy is cost-effective in Taiwan |
| Nagy [29] | LAIV: $27.5 | 22–28% | 80% | $6312 (€6000) | Societal perspective: ICER = $189/QALY Payer’s perspective: ICER = $6032/QALY | Vaccinating 2–17 Y with LAIV is cost-effective |
| Shim [20] | LAIV: $23.4 TIV: $14.6 | 58% | 83% | $159,123 | Public sector’s perspective: ICER = $53,960/QALY | Vaccinating 2–8 Y with LAIV is cost-effective over TIV. |
| Baguelin [30] | LAIV: $7.8 TIV: $7.8 | 50% | 70% | $30,791 (£25,000) | ICER: $9968/QALY | Vaccinating 2–16 Y with LAIV in addition to the current strategy is cost-effective. |
| Damm [31] | LAIV: $21.6 TIV: $11.4 | 50% | 80% | $52,600 (€50,000) | Narrow third-party payer: ICER = $2507/QALY Board third-party payer: ICER = $1359/QALY | Vaccinating 2–16 Y with LAIV in addition to the current strategy is cost-effective. |
| Pitman [32] | LAIV: $7.3 TIV: $7.3 | 50% | 80% | $24,632 (£20,000) | ICER: $309/QALY | Vaccinating 2–18 Y with LAIV in addition to the current strategy is cost-effective. |
| Tarride [19] | LAIV: $11.1 TIV: $7.6 | 37% (2–5 Y); 42% (6–9 Y); 100% (10–17 Y) | 60% | $39,152 (CAD$50,000) | Societal perspective: LAIV is the dominant strategy Payer’s perspective: LAIV is the dominant strategy | Vaccinating 2–16 Y with LAIV is cost-effective over TIV. |
| Prosser [23] | LAIV: $17.9 | NR | 84% | $150,000 | 6 M–23 M: $18,000/QALY 2 Y: $21,000/QALY 3–4 Y: $32,000/QALY | Vaccinating with LAIV is cost-effective for different age groups. Cost-effectiveness among children decreases with increasing age. |
| Prosser [21] | LAIV: $12.9 | NR | 84% | $150,000 | 6 M–23 M: $9000/QALY 2 Y: $15,000/QALY 3–4 Y: $25,000/QALY 5–11 Y: $72,000/QALY 12–17 Y: $109,000/QALY | Vaccinating with LAIV is cost-effective for different age groups. Cost-effectiveness decreases (i.e., higher ICER) when increasing the vaccination age among children. |
| Study No. | Checklist Question | De Boer 2021 [24] | Wenzel 2011 [25] | Thorrington 2017 [26] | Gerlier 2016 [27] | Gibson 2016 * [28] | Nagy 2016 [29] | Shim 2016 [30] | Baguelin 2015 [31] | Damm 2015 [32] | Pitman 2013 [33] | Tarride 2012 [19] | Prosser 2011 [23] | Prosser 2006 [21] | Total (% of Yes) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Is the study population clearly described? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 2 | Are competing alternatives clearly described? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 3 | Is a well-defined research question posed in answerable form? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 4 | Is the economic study design appropriate to the stated objective? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 5 | Are the structural assumptions and the validation methods of the model properly reported? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 6 | Is the chosen time horizon appropriate in order to include the relevant costs and consequences? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 7 | Is the actual perspective chosen appropriate? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 8 | Are all important and relevant costs for each alternative identified? | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 84.6 |
| 9 | Are all costs measured appropriately in physical units? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 10 | Are costs valued appropriately? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 11 | Are all important and relevant outcomes for each alternative identified? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 12 | Are all outcomes measured appropriately? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 13 | Are outcomes valued appropriately? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 14 | Is an appropriate incremental analysis of costs and outcomes of alternatives performed? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 15 | Are all future costs and outcomes discounted appropriately? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 84.6 |
| 16 | Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 17 | Do the conclusions follow from the data reported? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 100 |
| 18 | Does the study discuss the generalizability of the results to other settings and patient/client groups? | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 38.4 |
| 19 | Does the article/report indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 84.6 |
| 20 | Are ethical and distributional issues discussed appropriately? | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 38.4 |
| % of Yes | 90 | 85 | 100 | 95 | 95 | 90 | 90 | 90 | 95 | 90 | 90 | 95 | 90 | ||
| Overall quality | Good | Good | Excellent | Good | Good | Good | Good | Good | Good | Good | Good | Good | Good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, K.S.-K.; Wong, C.H.-L.; Choi, H.C.-W. Cost-Effectiveness of Intranasal Live-Attenuated Influenza Vaccine for Children: A Systematic Review. Vaccines 2022, 10, 1466. https://doi.org/10.3390/vaccines10091466
Chan KS-K, Wong CH-L, Choi HC-W. Cost-Effectiveness of Intranasal Live-Attenuated Influenza Vaccine for Children: A Systematic Review. Vaccines. 2022; 10(9):1466. https://doi.org/10.3390/vaccines10091466
Chicago/Turabian StyleChan, Kenneth Sik-Kwan, Charlene Hoi-Lam Wong, and Horace Cheuk-Wai Choi. 2022. "Cost-Effectiveness of Intranasal Live-Attenuated Influenza Vaccine for Children: A Systematic Review" Vaccines 10, no. 9: 1466. https://doi.org/10.3390/vaccines10091466
APA StyleChan, K. S.-K., Wong, C. H.-L., & Choi, H. C.-W. (2022). Cost-Effectiveness of Intranasal Live-Attenuated Influenza Vaccine for Children: A Systematic Review. Vaccines, 10(9), 1466. https://doi.org/10.3390/vaccines10091466






