Increased Production of Inflammatory Cytokines after Inoculation with Recombinant Zoster Vaccine in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Plan
2.2. Cytokine Measurement
2.3. Statistical Analysis
3. Results
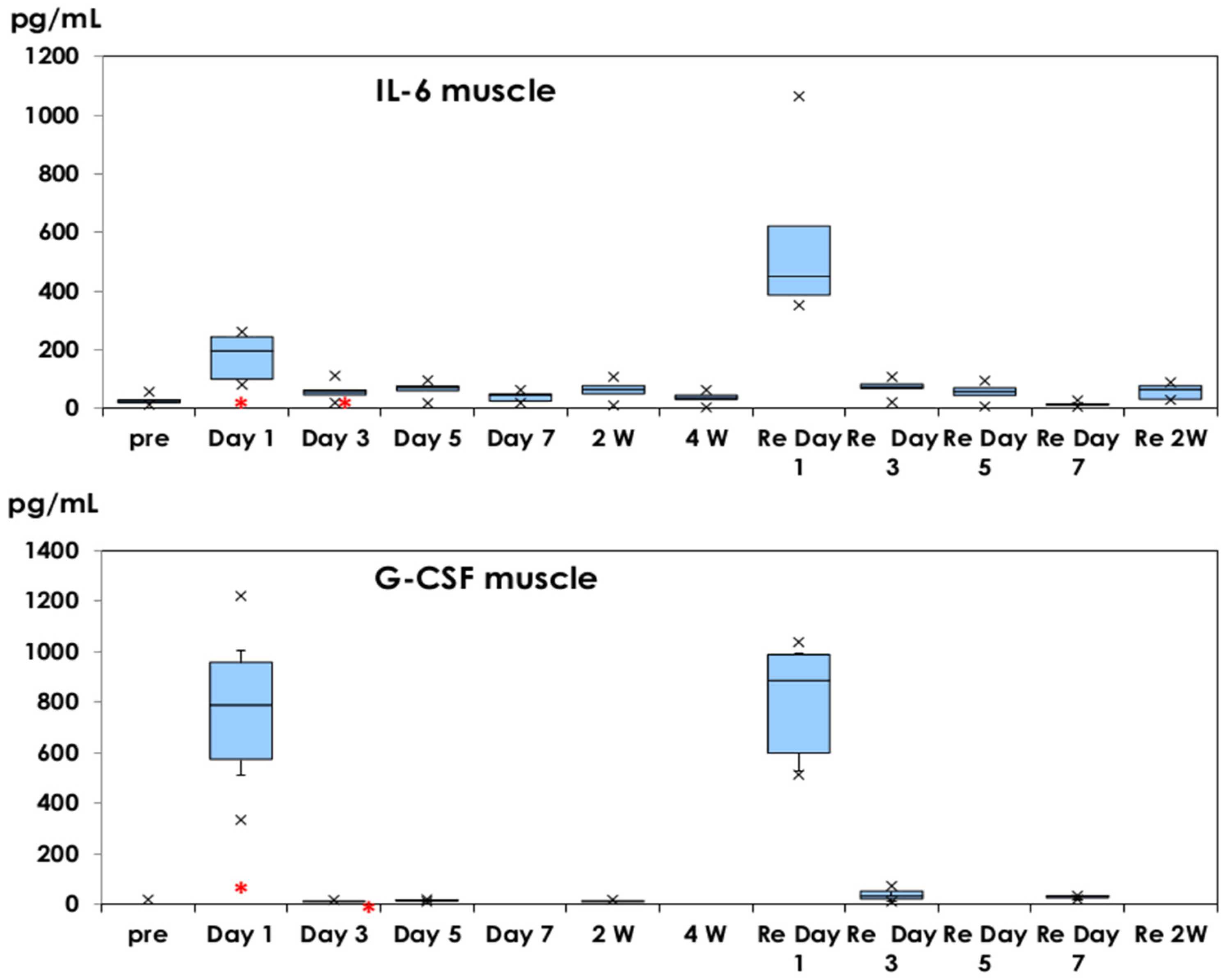
3.1. Inflammatory Cytokine Production in Muscle Tissues and Serum Samples
3.2. IL-5 and IFN-γ Profiles in Muscle Tissue and Serum Samples
3.3. IL-2, IL-4, TNF-α, and IL-10 in Muscle Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CD | cluster differentiation |
| DAMPs | pathogen-associated molecular patterns |
| DOPC | dioleoylphosphatidylcholine |
| G-CSF | granulocyte-colony stimulating factor |
| IFN | interferon |
| IL | interleukin |
| MPL | monophophoryl lipid A |
| NETs | neutrophile extracellular traps |
| PAMPs | pathogen-associated molecular patterns |
| QS21 | Quillaja Saponaria Molina |
| TNF | tumor necrosis factor |
| VZV | varicella zoster virus |
References
- Cohen, J.I.; Straus, S.E.; Arvin, A.M. Varicella-zoster virus replication, pathogenesis, and management. Fields Virol. 2006, 2, 2773–2818. [Google Scholar]
- Gershon, A.A.; Breuer, J.; Cohen, J.I.; Cohrs, R.J.; Gershon, M.D.; Gilden, D.; Grose, C.; Hambleton, S.; Kennedy, P.G.E.; Oxman, M.N.; et al. Varicella zoster virus infection. Nat. Rev. Dis. Prim. 2015, 1, 15016. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.W.; Rice, A.S.C. Postherpetic neuralgia. N. Engl. J. Med. 2014, 371, 1526–1533. [Google Scholar] [CrossRef]
- Kennedy, P.G.E.; Gershon, A.A. Clinical features of varicella-zoster virus infection. Viruses 2018, 10, 609. [Google Scholar] [CrossRef] [PubMed]
- Winthrop, K.L.; Novosad, S.A.; Baddley, J.W.; Calabrese, L.; Chiller, T.; Polgreen, P.; Bartalesi, F.; Lipman, M.; Mariette, X.; Lortholary, O.; et al. Opportunistic infections and biologic therapies in immune-mediated inflammatory diseases: Consensus recommendations for infection reporting during clinical trials and postmarketing surveillance. Ann. Rheum. Dis. 2015, 74, 2107–2116. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.; Zhang, J.X.; Seward, J.F. Near elimination of varicella deaths in the US after implementation of the vaccination program. Pediatrics 2011, 128, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.M.; Harpaz, R.; Joesoef, M.R.; Bialek, S.R. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann. Intern. Med. 2013, 159, 739–745. [Google Scholar] [CrossRef]
- Leung, J.; Harped, R.; Molinari, N.A.; Jumaan, A.; Zhou, F. Herpes zoster incidence among insured persons in the United States, 1993–2006: Evaluation of impact of varicella vaccination. Clin. Infect. Dis. 2011, 52, 332–340. [Google Scholar] [CrossRef]
- Oxman, M.N.; Levin, M.J.; Johnson, G.R.; Schmader, K.E.; Straus, S.E.; Gelb, L.D.; Arbeit, R.D.; Simberkoff, M.S.; Gershon, A.A.; Davis, L.E.; et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N. Engl. J. Med. 2005, 352, 2271–2284. [Google Scholar] [CrossRef]
- Chlibek, R.; Bayas, J.M.; Collins, H.; de la Pinta, M.L.R.; Ledent, E.; Mols, J.F.; Heineman, T.C. Safety and immunogenicity of an AS01-adjuvanted varicella-zoster virus subunit candidate vaccine against herpes zoster in adults ≥50 years of age. J. Infect. Dis. 2013, 208, 1953–1961. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Lal, H.; Kovac, M.; Chlibek, R.; Hwang, S.J.; Díez-Domingo, J.; Godeaux, O.; Levin, M.J.; McElhaney, J.E.; Puig-Barberà, J.V.; et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N. Engl. J. Med. 2016, 375, 1019–1032. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.M.Z.; Andriolo, B.N.G.; Torloni, M.R.; Soares, B.G.O.; de Oliveira Gomes, J.; Andriolo, R.B.; Cruz, E.C. Vaccines for preventing herpes zoster in older adults. Cochrane Database Syst. Rev. 2019, 2019, CD008858. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Collignon, C.; Bourguignon, P.; Wouters, S.; Fierens, K.; Fochesato, M.; Dendouga, N.; Langlet, C.; Malissen, B.; Lambrecht, B.N.; et al. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J. Immunol. 2014, 193, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, K.; Hineno, A.; Kinoshita, T.; Ishihara, S.; Ikeda, S. Suspected adverse effects after human papillomavirus vaccination: A temporal relationship between vaccine administration and the appearance of symptoms in Japan. Drug Saf. 2017, 40, 1219–1229. [Google Scholar] [CrossRef][Green Version]
- Suzuki, S.; Hosono, A. No association between HPV vaccine and reported post-vaccination symptoms in Japanese young women: Results of the Nagoya study. Papillomavirus Res. 2018, 5, 96–103. [Google Scholar] [CrossRef]
- Nakayama, T.; Kashiwagi, Y.; Kawashima, H. Long-term regulation of local cytokine production following immunization in mice. Microbiol. Immunol. 2018, 62, 124–131. [Google Scholar] [CrossRef]
- Nakayama, T. An inflammatory response is essential for the development of adaptive immunity-immunogenicity and immunotoxicity. Vaccine 2016, 34, 5815–5818. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Exp. Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Kielland, A.; Vosters, O.; Vanderheyde, N.; Schiavetti, F.; et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef]
- Talaat, K.; Halsey, N.A.; Cox, A.B.; Coles, C.L.; Durbin, A.O.; Ramakrishnan, A.; Bream, J.H. Rapid changes in serum cytokines and chemokines in response to inactivated influenza vaccination. Influenza Other Respir. Viruses 2018, 12, 202–210. [Google Scholar] [CrossRef]
- Leon, L.R. Invited review: Cytokine regulation of fever: Studies using gene knockout mice. J. Appl. Physiol. 2002, 92, 2648–2655. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, W.C.; Bruce, R.; Li, H.; Schleide, D.M.; Mulbury, M.J.; Bain, M.D.; Wallace, P.K.; Baumann, H.; Evans, S.S. Central role of IL-6 receptor signal-transducing chain gp130 in activation of L-selectin adhesion by fever-range thermal stress. Immunity 2004, 20, 59–70. [Google Scholar] [CrossRef]
- Kashiwagi, Y.; Miyata, A.; Kumagai, T.; Maehara, K.; Suzuki, E.; Nagai, T.; Ozaki, T.; Nishimura, N.; Okada, K.; Kawashima, H.; et al. Production of inflammatory cytokines in response to diphtheria-pertussis-tetanus (DPT), haemophilus influenzae type b (Hib), and 7-valent pneumococcal (PCV7) vaccines. Hum. Vaccines Immunother. 2013, 10, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Godeaux, O.; Kovac, M.; Shu, D.; Grupping, K.; Campora, L.; Douha, M.; Heineman, T.C.; Lal, H. Immunogenicity and safety of an adjuvanted herpes zoster subunit candidate vaccine in adults ≥50 years of age with a prior history of herpes zoster: A phase III, non-randomized, open-label clinical trial. Hum. Vaccines Immunother. 2016, 13, 1051–1058. [Google Scholar] [CrossRef]
- Gehling, A.M.; Kuszpit, K.; Bailey, E.J.; Allen-Worthington, K.H.; Fetterer, D.P.; Rico, P.J.; Bocan, T.M.; Hofer, C.C. Evaluation of volume of intramuscular injection into the caudal thigh muscles of female and male BALB/c Mice (Mus musculus). J. Am. Assoc. Lab. Anim. Sci. 2018, 67, 35–43. [Google Scholar]
- Rutella, S.; Pierelli, L.; Bonanno, G.; Sica, S.; Ameglio, F.; Capoluongo, E.; Mariotti, A.; Scambia, G.; d’Onofrio, G.; Leone, G. Role for granulocyte colony–stimulating factor in the generation of human T regulatory type 1 cells. Blood 2002, 100, 2562–2571. [Google Scholar] [CrossRef]
- Neumann, C.; Scheffold, A.; Rutz, S. Functions and regulation of T cell-derived interleukin-10. Sem. Immunol. 2019, 44, 101344. [Google Scholar] [CrossRef]
- He, L.; Sun, B.; Guo, Y.; Yan, K.; Liu, D.; Zang, Y.; Jiang, C.; Zhang, Y.; Kong, W. Immune response of C57BL/6J mice to herpes zoster subunit vaccines formulated with nanoemulsion-based and liposome-based adjuvants. Int. Immunopharmacol. 2021, 101, 108216. [Google Scholar] [CrossRef]
- Song, H.K.; Hwang, D.Y. Use of C57/BL/6N mice on the variety of immunological researches. Lab Anim. Res. 2017, 33, 119–123. [Google Scholar] [CrossRef]
- Schülke, S. Induction of interleukin-10 producing dendritic cells as a tool to suppress allergen-specific T helper 2 responses. Front. Immunol. 2018, 9, 455. [Google Scholar] [CrossRef]
- Le, N.P.K.; Herz, C.; Gomes, J.V.D.; Förster, N.; Antoniadou, K.; Mittermeier-Kleßinger, V.K.; Mewis, I.; Dawid, C.; Ulrichs, C.; Lamy, E. Comparative anti-inflammatory effects of Salix cortex extracts and acetylsalicylic acid in SARS-CoV-2 peptide and LPS-activated human in vitro systems. Int. J. Mol. Sci. 2021, 22, 6766. [Google Scholar] [CrossRef] [PubMed]
 : mean ± 1.0 SD. ×: outliers.
: mean ± 1.0 SD. ×: outliers.
 : mean ± 1.0 SD. ×: outliers.
: mean ± 1.0 SD. ×: outliers.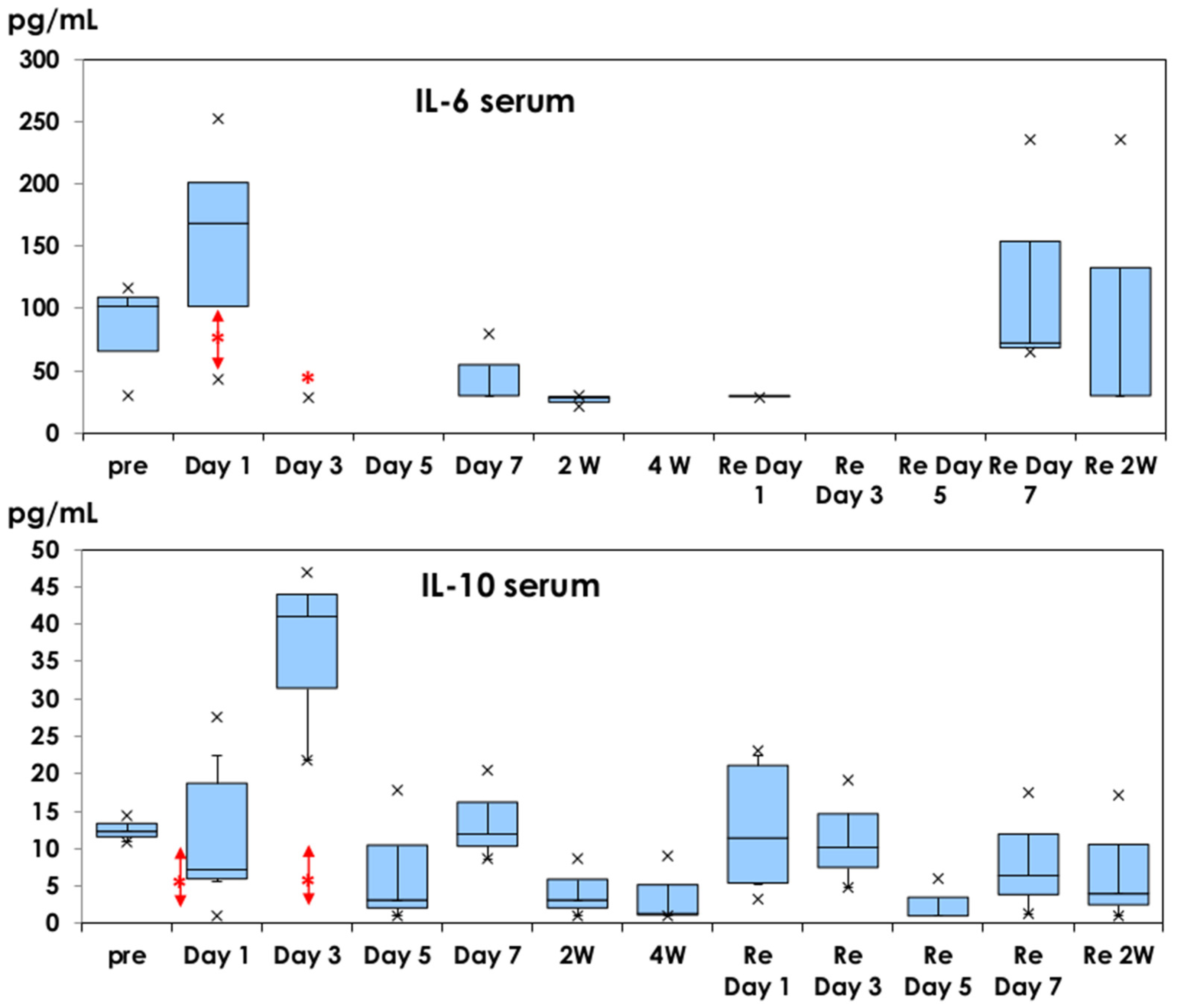
 : mean ± 1.0 SD. ×: outliers.
: mean ± 1.0 SD. ×: outliers.
 : mean ± 1.0 SD. ×: outliers.
: mean ± 1.0 SD. ×: outliers.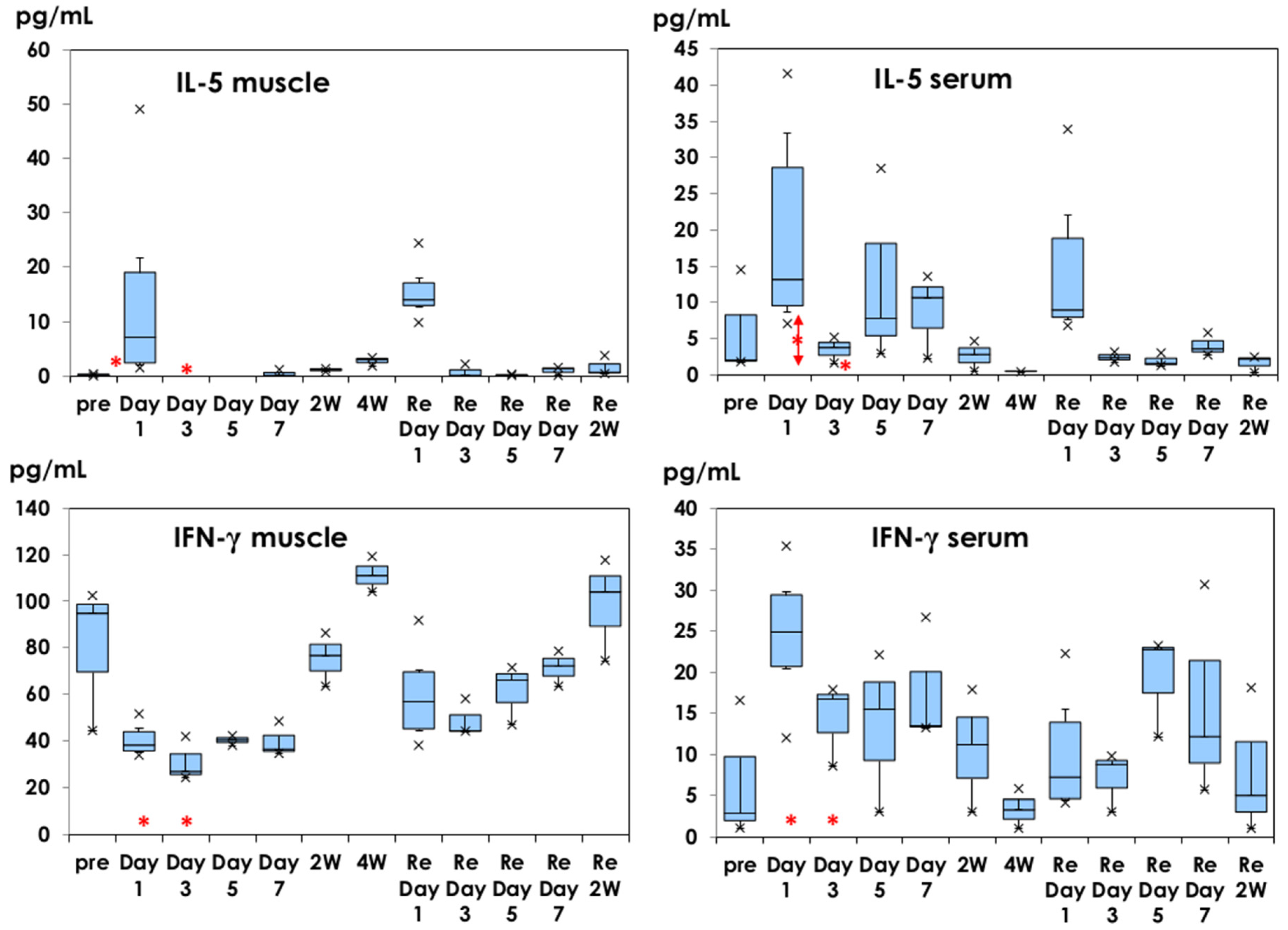

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, T.; Sawada, A.; Ito, T. Increased Production of Inflammatory Cytokines after Inoculation with Recombinant Zoster Vaccine in Mice. Vaccines 2022, 10, 1339. https://doi.org/10.3390/vaccines10081339
Nakayama T, Sawada A, Ito T. Increased Production of Inflammatory Cytokines after Inoculation with Recombinant Zoster Vaccine in Mice. Vaccines. 2022; 10(8):1339. https://doi.org/10.3390/vaccines10081339
Chicago/Turabian StyleNakayama, Tetsuo, Akihito Sawada, and Takeshi Ito. 2022. "Increased Production of Inflammatory Cytokines after Inoculation with Recombinant Zoster Vaccine in Mice" Vaccines 10, no. 8: 1339. https://doi.org/10.3390/vaccines10081339
APA StyleNakayama, T., Sawada, A., & Ito, T. (2022). Increased Production of Inflammatory Cytokines after Inoculation with Recombinant Zoster Vaccine in Mice. Vaccines, 10(8), 1339. https://doi.org/10.3390/vaccines10081339




