Clinical Progression and Outcome of Hospitalized Patients Infected with SARS-CoV-2 Omicron Variant in Shanghai, China
Abstract
:1. Introduction
2. Methods
2.1. Study Designs and Participants
2.2. Procedures
2.3. Laboratory Confirmation
2.4. Statistical Analysis
3. Results
3.1. Clinical and Laboratory Characteristics of Participants on Admission
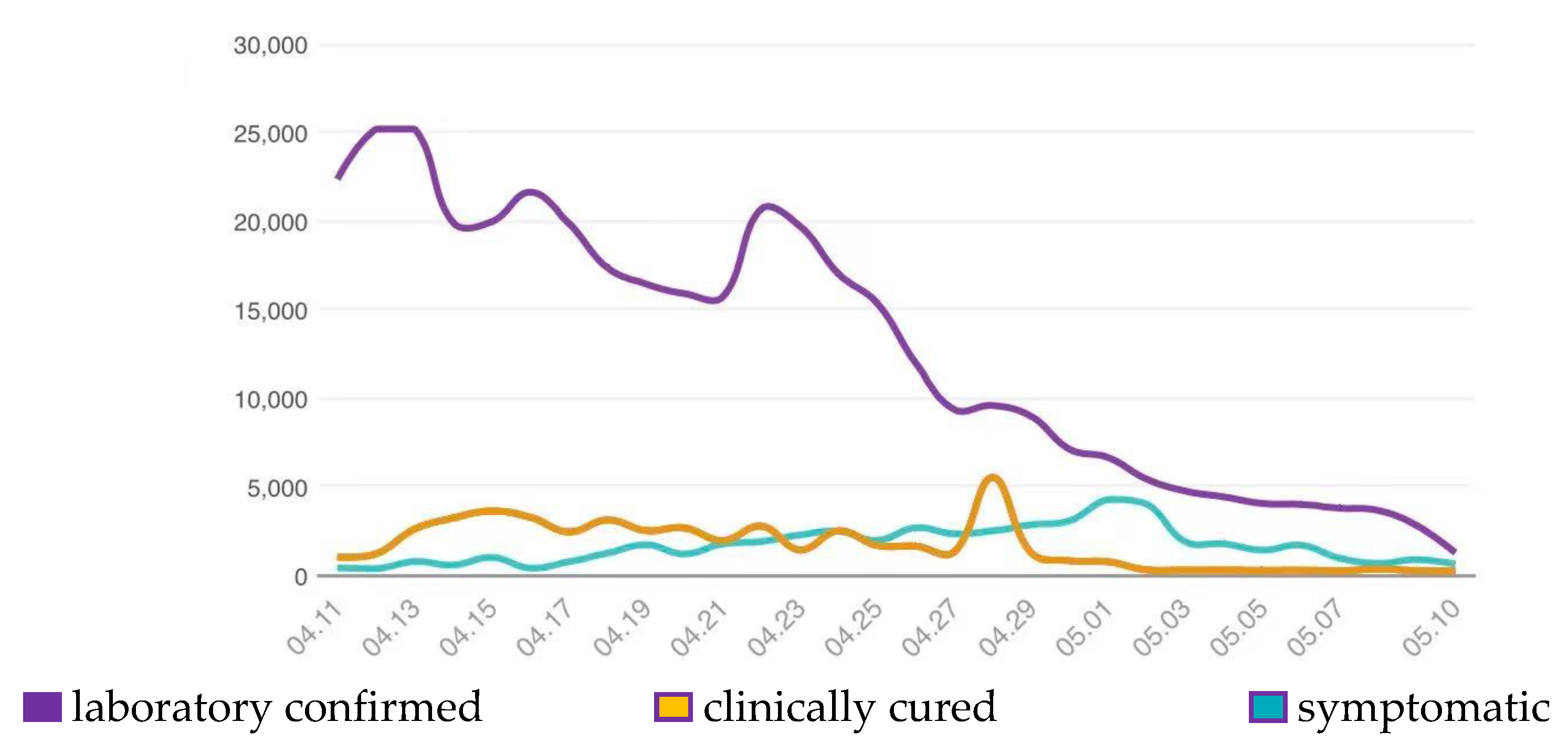
3.2. Clinical Outcomes of the Study Population
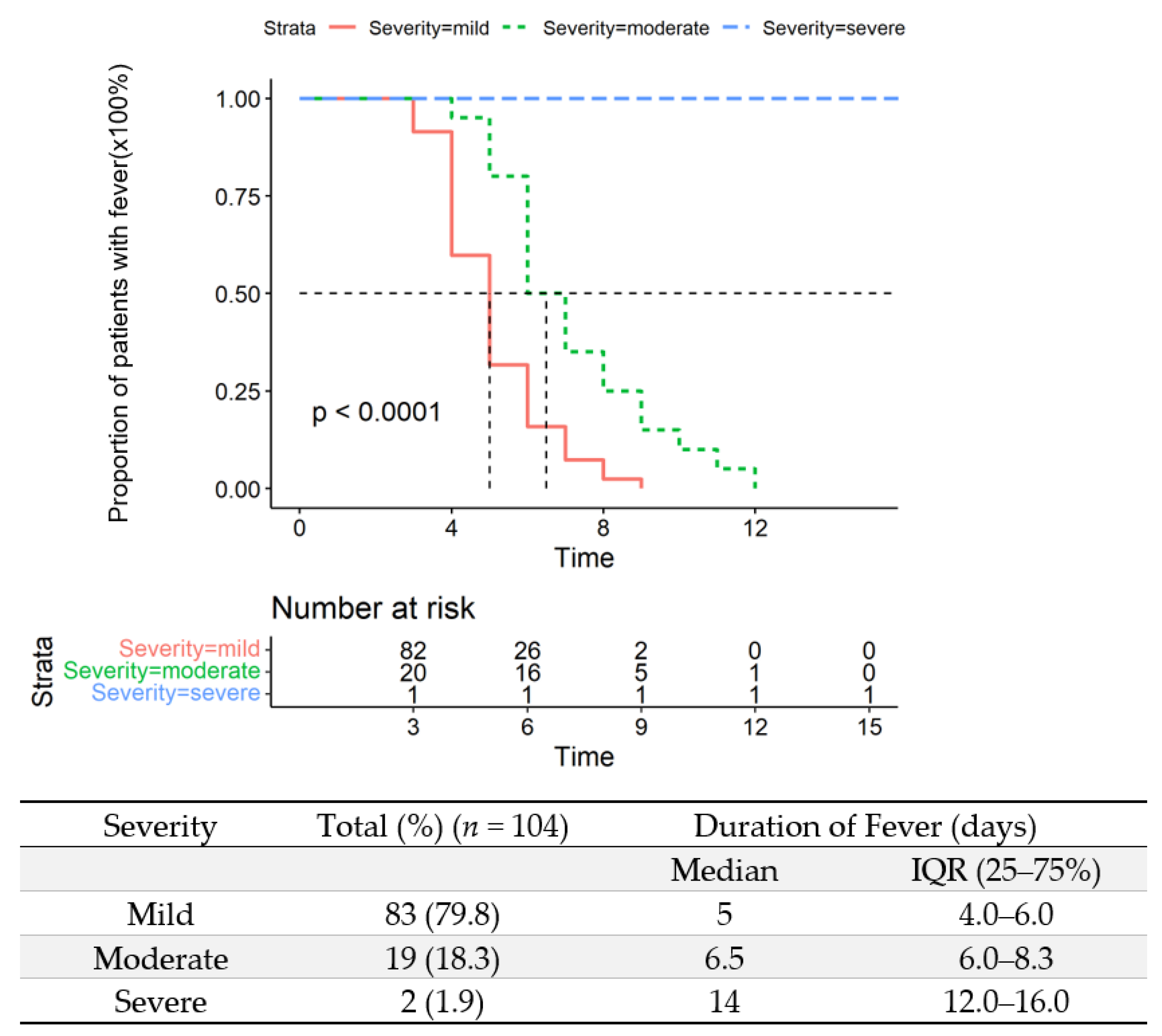
3.3. Duration of Fever in the Study Population
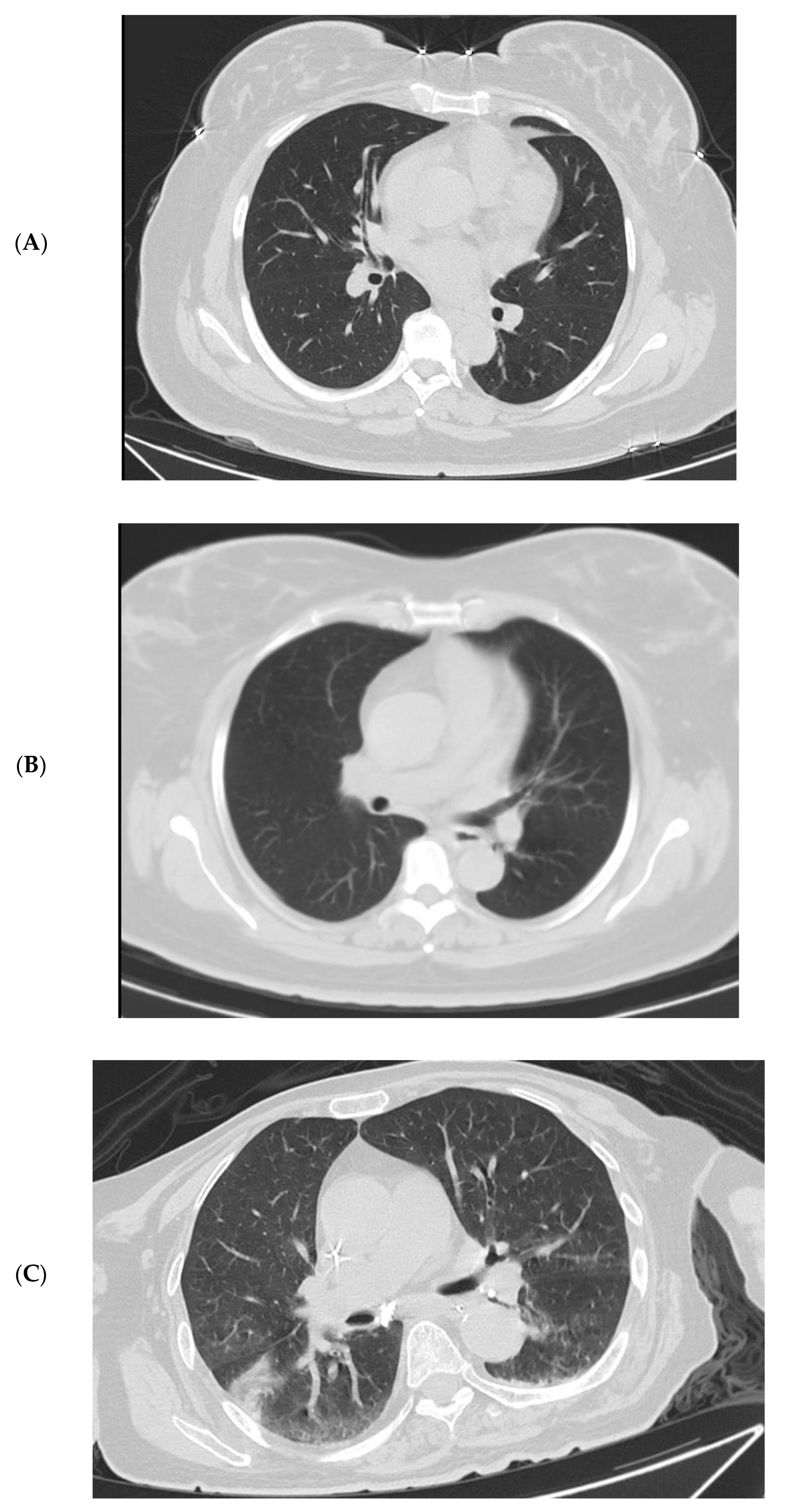
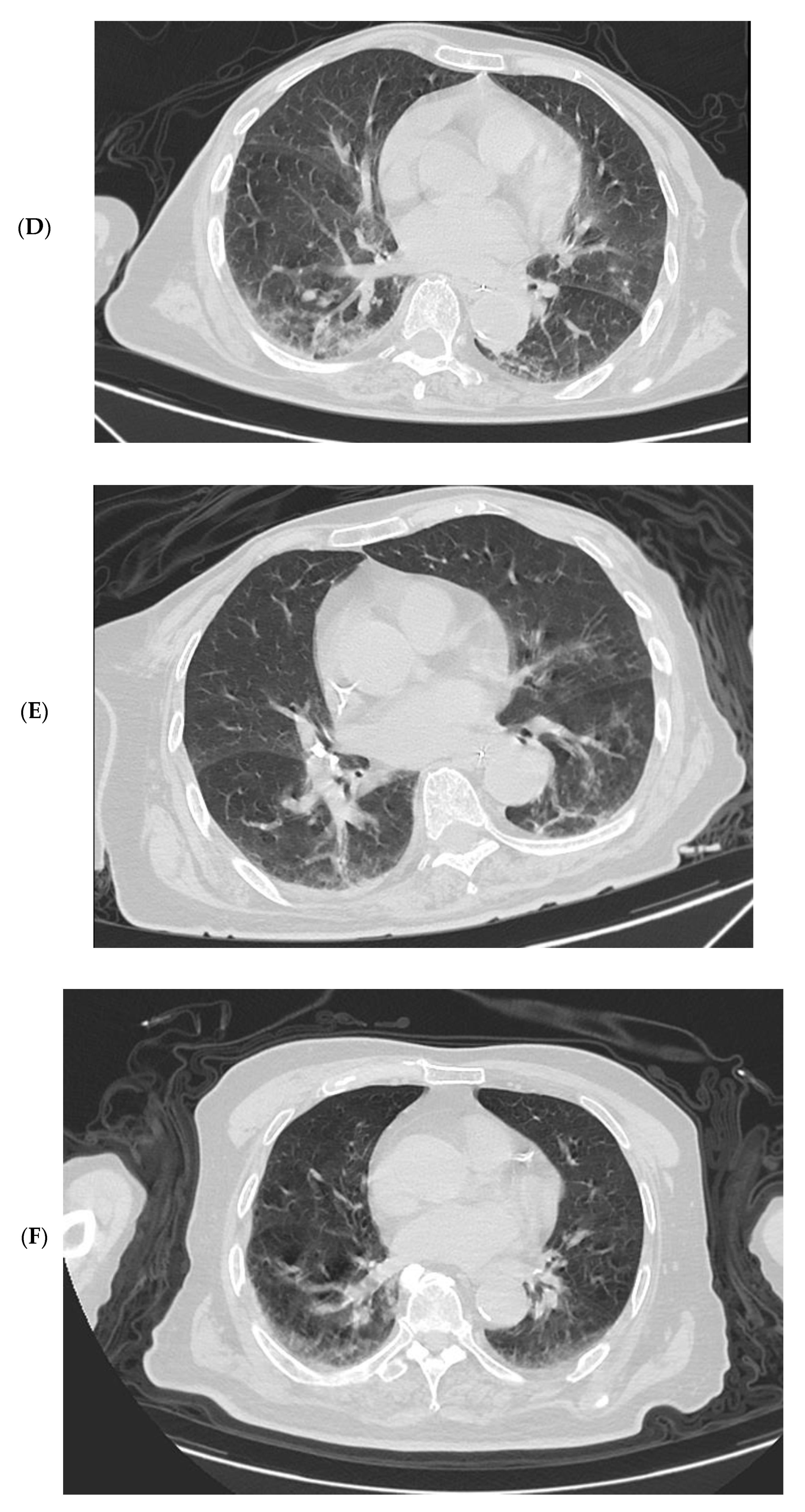
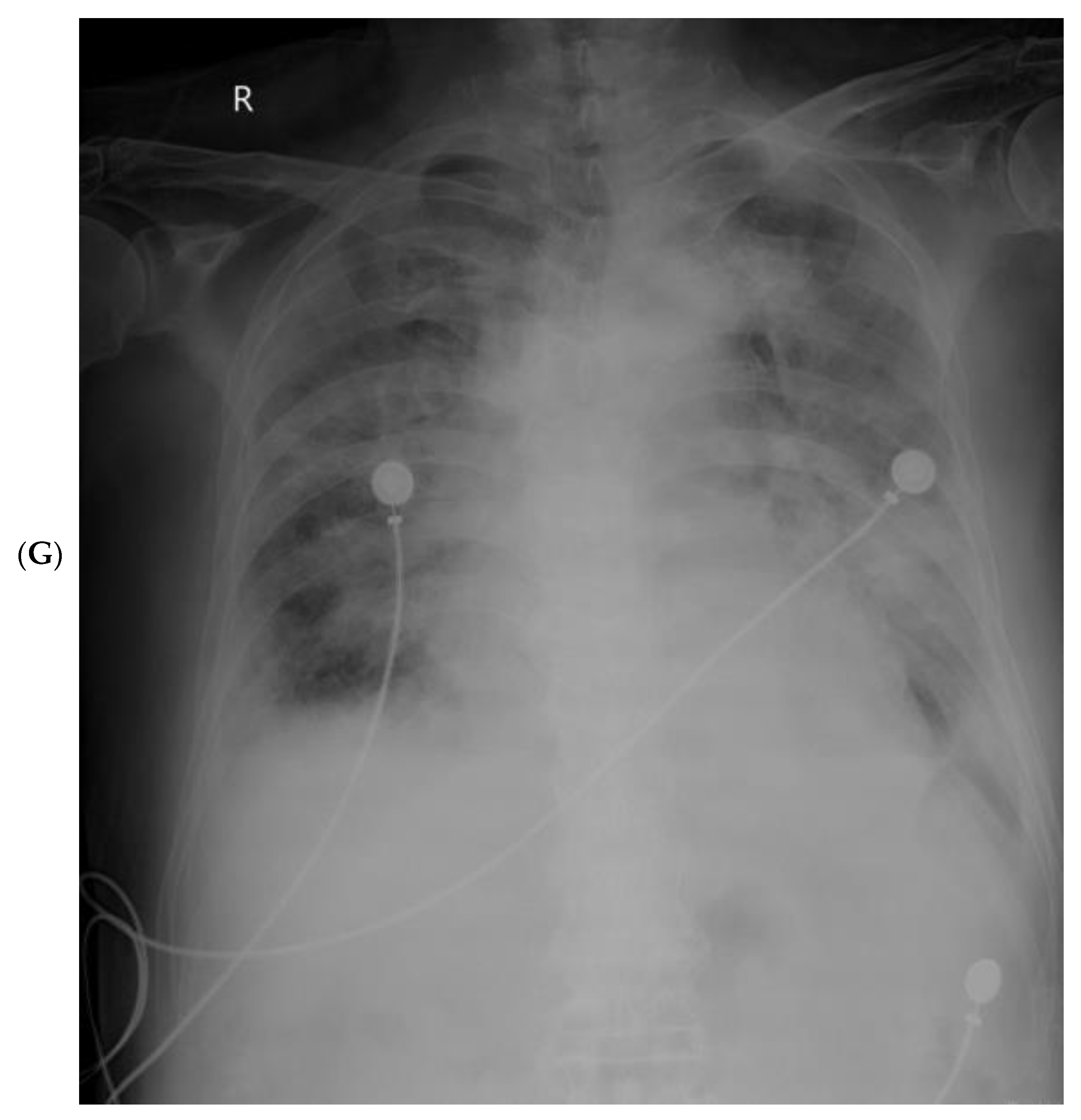
3.4. Imaging Changes in Disease Progression
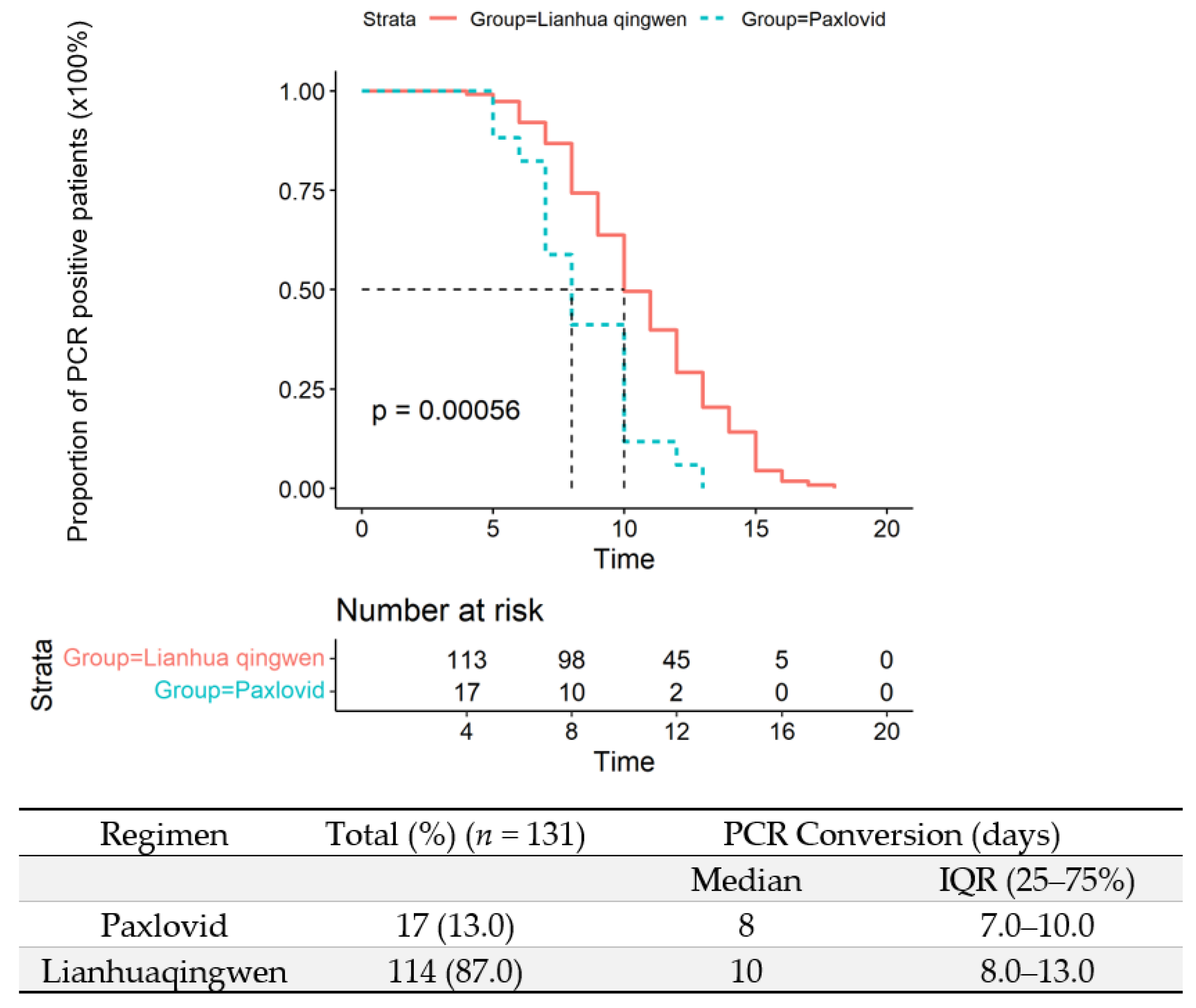
3.5. Viral Eradication in the Upper Respiratory Tract
3.6. Factors Related to the Severity of the Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, J.; Zeng, G. Letter to the editor: Epidemiology of the SARS-CoV-2 variant Omicron BA.2-vigilance needed. Euro Surveill 2022, 27, 22-00254. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Ratho, R.K. OMICRON (B.1.1.529): A new SARS-CoV-2 variant of concern mounting worldwide fear. J. Med. Virol. 2022, 94, 1821–1824. [Google Scholar] [CrossRef] [PubMed]
- Singhal, T. The Emergence of Omicron: Challenging Times Are Here Again! Indian J. Pediatr. 2022, 89, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Lee, B.; Choi, Y.Y.; Um, J.; Lee, K.S.; Sung, H.K.; Kim, Y.; Park, J.S.; Lee, M.; Jang, H.C.; et al. Clinical Characteristics of 40 Patients Infected With the SARS-CoV-2 Omicron Variant in Korea. J. Korean Med. Sci. 2022, 37, e31. [Google Scholar] [CrossRef]
- Lee, J.J.; Choe, Y.J.; Jeong, H.; Kim, M.; Kim, S.; Yoo, H.; Park, K.; Kim, C.; Choi, S.; Sim, J.; et al. Importation and Transmission of SARS-CoV-2 B.1.1.529 (Omicron) Variant of Concern in Korea, November 2021. J. Korean Med. Sci. 2021, 36, e346. [Google Scholar] [CrossRef]
- Team, C.C.-R. SARS-CoV-2 B.1.1.529 (Omicron) Variant-United States, December 1-8, 2021. MMWR Morb. Mortal. Wkly Rep. 2021, 70, 1731–1734. [Google Scholar] [CrossRef]
- COVID-19 Coronavirus Pandemic: Reported Cases and Deaths by Country or Territory. 2022. Available online: https://www.worldometers.info/coronavirus/ (accessed on 16 February 2022).
- Shanghai Municipal Health Commission. 2022. Available online: https://wsjkw.sh.gov.cn (accessed on 16 February 2022).
- Wang, Z.; Yang, L. In the age of Omicron variant: Paxlovid raises new hopes of COVID-19 recovery. J. Med. Virol. 2022, 94, 1766–1767. [Google Scholar] [CrossRef]
- Shen, P.; Li, J.; Tu, S.; Wu, Y.; Peng, Y.; Chen, G.; Chen, C. Positive effects of Lianhuaqingwen granules in COVID-19 patients: A retrospective study of 248 cases. J. Ethnopharmacol. 2021, 278, 114220. [Google Scholar] [CrossRef]
- Liu, M.; Gao, Y.; Yuan, Y.; Yang, K.; Shi, S.; Tian, J.; Zhang, J. Efficacy and safety of herbal medicine (Lianhuaqingwen) for treating COVID-19: A systematic review and meta-analysis. Integr. Med. Res. 2021, 10, 100644. [Google Scholar] [CrossRef]
- China NHCotPsRo. Notice on printing and distributing the COVID-19 Pneumonia Diagnosis and Treatment Plan (Ninth Trial Version) 2022. 2022. Available online: http://www.nhc.gov.cn/yzygj/s7653p/202203/b74ade1ba4494583805a3d2e40093d88.shtml (accessed on 26 May 2022). (In Chinese)
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Cann, S.A.H. Fever: Could A Cardinal Sign of COVID-19 Infection Reduce Mortality? Am. J. Med. Sci. 2021, 361, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, H.; Huang, S.; Shi, L.; Xing, Z.; Shen, J. Case Report of China/Tianjin’s First Novel Coronavirus Variant Omicron. Iran J. Immunol. 2022, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Maslo, C.; Friedland, R.; Toubkin, M.; Laubscher, A.; Akaloo, T.; Kama, B. Characteristics and Outcomes of Hospitalized Patients in South Africa During the COVID-19 Omicron Wave Compared With Previous Waves. JAMA 2022, 327, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Hou, Y.; Lin, Q.; Chen, L.; Ren, T. How China responds to Omicron. J. Infect. 2022, 85, 90–122. [Google Scholar] [CrossRef]
- Hui, K.P.Y.; Ho, J.C.W.; Cheung, M.C.; Ng, K.C.; Ching, R.H.H.; Lai, K.L.; Kam, T.T.; Gu, H.; Sit, K.Y.; Hsin, M.K.Y.; et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 2022, 603, 715–720. [Google Scholar] [CrossRef]
- Gupta, R. SARS-CoV-2 Omicron spike mediated immune escape and tropism shift. Res. Sq. 2022; Preprint. [Google Scholar] [CrossRef]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne transmission of respiratory viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef]
- Bennasrallah, C.; Zemni, I.; Dhouib, W.; Sriha, H.; Mezhoud, N.; Bouslama, S.; Taboubi, W.; Beji, M.O.; Kacem, M.; Abroug, H.; et al. Factors associated with a prolonged negative conversion of viral RNA in patients with COVID-19. Int. J. Infect. Dis. 2021, 105, 463–469. [Google Scholar] [CrossRef]
- Widders, A.; Broom, A.; Broom, J. SARS-CoV-2: The viral shedding vs infectivity dilemma. Infect. Dis. Health 2020, 25, 210–215. [Google Scholar] [CrossRef]
- He, X.; Lau, E.H.Y.; Wu, P.; Deng, X.; Wang, J.; Hao, X.; Lau, Y.C.; Wong, J.Y.; Guan, Y.; Tan, X.; et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat. Med. 2020, 26, 672–675. [Google Scholar] [CrossRef] [Green Version]
- Torjesen, I. Covid-19: Peak of viral shedding is later with omicron variant, Japanese data suggest. BMJ 2022, 376, o89. [Google Scholar] [CrossRef]
- Chen, J.; Qi, T.; Liu, L.; Ling, Y.; Qian, Z.; Li, T.; Li, F.; Xu, Q.; Zhang, Y.; Xu, S.; et al. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 2020, 80, e1–e6. [Google Scholar] [CrossRef]
- Graham, F. Daily briefing: Omicron struggles to infect the lungs. Nature, 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Kozlov, M. Omicron’s feeble attack on the lungs could make it less dangerous. Nature 2022, 601, 177. [Google Scholar] [CrossRef] [PubMed]
- Runfeng, L.; Yunlong, H.; Jicheng, H.; Weiqi, P.; Qinhai, M.; Yongxia, S.; Chufang, L.; Jin, Z.; Zhenhua, J.; Haiming, J.; et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol. Res. 2020, 156, 104761. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Hui, N.; Liu, Y.; Qiao, G.; Li, J.; Tian, L.; Ju, X.; Jia, M.; Liu, H.; Cao, W.; et al. Insights into forsythia honeysuckle (Lianhuaqingwen) capsules: A Chinese herbal medicine repurposed for COVID-19 pandemic. Phytomed. Plus 2021, 1, 100027. [Google Scholar] [CrossRef] [PubMed]
- Mesfin, Y.; Chen, D.; Bond, H.; Lam, V.; Cheung, J.K.; Wong, J.Y.; Taslim Ali, S.; Lau, E.H.Y.; Wu, P.; Leung, G.M.; et al. Epidemiology of infections with SARS-CoV-2 Omicron BA.2 variant in Hong Kong, January-March 2022. medRxiv 2022, 28, 1856–1858. [Google Scholar] [CrossRef]
- Rossler, A.; Riepler, L.; Bante, D.; von Laer, D.; Kimpel, J. SARS-CoV-2 Omicron Variant Neutralization in Serum from Vaccinated and Convalescent Persons. N. Engl. J. Med. 2022, 386, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Abas, A.H.; Marfuah, S.; Idroes, R.; Kusumawaty, D.; Fatimawali; Park, M.N.; Siyadatpanah, A.; Alhumaydhi, F.A.; Mahmud, S.; Tallei, T.E.; et al. Can the SARS-CoV-2 Omicron Variant Confer Natural Immunity against COVID-19? Molecules 2022, 27, 2221. [Google Scholar] [CrossRef]
- Butt, A.A.; Dargham, S.R.; Loka, S.; Shaik, R.M.; Chemaitelly, H.; Tang, P.; Hasan, M.R.; Coyle, P.V.; Yassine, H.M.; Al-Khatib, H.A.; et al. COVID-19 Disease Severity in Children Infected with the Omicron Variant. Clin. Infect. Dis. 2022, 75, e361–e367. [Google Scholar] [CrossRef]
- Martin, B.; DeWitt, P.E.; Russell, S.; Sanchez-Pinto, L.N.; Haendel, M.A.; Moffitt, R.; Bennett, T.D. Acute Upper Airway Disease in Children With the Omicron (B.1.1.529) Variant of SARS-CoV-2-A Report from the US National COVID Cohort Collaborative. JAMA Pediatr. 2022, 176, 819–821. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, X.; Chen, C.; Jiang, D.; Liu, X.; Zhou, Y.; Huang, C.; Zhou, Y.; Guan, Z.; Ding, C.; et al. Characteristics of Viral Shedding Time in SARS-CoV-2 Infections: A Systematic Review and Meta-Analysis. Front. Public Health 2021, 9, 652842. [Google Scholar] [CrossRef]
- Anjos, D.; Fiaccadori, F.S.; Servian, C.D.P.; da Fonseca, S.G.; Guilarde, A.O.; Borges, M.; Franco, F.C.; Ribeiro, B.M.; Souza, M. SARS-CoV-2 loads in urine, sera and stool specimens in association with clinical features of COVID-19 patients. J. Clin. Virol. Plus 2022, 2, 100059. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]



| Characteristics | All Patients (n = 226) | Disease Severity | p-Value | |||
|---|---|---|---|---|---|---|
| Asymptomatic (n = 4) | Mild (n = 180) | Moderate (n = 41) | Severe (n = 1) | |||
| Age | ||||||
| Median (IQR), y | 52.0 (32.0–68.0) | 51.5 (43.5–58.5) | 48.0 (28.5–62.5) | 71.0 (57.0–78.0) | 78.0 (78.0–78.0) | <0.001 |
| Distribution—No./total No. (%) | <0.001 | |||||
| 0–14 y | 28 (12.4) | 0 (0.0) | 28 (15.6) | 0 (0.0) | 0 | |
| 15–49 y | 73 (32.4) | 1 (25.0) | 66 (36.9) | 6 (14.6) | 0 | |
| 50–64 y | 52 (23.1) | 2 (50.0) | 42 (23.5) | 8 (19.5) | 0 | |
| ≥65 y | 72 (32.0) | 1 (25.0) | 43 (24.0) | 27 (65.9) | 1 (100) | |
| Female sex—No./total No. (%) | 118 (52.2) | 2 (50.0) | 99 (55.0) | 17 (41.5) | 0 | 0.251 |
| Smoking history—No./total No. (%) | 0.245 | |||||
| Smoker | 27 (11.9) | 0 (0.0) | 21 (11.7) | 6 (14.3) | ||
| Non-smoker | 199 (88.1) | 4 (100.0) | 159 (88.3) | 36 (85.7) | ||
| Median days from onset of symptoms to admission | 3.0 (2.0–3.0) | / | 3.0 (2.0–3.0) | 3.0 (2.0–4.0) | 2.0 (2.0–2.0) | 0.608 |
| Median vaccination doses (IQR) | 2.0 (0.0–3.0) | 3.0 (2.0–3.0) | 2.0 (0.0–3.0) | 0.0 (0.0–2.0) | 0.0 (0.0–0.0) | 0.009 |
| Fever—No./total No. (%) | 103 (45.6) | 0 (0.0) | 83 (46.1) | 20 (48.8) | 0 (0.0) | 0.226 |
| Median temperature (IQR)—°C | 38.4 (38.0–39.0) | / | 38.4 (38.0–39.0) | 38.4 (37.9–38.8) | 0.444 | |
| Duration of fever | 5.0 (4.0–6.0) | / | 5.0 (4.0–6.0) | 6.5 (6.0–8.3) | 14.0 (12.0–16.0) | <0.001 |
| Distribution of Temperature—No./total No. (%) | 0.280 | |||||
| <37.5 °C | 123 (54.4) | 4 (100.0) | 97 (53.9) | 21 (51.2) | 1 (100.0) | |
| 37.5–38.0 °C | 35 (15.5) | 0 (0.0) | 28 (15.6) | 7 (17.1) | 0 (0.0) | |
| 38.1–39.0 °C | 51 (22.6) | 0 (0.0) | 38 (21.1) | 13 (31.7) | 0 (0.0) | |
| >39.0 °C | 17 (7.52) | 0 (0.0) | 17 (9.4) | 0 (0.0) | 0 (0.0) | |
| Symptoms—No. (%) | ||||||
| Cough | 168 (74.3) | 0 (0.0) | 133 (73.9) | 34 (82.9) | 1 (100.0) | 0.005 |
| Sputum production | 111 (49.1) | 0 (0.0) | 85 (47.2) | 25 (61.0) | 1 (100.0) | 0.031 |
| Loss of olfaction | 16 (7.1) | 0 (0.0) | 14 (7.8) | 2 (4.9) | 0 (0.0) | 0.822 |
| Fatigue | 33 (14.6) | 0 (0.0) | 27 (15.0) | 6 (14.6) | 0 (0.0) | 1.000 |
| Dizziness and headache | 28 (12.4) | 0 (0.0) | 22 (12.2) | 6 (14.6) | 0 (0.0) | 0.801 |
| Shortness of breath | 4 (1.8) | 0 (0.0) | 3 (1.67) | 1 (2.44) | 0 (0.0) | 0.600 |
| Rhinorrhea | 18 (8.0) | 0 (0.0) | 16 (8.89) | 2 (4.88) | 0 (0.0) | 0.696 |
| Sore throat | 44 (19.5) | 0 (0.0) | 40 (22.2) | 4 (9.76) | 0 (0.0) | 0.212 |
| Diarrhea | 6 (2.7) | 0 (0.0) | 3 (1.67) | 3 (7.32) | 0 (0.0) | 0.196 |
| Myalgia/arthralgia | 11 (4.9) | 0 (0.0) | 8 (4.44) | 3 (7.32) | 0 (0.0) | 0.559 |
| Comorbidity—No. (%) | ||||||
| Respiratory system diseases | 6 (2.7) | 0 (0.0) | 4 (2.2) | 2 (4.9) | 0 (0.0) | 0.396 |
| Type 2 diabetes | 30 (13.3) | 0 (0.0) | 18 (10.0) | 12 (29.3) | 0 (0.0) | 0.012 |
| Hypertension | 76 (33.6) | 1 (25.0) | 50 (27.8) | 24 (58.5) | 1 (100.0) | <0.001 |
| Non-hypertension cardiovascular disease | 13 (5.8) | 0 (0.0) | 10 (5.6) | 3 (7.3) | 0 (0.0) | 0.787 |
| Cerebrovascular disease | 22 (9.7) | 0 (0.0) | 9 (5.00) | 13 (31.7) | 0 (0.0) | <0.001 |
| Hepatitis B virus infection | 1 (0.4) | 0 (0.0) | 1 (0.6) | 0 (0.0) | 0 (0.0) | 1.000 |
| Cancer | 14 (6.2) | 0 (0.0) | 6 (3.3) | 7 (17.1) | 1 (100.0) | 0.001 |
| Chronic renal disease | 2 (0.9) | 0 (0.0) | 2 (1.1) | 0 (0.0) | 0 (0.0) | 1.000 |
| Endocrine disorder | 2 (0.9) | 0 (0.0) | 2 (1.1) | 0 (0.0) | 0 (0.0) | 1.000 |
| Asthma | 2 (0.9) | 0 (0.0) | 1 (0.6) | 1 (2.4) | 0 (0.0) | 1.000 |
| Parkinson’s disease | 2 (0.9) | 0 (0.0) | 1 (0.6) | 1 (2.4) | 0 (0.0) | 0.366 |
| Immunodeficiency | 1 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 0.204 |
| Laboratory Findings | Reference Range | Median (IQR), on Hospital Admission (n = 226) | Median (IQR) | p-Value | |||
|---|---|---|---|---|---|---|---|
| Disease Severity | |||||||
| Asymptomatic (n = 4) | Mild (n = 180) | Moderate (n = 41) | Severe (n = 1) | ||||
| WBC (×109/L) | 3.5–9.5 | 4.6 (3.7–6.0) | 5.4 (4.6–6.1) | 4.5 (3.7–5.9) | 5.20 (3.7–6.2) | 3.30 (3.3–3.3) | 0.432 |
| Hb (g/L) | 130–175 | 130.0 (121.0–141.0) | 138.0 (131.0–143.0) | 130.0 (122.0–141.0) | 124.0 (116.0–135.0) | 103.0 (103.0–103.0) | 0.052 |
| PLT (×109/L) | 125–350 | 188.0 (139.0–227.0) | 180.0 (154.0–192.0) | 188.0 (140.0–232.0) | 174.0 (138.0–224.0) | 196.0 (196.0–196.0) | 0.618 |
| Lymphocyte (×109/L) | 1.1–3.2 | 1.3 (0.9–1.7) | 1.1 (0.9–1.6) | 1.3 (1.0–1.7) | 1.1 (0.9–1.4) | 0.6 (0.6–0.6) | 0.034 |
| Ferritin (ng/mL) | 4.63–204 | 111.0 (47.0–218.0) | 122.0 (81.8–203.0) | 99.3 (40.2–187.0) | 238.0 (142.0–336.0) | 296.0 (296.0–296.0) | <0.001 |
| ESR (mm/h) | <15 | 11.0 (8.0–15.0) | 8.0 (7.3–10.8) | 10.0 (8.0–12.0) | 20.0 (14.0–29.0) | 24.0 (24.0–24.0) | <0.001 |
| CRP (mg/L) | 0–8 | 2.9 (0.4–7.0) | 3.4 (0.7–7.7) | 2.0 (0.4–5.5) | 9.7 (3.8–24.6) | 71.7 (71.7–71.7) | <0.001 |
| PCT (ng/mL) | ≤0.25 | 0.0 (0.0–0.1) | 0.1 (0.0–0.1) | 0.0 (0.0–0.1) | 0.1 (0.0–0.2) | 1.3 (1.3–1.3) | <0.001 |
| IL-6 (pg/mL) | 0–10 | 4.7 (3.6–8.5) | 6.6 (3.9–10.1) | 4.2 (3.3–6.4) | 13.8 (5.9–24.5) | 23.6 (23.6–23.6) | <0.001 |
| D-dimer (mg/L) | 0–0.5 | 0.3 (0.2–0.6) | 0.2 (0.2–0.3) | 0.3 (0.2–0.5) | 0.6 (0.3–1.2) | 1.4 (1.4–1.4) | <0.001 |
| TG (mmol/L) | 0–1.8 | 1.2 (0.9–1.5) | 1.0 (1.0–1.2) | 1.2 (0.9–1.5) | 1.2 (0.8–1.8) | 1.1 (1.1–1.1) | 0.841 |
| TC (mmol/L) | 2.84–6.2 | 3.8 (3.2–4.4) | 3.6 (3.5–3.9) | 3.8 (3.1–4.3) | 4.1 (3.7–4.6) | 3.1 (3.1–3.1) | 0.370 |
| HDL (mmol/L) | 1.04–1.68 | 1.1 (0.9–1.4) | 1.4 (1.1–1.5) | 1.2 (1.0–1.5) | 1.0 (0.9–1.1) | 1.1 (1.1–1.1) | 0.004 |
| LDL (mmol/L) | ≤3.3 | 2.3 (1.9–2.7) | 2.1 (1.8–2.4) | 2.3 (1.9–2.8) | 2.3 (1.8–2.7) | 1.4 (1.4–1.4) | 0.500 |
| Total bilirubin (umol/L) | 3.4–20.5 | 8.3 (6.3–12.8) | 11.8 (9.8–17.8) | 7.9 (6.3–11.7) | 9.3 (7.3–15.4) | 17.6 (17.6–17.6) | 0.031 |
| Pro-BNP (pg/mL) | ≤589 | 36.9 (27.5–93.0) | 35.6 (27.7–40.5) | 33.5 (25.5–57.0) | 89.8 (47.9–222.0) | 255.0 (255.0–255.0) | <0.001 |
| ALT (IU/L) | <50 | 15.0 (12.0–23.0) | 11.5 (8.0–16.2) | 16.0 (12.0–23.0) | 15.0 (12.0–25.0) | 11.0 (11.0–11.0) | 0.404 |
| AST (IU/L) | 17–59 | 23.0 (18.0–30.0) | 17.0 (14.8–21.0) | 22.0 (18.0–30.0) | 24.0 (18.0–29.0) | 45.0 (45.0–45.0) | 0.222 |
| Albumin (g/L) | 35–53 | 39.0 (37.0–41.0) | 41.0 (40.2–41.2) | 40.0 (38.0–41.0) | 36.0 (33.0–39.0) | 28.0 (28.0–28.0) | <0.001 |
| LDH (IU/L) | 50–240 | 157.0 (132.0–201.0) | 156.0 (131.0–183.0) | 154.0 (129.0–194.0) | 198.0 (156.0–255.0) | 675.0 (675.0–675.0) | <0.001 |
| Lactate (mmol/L) | 0.7–2.1 | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 1.0 (0.8–1.2) | 1.4 (1.2–1.7) | 2.0 (2.0–2.0) | <0.001 |
| e-GFR (mL/min/1.73 m2) | 46–92 | 99.6 (87.5–117.0) | 98.5 (87.3–109.0) | 103.0 (90.9–121.0) | 85.4 (76.1–97.2) | 92.2 (92.2–92.2) | <0.001 |
| Potassium (mmol/L) | 3.5–5.1 | 4.0 (3.7–4.3) | 3.8 (3.8–3.9) | 4.0 (3.7–4.3) | 3.9 (3.5–4.4) | 3.7 (3.7–3.7) | 0.552 |
| Sodium (mmol/L) | 135–147 | 139.0 (137.0–141.0) | 139.0 (139.0–140.0) | 139.0 (137.0–141.0) | 139.0 (137.0–141.0) | 139.0 (139.0–139.0) | 0.969 |
| Chloride (mmol/L) | 98–107 | 104.0 (101.0–106.0) | 105.0 (103.0–106.0) | 104.0 (101.0–107.0) | 105.0 (102.0–106.0) | 103.0 (103.0–103.0) | 0.905 |
| Univariate Logistic Regression | Multivariate Logistic Regression | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p-Value | OR | 95%CI | p-Value | |
| Age | 1.06 | 1.03–1.08 | <0.001 | 1.03 | 0.99–1.08 | 0.138 |
| Female | 0.58 | 0.29–1.15 | 0.120 | 0.51 | 0.20–1.24 | 0.143 |
| Fever | 1.11 | 0.56–2.19 | 0.757 | 1.97 | 0.75–5.35 | 0.172 |
| Comorbidity | 5.46 | 2.46–12.12 | <0.001 | 1.11 | 0.33–3.69 | 0.867 |
| WBC | 1.09 | 0.94–1.26 | 0.266 | 0.95 | 0.74–1.20 | 0.674 |
| Lymphocytes | 0.48 | 0.25–0.91 | 0.025 | 0.70 | 0.27–1.65 | 0.459 |
| CRP | 1.03 | 1.01–1.05 | 0.004 | 0.99 | 0.96–1.02 | 0.387 |
| ESR | 1.08 | 1.04–1.13 | <0.001 | 1.05 | 1.02–1.10 | 0.007 |
| Albumin | 0.81 | 0.73–0.89 | <0.001 | 0.95 | 0.84–1.07 | 0.363 |
| ALT | 1.00 | 0.99–1.01 | 0.861 | 1.00 | 0.97–1.04 | 0.908 |
| AST | 1.00 | 0.99–1.00 | 0.920 | 1.00 | 0.97–1.02 | 0.703 |
| Lactate | 6.51 | 2.72–15.58 | <0.001 | 2.34 | 0.67–8.25 | 0.180 |
| e-GFR | 0.97 | 0.95–0.98 | <0.001 | 0.99 | 0.97–1.02 | 0.606 |
| Dose of vaccinations | ||||||
| 0 | 1.00 | 1.00 | ||||
| 1 | 1.46 | 0.25–8.49 | 0.676 | 2.43 | 0.17–28.85 | 0.492 |
| 2 | 0.63 | 0.28–1.46 | 0.282 | 0.71 | 0.21–2.25 | 0.571 |
| 3 | 0.28 | 0.11–0.73 | 0.009 | 0.52 | 0.15–1.73 | 0.296 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, J.; Fan, R.; Hu, J.; Zhang, T.; Lee, C.; Huang, X.; Wang, F.; Liang, H.; Jin, Y.; Jiang, Y.; et al. Clinical Progression and Outcome of Hospitalized Patients Infected with SARS-CoV-2 Omicron Variant in Shanghai, China. Vaccines 2022, 10, 1409. https://doi.org/10.3390/vaccines10091409
Shao J, Fan R, Hu J, Zhang T, Lee C, Huang X, Wang F, Liang H, Jin Y, Jiang Y, et al. Clinical Progression and Outcome of Hospitalized Patients Infected with SARS-CoV-2 Omicron Variant in Shanghai, China. Vaccines. 2022; 10(9):1409. https://doi.org/10.3390/vaccines10091409
Chicago/Turabian StyleShao, Jiasheng, Rong Fan, Jianrong Hu, Tiejun Zhang, Catherine Lee, Xuyuan Huang, Fei Wang, Haiying Liang, Ye Jin, Ying Jiang, and et al. 2022. "Clinical Progression and Outcome of Hospitalized Patients Infected with SARS-CoV-2 Omicron Variant in Shanghai, China" Vaccines 10, no. 9: 1409. https://doi.org/10.3390/vaccines10091409
APA StyleShao, J., Fan, R., Hu, J., Zhang, T., Lee, C., Huang, X., Wang, F., Liang, H., Jin, Y., Jiang, Y., Gu, Y., & Huang, G. (2022). Clinical Progression and Outcome of Hospitalized Patients Infected with SARS-CoV-2 Omicron Variant in Shanghai, China. Vaccines, 10(9), 1409. https://doi.org/10.3390/vaccines10091409





