Lessons Learned from the Development and Roll-Out of the rVSVΔG-ZEBOV-GP Zaire ebolavirus Vaccine to Inform Marburg Virus and Sudan ebolavirus Vaccines
Abstract
1. Introduction
2. Clinical and Non-Clinical Development of rVSVΔG-ZEBOV-GP Pre- and Post-Product Registration
2.1. Early Characterization and Clinical Development Leading to Registration of rVSVΔG-ZEBOV-GP
2.2. Post-Product Registration Advancements in Non-Clinical and Clinical Development
2.2.1. Pediatric Populations
2.2.2. HIV-Positive Individuals
2.2.3. Impact of Booster Doses
2.2.4. Human Studies Assessing Durability of Immunogenicity and Correlates of Protection
2.2.5. NHP Studies Assessing Correlates and Durability of Protection
2.3. Bridging to Marburg and Sudan Vaccine Viruses
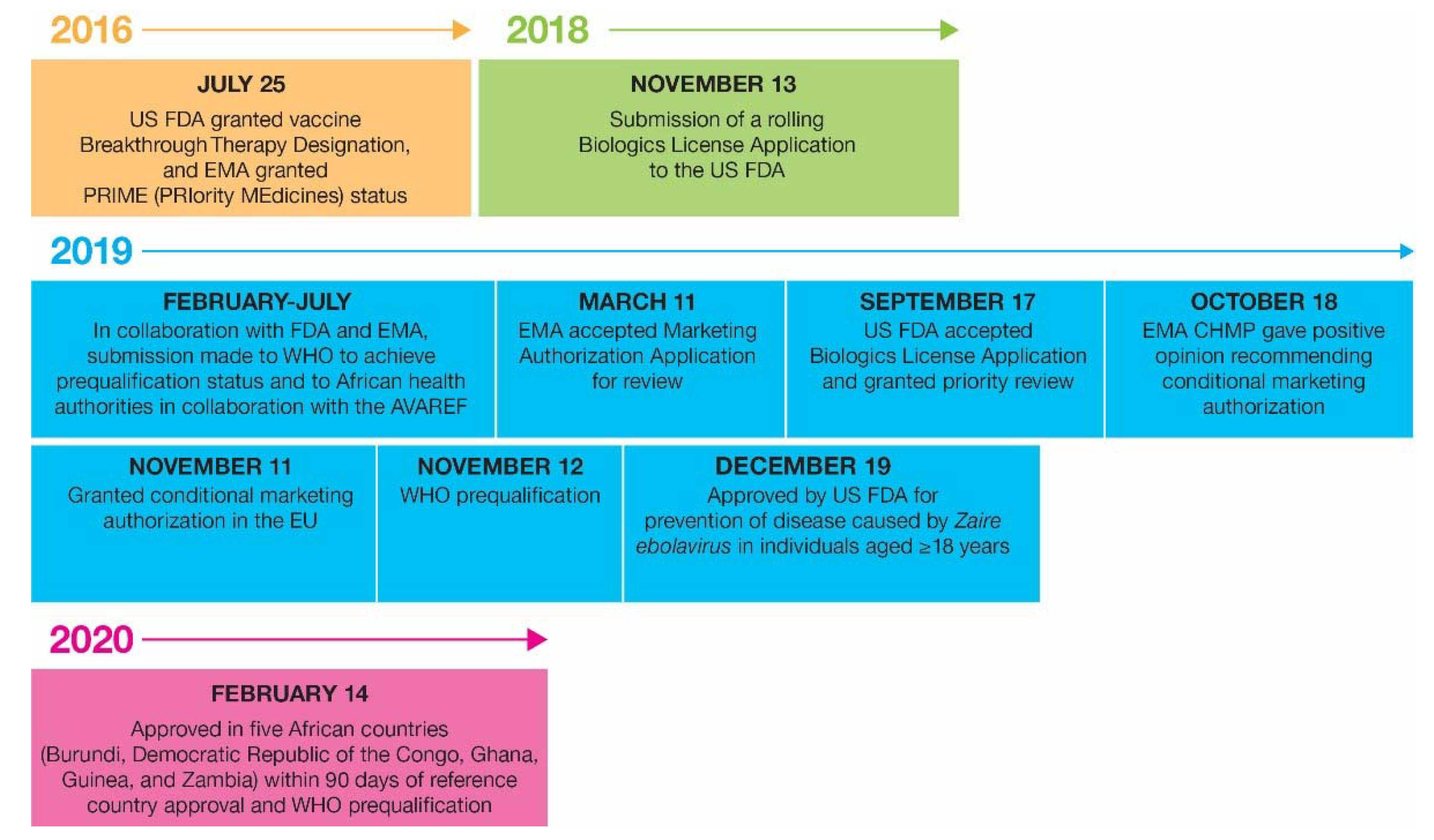
3. Regulatory Aspects
3.1. Initial Product Registration and Post-Approval Changes
3.2. Expansion of Indication to Include Pediatric and HIV-Positive Individuals
3.3. Labeling Harmonization to Facilitate Distribution of Stockpiled or Pandemic Vaccines
4. Use of rVSVΔG-ZEBOV-GP in Ebola Outbreak Response
5. Manufacturing and Stockpiling
5.1. General Business Model and Sustainability
5.2. Supply/Demand Planning
5.3. Supply Chain Design—Manufacturing Facility
5.4. Supply Chain Design—Stockpile Enablement
6. Authority Recommendations for rVSVΔG-ZEBOV-GP
7. Environmental Risk Assessment for rVSVΔG-ZEBOV-GP
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Ebola Response Team; Agua-Agum, J.; Allegranzi, B.; Ariyarajah, A.; Aylward, R.; Blake, I.M.; Barboza, P.; Bausch, D.; Brennan, R.J.; Clement, P.; et al. After Ebola in West Africa--Unpredictable Risks, Preventable Epidemics. N. Engl. J. Med. 2016, 375, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Garbutt, M.; Liebscher, R.; Wahl-Jensen, V.; Jones, S.; Möller, P.; Wagner, R.; Volchkov, V.; Klenk, H.D.; Feldmann, H.; Ströher, U. Properties of replication-competent vesicular stomatitis virus vectors expressing glycoproteins of filoviruses and arenaviruses. J. Virol. 2004, 78, 5458–5465. [Google Scholar] [CrossRef] [PubMed]
- Merck Sharp & Dohme L.L.C. ERVEBO® Prescribing Information. 2019. Available online: https://www.fda.gov/media/133748/download (accessed on 15 March 2022).
- European Medicines Agency. Ervebo: Summary of Product Characteristics. 2019. Available online: https://www.ema.europa.eu/en/documents/product-information/ervebo-epar-product-information_en.pdf (accessed on 6 April 2022).
- Regules, J.A.; Beigel, J.H.; Paolino, K.M.; Voell, J.; Castellano, A.R.; Hu, Z.; Munoz, P.; Moon, J.; Ruck, R.; Bennet, J.; et al. A Recombinant Vesicular Stomatitis Virus Ebola Vaccine. N. Engl. J. Med. 2017, 376, 330–341. [Google Scholar] [CrossRef] [PubMed]
- Heppner, D.G., Jr.; Kemp, T.L.; Martin, B.K.; Ramsey, W.J.; Nichols, R.; Dasen, E.J.; Link, C.J.; Das, R.; Xu, Z.J.; Sheldon, E.A.; et al. Safety and immunogenicity of the rVSVΔG-ZEBOV-GP Ebola virus vaccine candidate in healthy adults: A phase 1b randomised, multicentre, double-blind, placebo-controlled, dose-response study. Lancet Infect. Dis. 2017, 17, 854–866. [Google Scholar] [CrossRef]
- ElSherif, M.S.; Brown, C.; MacKinnon-Cameron, D.; Li, L.; Racine, T.; Alimonti, J.; Rudge, T.L.; Sabourin, C.; Silvera, P.; Hooper, J.W.; et al. Assessing the safety and immunogenicity of recombinant vesicular stomatitis virus Ebola vaccine in healthy adults: A randomized clinical trial. CMAJ 2017, 189, E819–E827. [Google Scholar] [CrossRef]
- Agnandji, S.T.; Huttner, A.; Zinser, M.E.; Njuguna, P.; Dahlke, C.; Fernandes, J.F.; Yerly, S.; Dayer, J.A.; Kraehling, V.; Kasonta, R.; et al. Phase 1 Trials of rVSV Ebola Vaccine in Africa and Europe. N. Engl. J. Med. 2016, 374, 1647–1660. [Google Scholar] [CrossRef]
- Huttner, A.; Dayer, J.A.; Yerly, S.; Combescure, C.; Auderset, F.; Desmeules, J.; Eickmann, M.; Finckh, A.; Goncalves, A.R.; Hooper, J.W.; et al. The effect of dose on the safety and immunogenicity of the VSV Ebola candidate vaccine: A randomised double-blind, placebo-controlled phase 1/2 trial. Lancet Infect. Dis. 2015, 15, 1156–1166. [Google Scholar] [CrossRef]
- Agnandji, S.T.; Fernandes, J.F.; Bache, E.B.; Obiang Mba, R.M.; Brosnahan, J.S.; Kabwende, L.; Pitzinger, P.; Staarink, P.; Massinga-Loembe, M.; Krähling, V.; et al. Safety and immunogenicity of rVSVΔG-ZEBOV-GP Ebola vaccine in adults and children in Lambaréné, Gabon: A phase I randomised trial. PLoS Med. 2017, 14, e1002402. [Google Scholar] [CrossRef]
- Dahlke, C.; Kasonta, R.; Lunemann, S.; Krähling, V.; Zinser, M.E.; Biedenkopf, N.; Fehling, S.K.; Ly, M.L.; Rechtien, A.; Stubbe, H.C.; et al. Dose-dependent T-cell Dynamics and Cytokine Cascade Following rVSV-ZEBOV Immunization. EBioMedicine 2017, 19, 107–118. [Google Scholar] [CrossRef]
- Kennedy, S.B.; Bolay, F.; Kieh, M.; Grandits, G.; Badio, M.; Ballou, R.; Eckes, R.; Feinberg, M.; Follmann, D.; Grund, B.; et al. Phase 2 Placebo-Controlled Trial of Two Vaccines to Prevent Ebola in Liberia. N. Engl. J. Med. 2017, 377, 1438–1447. [Google Scholar] [CrossRef]
- Henao-Restrepo, A.M.; Camacho, A.; Longini, I.M.; Watson, C.H.; Edmunds, W.J.; Egger, M.; Carroll, M.W.; Dean, N.E.; Diatta, I.; Doumbia, M.; et al. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ca Suffit!). Lancet 2017, 389, 505–518. [Google Scholar] [CrossRef]
- Samai, M.; Seward, J.F.; Goldstein, S.T.; Mahon, B.E.; Lisk, D.R.; Widdowson, M.A.; Jalloh, M.I.; Schrag, S.J.; Idriss, A.; Carter, R.J.; et al. The Sierra Leone Trial to Introduce a Vaccine Against Ebola: An Evaluation of rVSVΔG-ZEBOV-GP Vaccine Tolerability and Safety During the West Africa Ebola Outbreak. J. Infect Dis. 2018, 217, S6–S15. [Google Scholar] [CrossRef]
- Halperin, S.A.; Arribas, J.R.; Rupp, R.; Andrews, C.P.; Chu, L.; Das, R.; Simon, J.K.; Onorato, M.T.; Liu, K.; Martin, J.; et al. Six-Month Safety Data of Recombinant Vesicular Stomatitis Virus-Zaire Ebola Virus Envelope Glycoprotein Vaccine in a Phase 3 Double-Blind, Placebo-Controlled Randomized Study in Healthy Adults. J. Infect. Dis. 2017, 215, 1789–1798. [Google Scholar] [CrossRef] [PubMed]
- Halperin, S.A.; Das, R.; Onorato, M.T.; Liu, K.; Martin, J.; Grant-Klein, R.J.; Nichols, R.; Coller, B.A.; Helmond, F.A.; Simon, J.K.; et al. Immunogenicity, Lot Consistency, and Extended Safety of rVSVΔG-ZEBOV-GP Vaccine: A Phase 3 Randomized, Double-Blind, Placebo-Controlled Study in Healthy Adults. J. Infect. Dis. 2019, 220, 1127–1135. [Google Scholar] [CrossRef]
- Higgs, E.S.; Dubey, S.A.; Coller, B.A.G.; Simon, J.K.; Bollinger, L.; Sorenson, R.A.; Wilson, B.; Nason, M.C.; Hensley, L.E. Accelerating Vaccine Development During the 2013-2016 West African Ebola Virus Disease Outbreak. Curr. Top. Microbiol. Immunol. 2017, 411, 229–261. [Google Scholar] [CrossRef]
- Ebola ça Suffit Ring Vaccination Trial Consortium. The ring vaccination trial: A novel cluster randomised controlled trial design to evaluate vaccine efficacy and effectiveness during outbreaks, with special reference to Ebola. BMJ 2015, 351, h3740. [Google Scholar] [CrossRef][Green Version]
- European Medicines Agency. First Vaccine to Protect against Ebola. 2019. Available online: https://www.ema.europa.eu/en/news/first-vaccine-protect-against-ebola (accessed on 16 March 2022).
- Geisbert, T.W.; Geisbert, J.B.; Leung, A.; Daddario-DiCaprio, K.M.; Hensley, L.E.; Grolla, A.; Feldmann, H. Single-injection vaccine protects nonhuman primates against infection with marburg virus and three species of ebola virus. J. Virol. 2009, 83, 7296–7304. [Google Scholar] [CrossRef]
- Jones, S.M.; Feldmann, H.; Stroher, U.; Geisbert, J.B.; Fernando, L.; Grolla, A.; Klenk, H.D.; Sullivan, N.J.; Volchkov, V.E.; Fritz, E.A.; et al. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 2005, 11, 786–790. [Google Scholar] [CrossRef]
- Marzi, A.; Engelmann, F.; Feldmann, F.; Haberthur, K.; Shupert, W.L.; Brining, D.; Scott, D.P.; Geisbert, T.W.; Kawaoka, Y.; Katze, M.G.; et al. Antibodies are necessary for rVSV/ZEBOV-GP-mediated protection against lethal Ebola virus challenge in nonhuman primates. Proc. Natl. Acad. Sci. USA 2013, 110, 1893–1898. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Fernando, L.; Alimonti, J.B.; Melito, P.L.; Feldmann, F.; Dick, D.; Stroher, U.; Feldmann, H.; Jones, S.M. Mucosal immunization of cynomolgus macaques with the VSVDeltaG/ZEBOVGP vaccine stimulates strong ebola GP-specific immune responses. PLoS ONE 2009, 4, e5547. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Ervebo: EPAR—Product Information. 2019. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ervebo (accessed on 15 March 2022).
- Monath, T.P.; Fast, P.E.; Modjarrad, K.; Clarke, D.K.; Martin, B.K.; Fusco, J.; Nichols, R.; Heppner, D.G.; Simon, J.K.; Dubey, S.; et al. rVSVDeltaG-ZEBOV-GP (also designated V920) recombinant vesicular stomatitis virus pseudotyped with Ebola Zaire Glycoprotein: Standardized template with key considerations for a risk/benefit assessment. Vaccine X 2019, 1, 100009. [Google Scholar] [CrossRef] [PubMed]
- Marzi, A.; Robertson, S.J.; Haddock, E.; Feldmann, F.; Hanley, P.W.; Scott, D.P.; Strong, J.E.; Kobinger, G.; Best, S.M.; Feldmann, H. EBOLA VACCINE. VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain. Science 2015, 349, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Tell, J.G.; Coller, B.G.; Dubey, S.A.; Jenal, U.; Lapps, W.; Wang, L.; Wolf, J. Environmental Risk Assessment for rVSVΔG-ZEBOV-GP, a Genetically Modified Live Vaccine for Ebola Virus Disease. Vaccines 2020, 8, 779. [Google Scholar] [CrossRef] [PubMed]
- Badio, M.; Lhomme, E.; Kieh, M.; Beavogui, A.H.; Kennedy, S.B.; Doumbia, S.; Leigh, B.; Sow, S.O.; Diallo, A.; Fusco, D.; et al. Partnership for Research on Ebola VACcination (PREVAC): Protocol of a randomized, double-blind, placebo-controlled phase 2 clinical trial evaluating three vaccine strategies against Ebola in healthy volunteers in four West African countries. Trials 2021, 22, 86. [Google Scholar] [CrossRef]
- Kieh, M.; Richert, L.; Beavogui, A.H.; Grund, B.; Leigh, B.; D’Ortenzio, E.; Doumbia, S.; Sow, S.; Hensley, L.; Levy, Y.; et al. PREVAC RCT: Effects of 3 Ebola vaccine strategies in West African adults and children. In Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI), 12–24 February 2022; virtually; Abstract 323. Available online: https://www.croiconference.org/abstract/prevac-rct-effects-of-3-ebola-vaccine-strategies-in-west-african-adults-and-children/ (accessed on 16 June 2022).
- Kieh, M.; Richert, L.; Beavogui, A.H.; Grund, B.; Leigh, B.; D’Ortenzio, E.; Doumbia, S.; Sow, S.; Hensley, L.; Levy, Y.; et al. PREVAC RCT: Effects of 3 Ebola vaccine strategies in West African adults and children. In Proceedings of the Conference on Retroviruses and Opportunistic Infections (CROI), 12–24 February 2022. virtually; Poster 00323. [Google Scholar]
- Grais, R.F.; Kennedy, S.B.; Mahon, B.E.; Dubey, S.A.; Grant-Klein, R.J.; Liu, K.; Hartzel, J.; Coller, B.A.; Welebob, C.; Hanson, M.E.; et al. Estimation of the correlates of protection of the rVSVΔG-ZEBOV-GP Zaire ebolavirus vaccine: A post-hoc analysis of data from phase 2/3 clinical trials. Lancet Microbe 2021, 2, e70–e78. [Google Scholar] [CrossRef]
- Kilgore, N.; Nuzum, E.O. An interagency collaboration to facilitate development of filovirus medical countermeasures. Viruses 2012, 4, 2312–2316. [Google Scholar] [CrossRef]
- Rudge, T.L., Jr.; Sankovich, K.A.; Niemuth, N.A.; Anderson, M.S.; Badorrek, C.S.; Skomrock, N.D.; Cirimotich, C.M.; Sabourin, C.L. Development, qualification, and validation of the Filovirus Animal Nonclinical Group anti-Ebola virus glycoprotein immunoglobulin G enzyme-linked immunosorbent assay for human serum samples. PLoS ONE 2019, 14, e0215457. [Google Scholar] [CrossRef]
- Niemuth, N.A.; Rudge, T.L., Jr.; Sankovich, K.A.; Anderson, M.S.; Skomrock, N.D.; Badorrek, C.S.; Sabourin, C.L. Method feasibility for cross-species testing, qualification, and validation of the Filovirus Animal Nonclinical Group anti-Ebola virus glycoprotein immunoglobulin G enzyme-linked immunosorbent assay for non-human primate serum samples. PLoS ONE 2020, 15, e0241016. [Google Scholar] [CrossRef]
- Antonello, J.; Grant-Klein, R.J.; Nichols, R.; Kennedy, S.B.; Dubey, S.; Simon, J.K. Serostatus cutoff levels and fold increase to define seroresponse to recombinant vesicular stomatitis virus—Zaire Ebola virus envelope glycoprotein vaccine: An evidence-based analysis. Vaccine 2020, 38, 4885–4891. [Google Scholar] [CrossRef]
- Amanna, I.J.; Messaoudi, I.; Slifka, M.K. Protective immunity following vaccination: How is it defined? Hum. Vaccine 2008, 4, 316–319. [Google Scholar] [CrossRef]
- International Coalition of Medicines Regulatory Authorities. Global Regulatory Workshop on COVID-19 Vaccine Development. 2020. Available online: https://www.icmra.info/drupal/en/news/March2020/summary (accessed on 9 May 2022).
- U.S. Food and Drug Administration. Development and Licensure of Vaccines to Prevent COVID-19: Guidance for Industry. 2019. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-and-licensure-vaccines-prevent-covid-19 (accessed on 9 May 2022).
- World Health Organization. Evaluation of the Quality, Safety and Efficacy of Messenger RNA Vaccines for the Prevention of Infectious Diseases: Regulatory Considerations. 2021. Available online: https://www.who.int/publications/m/item/evaluation-of-the-quality-safety-and-efficacy-of-messenger-rna-vaccines-for-the-prevention-of-infectious-diseases-regulatory-considerations (accessed on 9 May 2022).
- World Health Organization. Roadmap for Introduction and Roll-Out of Merck rVSV-ZEBOV Ebola Virus Disease Vaccine in African Countries. 2019. Available online: https://www.who.int/publications/m/item/merck-EVD-vax-intro-roadmap (accessed on 15 March 2022).
- World Health Organization. Lessons Learnt in Expediting Prequalification and Registration of Ebola Zaire Vaccine. 2020. Available online: https://apps.who.int/iris/handle/10665/333730 (accessed on 15 March 2022).
- World Health Organization. Ebola. North Kivu/Ituri, Democratic Republic of the Congo, August 2018–June 2020. 2020. Available online: https://www.who.int/emergencies/situations/Ebola-2019-drc- (accessed on 15 March 2022).
- World Health Organization. Ebola-African Region (AFRO), Democratic Republic of the Congo. 2020. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON284 (accessed on 15 March 2022).
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, October 2018-Conclusions and Recommendations. 2018. Available online: https://www.who.int/publications/i/item/55th-report-of-the-who-expert-committee-on-specifications-for-pharmaceutical-preparations (accessed on 15 March 2022).
- Rupani, N.; Ngole, M.E.; Lee, J.A.; Aluisio, A.R.; Gainey, M.; Perera, S.M.; Ntamwinja, L.K.; Matafali, R.M.; Muhayangabo, R.F.; Makoyi, F.N.; et al. Effect of Recombinant Vesicular Stomatitis Virus-Zaire Ebola Virus Vaccination on Ebola Virus Disease Illness and Death, Democratic Republic of the Congo. Emerg. Infect. Dis. 2022, 28, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.; Pagliusi, S.; Park, R.; Wilmansyah, T.; Jadhav, S.; Santana, P.C.; Krishnamurthy, K.R.; Yang, L. The importance of vaccine stockpiling to respond to epidemics and remediate global supply shortages affecting immunization: Strategic challenges and risks identified by manufacturers. Vaccine X 2021, 9, 100119. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Coordinating Group (ICG) on Vaccine Provision. 2022. Available online: https://www.who.int/groups/icg (accessed on 6 April 2022).
- World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, 22–24 March 2021: Conclusions and Recommendations. 2021. Available online: https://www.who.int/publications/i/item/meeting-of-the-strategic-advisory-group-of-experts-on-immunization-22-24-march-2021-conclusions-and-recommendations (accessed on 15 March 2022).
- Davis, H.; Dow, T.; Isopi, L.; Blue, J.T. Examination of the effect of agitation on the potency of the Ebola Zaire vaccine rVSVΔG-ZEBOV-GP. Vaccine 2020, 38, 2643–2645. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Cossaboom, C.M.; Whitesell, A.N.; Dyal, J.W.; Joyce, A.; Morgan, R.L.; Campos-Outcalt, D.; Person, M.; Ervin, E.; Yu, Y.C.; et al. Use of Ebola Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm. Rep. 2021, 70, 1–12. [Google Scholar] [CrossRef]
- Malenfant, J.H.; Joyce, A.; Choi, M.J.; Cossaboom, C.M.; Whitesell, A.N.; Harcourt, B.H.; Atmar, R.L.; Villanueva, J.M.; Bell, B.P.; Hahn, C.; et al. Use of Ebola Vaccine: Expansion of Recommendations of the Advisory Committee on Immunization Practices To Include Two Additional Populations-United States, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 290–292. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Determining the Need for and Content of Environmental Assessments for Gene Therapies, Vectored Vaccines, and Related Recombinant Viral or Microbial Products. Guidance for Industry. 2015. Available online: https://www.fda.gov/media/91425/download (accessed on 15 March 2022).
- European Union. Directive 2001/18/EC of the European Parliament and of the Council of 12 March 2001 on the Deliberate Release into the Environment of Genetically Modified Organisms and Repealing Council Directive 90/220/EEC-Commission Declaration. 2001. Available online: https://eur-lex.europa.eu/eli/dir/2001/18/oj (accessed on 15 March 2022).
- Jones, S.M.; Stroher, U.; Fernando, L.; Qiu, X.; Alimonti, J.; Melito, P.; Bray, M.; Klenk, H.D.; Feldmann, H. Assessment of a vesicular stomatitis virus-based vaccine by use of the mouse model of Ebola virus hemorrhagic fever. J. Infect. Dis. 2007, 196, S404–S412. [Google Scholar] [CrossRef]
- Bergren, N.A.; Miller, M.R.; Monath, T.P.; Kading, R.C. Assessment of the ability of V920 recombinant vesicular stomatitis-Zaire ebolavirus vaccine to replicate in relevant arthropod cell cultures and vector species. Hum. Vaccines Immunother. 2018, 14, 994–1002. [Google Scholar] [CrossRef]
- Tsuda, Y.; Safronetz, D.; Brown, K.; LaCasse, R.; Marzi, A.; Ebihara, H.; Feldmann, H. Protective efficacy of a bivalent recombinant vesicular stomatitis virus vaccine in the Syrian hamster model of lethal Ebola virus infection. J. Infect. Dis. 2011, 204, S1090–S1097. [Google Scholar] [CrossRef]
- Mire, C.E.; Miller, A.D.; Carville, A.; Westmoreland, S.V.; Geisbert, J.B.; Mansfield, K.G.; Feldmann, H.; Hensley, L.E.; Geisbert, T.W. Recombinant vesicular stomatitis virus vaccine vectors expressing filovirus glycoproteins lack neurovirulence in nonhuman primates. PLoS Negl. Trop. Dis. 2012, 6, e1567. [Google Scholar] [CrossRef]
- de Wit, E.; Marzi, A.; Bushmaker, T.; Brining, D.; Scott, D.; Richt, J.A.; Geisbert, T.W.; Feldmann, H. Safety of recombinant VSV-Ebola virus vaccine vector in pigs. Emerg. Infect. Dis. 2015, 21, 702–704. [Google Scholar] [CrossRef]
- Morozov, I.; Monath, T.P.; Meekins, D.A.; Trujillo, J.D.; Sunwoo, S.Y.; Urbaniak, K.; Kim, I.J.; Narayanan, S.K.; Indran, S.V.; Ma, W.; et al. High dose of vesicular stomatitis virus-vectored Ebola virus vaccine causes vesicular disease in swine without horizontal transmission. Emerg. Microbes Infect. 2021, 10, 651–663. [Google Scholar] [CrossRef] [PubMed]

| Agency and Date of Recommendations | Recommendations |
|---|---|
| WHO SAGE [48] March 2021 |
|
| U.S. CDC ACIP [50] February 2020 |
|
| U.S. CDC ACIP [51] November 2021 |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coller, B.-A.G.; Lapps, W., Jr.; Yunus, M.; Bruno, S.; Eichberg, M.J.; Lee, A.W.-T.; Liu, K.; Drury, R.; Millogo, J.; Macareo, L.R.; et al. Lessons Learned from the Development and Roll-Out of the rVSVΔG-ZEBOV-GP Zaire ebolavirus Vaccine to Inform Marburg Virus and Sudan ebolavirus Vaccines. Vaccines 2022, 10, 1446. https://doi.org/10.3390/vaccines10091446
Coller B-AG, Lapps W Jr., Yunus M, Bruno S, Eichberg MJ, Lee AW-T, Liu K, Drury R, Millogo J, Macareo LR, et al. Lessons Learned from the Development and Roll-Out of the rVSVΔG-ZEBOV-GP Zaire ebolavirus Vaccine to Inform Marburg Virus and Sudan ebolavirus Vaccines. Vaccines. 2022; 10(9):1446. https://doi.org/10.3390/vaccines10091446
Chicago/Turabian StyleColler, Beth-Ann G., William Lapps, Jr., Mahum Yunus, Samantha Bruno, Michael J. Eichberg, Andrew Wen-Tseng Lee, Kenneth Liu, Rosybel Drury, Jules Millogo, Louis Robert Macareo, and et al. 2022. "Lessons Learned from the Development and Roll-Out of the rVSVΔG-ZEBOV-GP Zaire ebolavirus Vaccine to Inform Marburg Virus and Sudan ebolavirus Vaccines" Vaccines 10, no. 9: 1446. https://doi.org/10.3390/vaccines10091446
APA StyleColler, B.-A. G., Lapps, W., Jr., Yunus, M., Bruno, S., Eichberg, M. J., Lee, A. W.-T., Liu, K., Drury, R., Millogo, J., Macareo, L. R., Armstrong, T. H., Blue, J. T., Isopi, L. A., Hughes, M., VanRheenen, S. M., Deutsch, J., Tell, J. G., & Dubey, S. A. (2022). Lessons Learned from the Development and Roll-Out of the rVSVΔG-ZEBOV-GP Zaire ebolavirus Vaccine to Inform Marburg Virus and Sudan ebolavirus Vaccines. Vaccines, 10(9), 1446. https://doi.org/10.3390/vaccines10091446





