Fast Tracking—Vaccine Safety, Efficacy, and Lessons Learned: A Narrative Review
Abstract
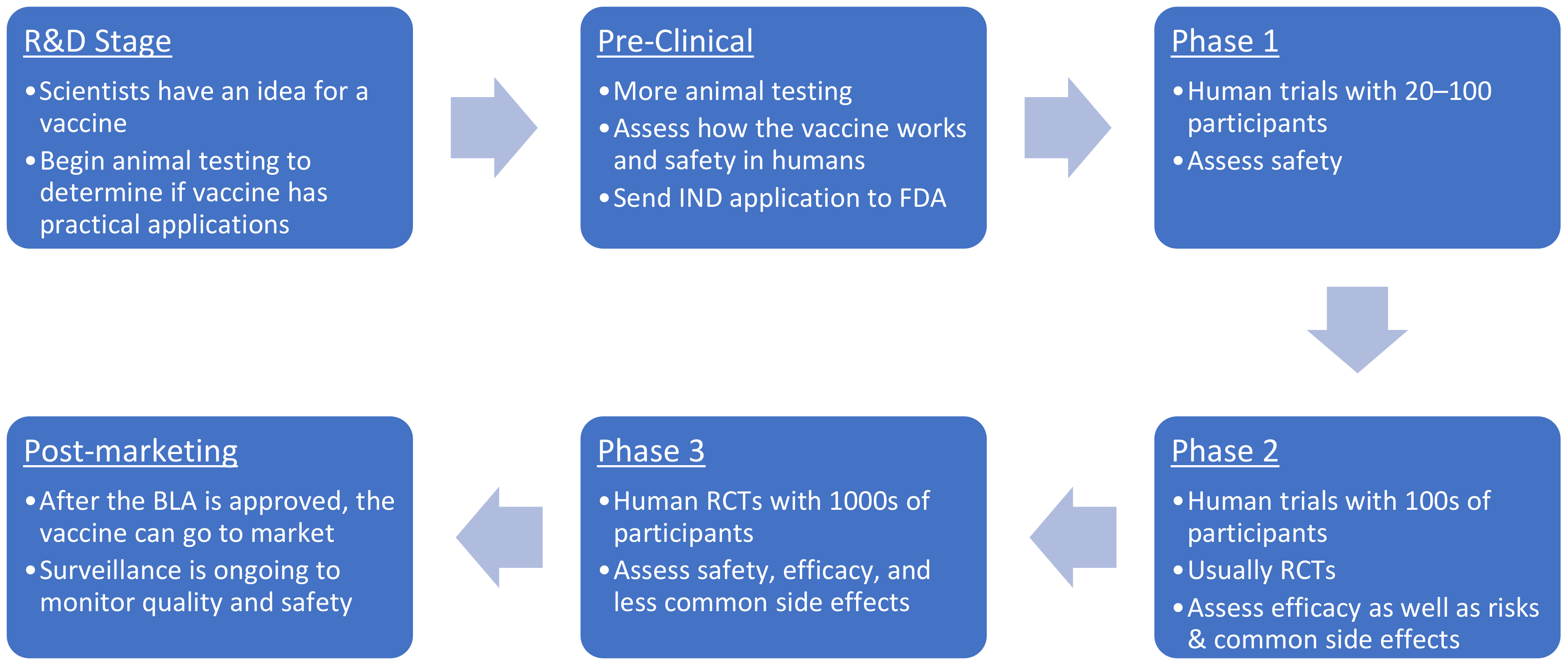
1. Introduction
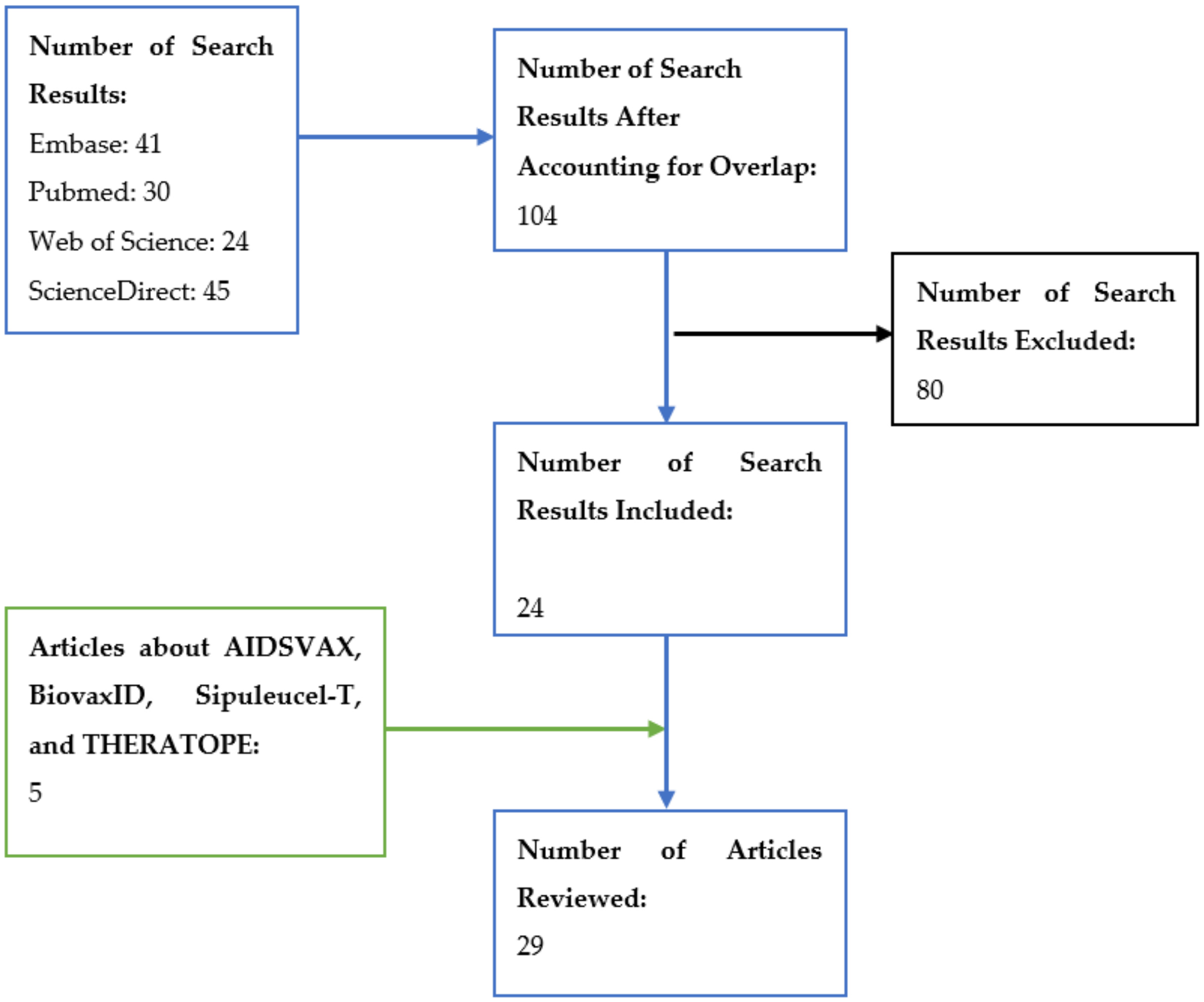
2. Materials and Methods
3. Results
3.1. Recent History and Previous Fast-Tracked Vaccines
3.2. Efficacy
3.3. Safety
3.4. Lessons Learned
4. Discussion
4.1. Efficacy
4.2. Safety
4.3. Lessons Learned
4.4. In the Context of COVID-19
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaccine Development–101|FDA. Available online: https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/vaccine-development-101 (accessed on 10 May 2021).
- The Journey of Your Child’s Vaccine (Infographic)|CDC. Available online: https://www.cdc.gov/vaccines/parents/infographics/journey-of-child-vaccine.html (accessed on 10 May 2021).
- Vaccine Safety Datalink (VSD)|VSD|Monitoring|Ensuring Safety|Vaccine Safety CDC. Available online: https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html (accessed on 10 May 2021).
- Fast Track, Breakthrough Therapy, Accelerated Approval, Priority Review. Available online: https://www.fda.gov/patients/learn-about-drug-and-device-approvals/fast-track-breakthrough-therapy-accelerated-approval-priority-review (accessed on 10 May 2021).
- Priority Review|FDA. Available online: https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/priority-review (accessed on 10 May 2021).
- Breakthrough Therapy|FDA. Available online: https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/breakthrough-therapy (accessed on 10 May 2021).
- Accelerated Approval|FDA. Available online: https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/accelerated-approval (accessed on 10 May 2021).
- Fast Track|FDA. Available online: https://www.fda.gov/patients/fast-track-breakthrough-therapy-accelerated-approval-priority-review/fast-track (accessed on 10 May 2021).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 11 July 2022).
- La Torre, G.; Backhaus, I.; Mannocci, A. Rating for narrative reviews: Concept and development of the International Narrative Systematic Assessment tool. Senses Sci. 2015, 2, 31–35. [Google Scholar] [CrossRef]
- Søborg, C.; Mølbak, K.; Doherty, T.M.; Ulleryd, P.; Brooks, T.; Coenen, C.; Van der Zeijst, B. Vaccines in a hurry. Vaccine 2009, 27, 3295–3298. [Google Scholar] [CrossRef] [PubMed]
- Muzumdar, J.M.; Cline, R.R. Vaccine supply, demand, and policy: A primer. J. Am. Pharm. Assoc. 2009, 49, e87–e99. [Google Scholar] [CrossRef]
- Thomas, S.J.; L’Azou, M.; Barrett, A.D.; Jackson, N.A. Fast-Track Zika Vaccine Development—Is It Possible? N. Engl. J. Med. 2016, 375, 1212–1216. [Google Scholar] [CrossRef]
- Limaye, R.J.; Sauer, M.; Truelove, S.A. Politicizing public health: The powder keg of rushing COVID-19 vaccines. Hum. Vaccines Immunother. 2021, 17, 1662–1663. [Google Scholar] [CrossRef]
- Rappuoli, R.; De Gregorio, E.; Del Giudice, G.; Phogat, S.; Pecetta, S.; Pizza, M.; Hanon, E. Vaccinology in the post-COVID-19 era. Proc. Natl. Acad. Sci. USA 2021, 118, e2020368118. [Google Scholar] [CrossRef]
- Steiner-Monard, V.; Kamaka, K.; Karoui, O.; Roethlisberger, S.; Audran, R.; Daubenberger, C.; Fayet-Mello, A.; Erdmann-Voisin, A.; Felger, I.; Geiger, K.; et al. The Candidate Blood-stage Malaria Vaccine P27A Induces a Robust Humoral Response in a Fast Track to the Field Phase 1 Trial in Exposed and Nonexposed Volunteers. Clin. Infect. Dis. 2019, 68, 466–474. [Google Scholar] [CrossRef]
- Sirima, S.B.; Durier, C.; Kara, L.; Houard, S.; Gansane, A.; Loulergue, P.; Bahuaud, M.; Benhamouda, N.; Nebié, I.; Faber, B.; et al. Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1-DiCo malaria vaccine adjuvanted with GLA-SE or Alhydrogel® in European and African adults: A phase 1a/1b, randomized, double-blind multi-centre trial. Vaccine 2017, 35, 6218–6227. [Google Scholar] [CrossRef]
- de Whalley, P.C.; Pollard, A.J. Pandemic influenza A (H1N1) 2009 vaccination in children: A UK perspective. J. Paediatr. Child Health 2013, 49, E183–E188. [Google Scholar] [CrossRef]
- McVernon, J.; Nolan, T. Panvax®: A monovalent inactivated unadjuvanted vaccine against pandemic influenza A (H1N1) 2009. Expert Rev. Vaccines 2011, 10, 35–43. [Google Scholar] [CrossRef]
- Cox, M.M.; Hashimoto, Y. A fast track influenza virus vaccine produced in insect cells. J. Invertebr. Pathol. 2011, 107, S31–S41. [Google Scholar] [CrossRef]
- Cox, M.M.; Hollister, J.R. FluBlok, a next generation influenza vaccine manufactured in insect cells. Biologicals 2009, 37, 182–189. [Google Scholar] [CrossRef]
- Ball, K. The enigma of the H1N1 flu: Are you ready? AORN J. 2009, 90, 852–866. [Google Scholar] [CrossRef]
- Update on Influenza A (H1N1) 2009 Monovalent Vaccines. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5839a3.htm (accessed on 10 May 2021).
- Reinis, M. BiovaxID, a personalized therapeutic vaccine against B-cell lymphomas. Curr. Opin. Mol. Ther. 2008, 10, 526–534. [Google Scholar]
- Adis International Ltd. Cancer vaccine THERATOPE—Biomira. Drugs R D 2003, 4, 236–240. [Google Scholar] [CrossRef]
- Zeichner, S.B. The failed Theratope vaccine: 10 years later. J. Am. Osteopath. Assoc. 2012, 112, 482–483. [Google Scholar]
- Pérez-Vargas, J.; Isa, P.; López, S.; Arias, C.F. Rotavirus vaccine: Early introduction in Latin America-risks and benefits. Arch. Med. Res. 2006, 37, 1–10. [Google Scholar] [CrossRef]
- Adis International Ltd. Sipuleucel-T: APC 8015, APC-8015, prostate cancer vaccine—Dendreon. Drugs R D 2006, 7, 197–201. [Google Scholar] [CrossRef]
- Higano, C.S.; Schellhammer, P.F.; Small, E.J.; Burch, P.A.; Nemunaitis, J.; Yuh, L.; Provost, N.; Frohlich, M.W. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 2009, 115, 3670–3679. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Roy Berger, E.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef]
- Barker, C.I.; Snape, M.D. Pandemic influenza A H1N1 vaccines and narcolepsy: Vaccine safety surveillance in action. Lancet Infect. Dis. 2014, 14, 227–238. [Google Scholar] [CrossRef]
- Wijnans, L.; de Bie, S.; Dieleman, J.; Bonhoeffer, J.; Sturkenboom, M. Safety of pandemic H1N1 vaccines in children and adolescents. Vaccine 2011, 29, 7559–7571. [Google Scholar] [CrossRef]
- Adis International Ltd. HIV gp120 vaccine—VaxGen: AIDSVAX, AIDSVAX B/B, AIDSVAX B/E, HIV gp120 vaccine—Genentech, HIV gp120 vaccine AIDSVAX—VaxGen, HIV vaccine AIDSVAX—VaxGen. Drugs R D 2003, 4, 249–253. [Google Scholar] [CrossRef]
- Understanding the Results of the AIDSVAX Trial|AVAC. Available online: https://www.avac.org/resource/understanding-results-aidsvax-trial (accessed on 10 May 2021).
- Wong, J.P.; Christopher, M.E.; Viswanathan, S.; Dai, X.; Salazar, A.M.; Sun, L.Q.; Wang, M. Antiviral role of toll-like receptor-3 agonists against seasonal and avian influenza viruses. Curr. Pharm. Des. 2009, 15, 1269–1274. [Google Scholar] [CrossRef]
- Geisbert, T.W. Emergency treatment for exposure to Ebola virus: The need to fast-track promising vaccines. JAMA 2015, 313, 1221–1222. [Google Scholar] [CrossRef][Green Version]
- Mooney, T.; Smout, E.; Leigh, B.; Greenwood, B.; Enria, L.; Ishola, D.; Manno, D.; Samai, M.; Douoguih, M.; Watson-Jones, D. EBOVAC-Salone: Lessons learned from implementing an Ebola vaccine trial in an Ebola-affected country. Clin. Trials 2018, 15, 436–443. [Google Scholar] [CrossRef]
- Feldmann, H.; Feldmann, F.; Marzi, A. Ebola: Lessons on Vaccine Development. Annu. Rev. Microbiol. 2018, 72, 423–446. [Google Scholar] [CrossRef]
- Watle, S.V.; Norheim, G.; Røttingen, J.A. Ebola vaccines—Where are we? Hum. Vaccin. Immunother. 2016, 12, 2700–2703. [Google Scholar] [CrossRef][Green Version]
- Dhanda, S.; Osborne, V.; Lynn, E.; Shakir, S. Postmarketing studies: Can they provide a safety net for COVID-19 vaccines in the UK? BMJ Evid. Based Med. 2022, 27, 1–6. [Google Scholar] [CrossRef]
- Dutta, A.K. Vaccine Against Covid-19 Disease—Present Status of Development. Indian J. Pediatr. 2020, 87, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S.; Firouzabadi, N.; Dehshahri, A.; Vazin, A. A focused review on technologies, mechanisms, safety, and efficacy of available COVID-19 vaccines. Int. Immunopharmacol. 2021, 100, 108162. [Google Scholar] [CrossRef] [PubMed]
- Lythgoe, M.P.; Middleton, P. Comparison of COVID-19 Vaccine Approvals at the US Food and Drug Administration, European Medicines Agency, and Health Canada. JAMA Netw. Open 2021, 4, e2114531. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Jung, S.Y.; Ahn, J.G.; Park, S.J.; Shoenfeld, Y.; Kronbichler, A.; Koyanagi, A.; Dragioti, E.; Tizaoui, K.; Hong, S.H. Comparative safety of mRNA COVID-19 vaccines to influenza vaccines: A pharmacovigilance analysis using WHO international database. J. Med. Virol. 2022, 94, 1085–1095. [Google Scholar] [CrossRef]


| Article | Risk of Bias | Include or Exclude? |
|---|---|---|
| De Wit et al., 2015 | Some concerns | Exclude |
| Langenberg et al., 2020 | Some concerns | Exclude |
| Mire et al., 2015 | Low risk of bias | Exclude |
| Steiner-Monard et al., 2019 | Low risk of bias | Include |
| Higano et al., 2009 | Low risk of bias | Include |
| Kantoff et al., 2010 | Low risk of bias | Include |
| Sirima et al., 2017 | Low risk of bias/Some concerns | Include |
| Gengenbacher et al., 2014 | High risk of bias | Exclude |
| Publication Date | Study Name | Fast-Tracked Vaccine | Patient Population | Intervention | Results |
|---|---|---|---|---|---|
| 2019 | The Candidate Blood-stage Malaria Vaccine P27A Induces a Robust Humoral Response in a Fast Track to the Field Phase 1 Trial in Exposed and Nonexposed Volunteers [17] | P27A peptide vaccine against malaria | 16 malaria non-exposed and 40 malaria-exposed subjects | P27A antigen IM adjuvanted with Alhydrogel, glucopyranosil lipid adjuvant stable emulsion, or control rabies vaccine (Verorab) | Specific humoral immune response represented by mixed Th1and Th2 cell mediated immunity as well as p27A-induced IgG antibody response able to inhibit parasite growth. |
| 2017 | Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1-DiCo malaria vaccine adjuvanted with GLA-SE or Alhydrogel® in European and African adults: A phase 1a/1b, randomized, double-blind multi-center trial [18] | Recombinant Plasmodium falciparum AMA1-DiCo malaria vaccine adjuvanted with GLA-SE or Alhydrogel® | Healthy European and African adults | European Adults: Intramuscular injection of AMA1-DiCo with either Alhydrogel® (n = 15) or GLA-SE (n = 15). African Adults: Intramuscular injection of AMA1-DiCo/GLA-SE (n = 18) or placebo (n = 18). AMA1-DiCo (50 mg) was administered intramuscularly at baseline, Week 4 and 26. | The main immunogenic response noted was an increase in IgG. The AMA1-DiCo malaria vaccine with Alhydrogel® group caused a 100-fold IgG increase from baseline and a 200–300-fold IgG increase when adjuvanted with GLA-SE. In African volunteers, immunization resulted in increased IgG levels that surpassed those of the European volunteers by 4-fold. Volunteers immunized also displayed a strong Th2 cell response that was present in more than 50% of the volunteers and detected by an IL-5 ELISPOT assay |
| 2013 | Pandemic influenza A (H1N1) 2009 vaccination in children: A UK perspective [19] | Adjuvanted vaccine AS03 for H1N1 | 6 months to 12 years old <10 years old 10–24 years old 30–60 years old | 2 doses regimen of the Adjuvanted vaccine AS03 for children Review does not describe intervention for adults. | 77% effectiveness in children <10 years old. 100% effectiveness in ages 10–24 years old. 89–92% in children (6 months to 12 years) and 69–89% in adults (30–60 years). |
| 2011 | Panvax®: a monovalent inactivated unadjuvanted vaccine against pandemic influenza A (H1N1) 2009 [20] | Panvax® vaccine | Adults and children (6 months to 64 years old) | 15-μg dose of Panvax®, a monovalent inactivated adjuvanted vaccine | Patients established > 90% seroprotection. |
| 2011 | A fast-tracked influenza virus vaccine produced in insect cells [21] | FluBlok vaccine | Study PSC01: Healthy adults aged 18–49 years old; enrolled during 2004–2005 flu season PSC04: Healthy adults aged 18–49 years old; enrolled during 2007–2008 flu season | Study PSC01: Subjects were vaccinated with either FluBlok 135 μg (n = 153) or 75 μg (n = 151), or a saline placebo (n = 154) Study PSC04: Subjects were randomized to receive FluBlok 135 μg (n = 2344) or placebo (n = 2304) | Protective efficacy was established against culture-confirmed CDC-ILI was 85.5% overall (95% CI 23.7, 98.5). This study also revealed statistically significant reduction in culture-confirmed CDC-ILI between subjects who received FluBlok (135 μg) vs. placebo (p = 0.0146). |
| 2009 | FluBlok, a next generation influenza vaccine manufactured in insect cells [22] | FluBlok vaccine | Study PSC01: Healthy adults aged 18–49 years old; enrolled during 2004–2005 flu season Study PSC03: Healthy adults age ≥ 65 years old; enrolled during 2006–2007 flu season Study PSC04: Healthy adults aged 18–49 years old; enrolled during 2007–2008 flu season Study PSC06: Healthy adults aged 50–64 years old; enrolled during 2007–2008 flu season | Study PSC01: Subjects were randomized to receive FluBlok 135 μg (n = 153) or 75 μg (n = 151), or placebo (n = 154) Study PSC03: Subjects were randomized to receive FluBlok 135 μg (n = 431) or trivalent, inactivated influenza virus vaccine (Fluzone®) (n = 430) Study PSC04: Subjects were randomized to receive FluBlok 135 μg (n = 2344) or placebo (n = 2304) Study PSC06: Subjects were randomized to receive FluBlok 135 μg (n = 299) or a trivalent, inactivated influenza virus vaccine (Fluzone®) (n = 302) | Study PSC01: FluBlok provided 87% seroprotection against A/H1N1 and 100% seroprotection against A/H3N2 (95% CI 98, 100) with the 135-μg dose. Study PSC03: FluBlok provided 95% seroprotection against A/H1N1 (95% CI 92, 97) and 97% seroprotection against A/H3N2 (95% CI 94, 98) Study PSC04: FluBlok provided 98% seroprotection against A/H1N1 (95% CI 97, 99) and 96% seroprotection against A/H3N2 (95% CI 94, 98). Study PSC06: Flublok provided 96% seroprotection against A/H1N1 (95% CI 94, 98) and 85% seroprotection against A/H3N2 (95% CI 81, 89). |
| 2009 | H1N1 vaccine | Adults aged ≥ 18 years old | One 15-μg injection of the H1N1 vaccine | After 21 days, 97% of these adults had enough antibodies for optimal protection against the virus. Antibody titers of 1:40 or more (hemagglutination-inhibition assay) were observed in 116 (97%) of 120 adults who received the 15-μg dose. | |
| 2008 | BiovaxID, a personalized therapeutic vaccine against B-cell lymphomas [25] | BiovaxID vaccine | Patients with follicular non-Hodgkin’s lymphoma in primary or secondary remission after chemotherapy treatment | Phase I Intervention: five subcutaneous immunizations of BiovaxID to 41 patients with follicular non-Hodgkin’s lymphoma Phase II Intervention: four monthly subcutaneous injections of BiovaxID with a sample size of 20 in complete remission after six or more monthly cycles of proMACE chemotherapy. | Phase 1 Result: 85% had antibody responses, 35% had cellular responses, and 87% of the patients who responded to the vaccine were able to remain tumor free. Phase I also revealed a progression-free period for vaccinated patients with a response of 7.9 years Phase II Result: Phase II of this trial yielded results showing 45% disease-free survival and a 95% overall survival among patients. BiovaxID treatment after 36 months showed approximately 100% improvement in median disease-free survival. (p = 0.024). |
| 2003 | Cancer Vaccine THERATOPE®—Biomira [26] | THERATOPE® | Patients with ovarian and breast cancer receiving HDC/ASCT | Five doses of THERATOPE® after HDC/ASCT | Patients who did not receive THERATOPE® had a 2-fold and 1.7-fold higher risk of death and relapse, respectively. |
| 2012 | The Failed Theratope Vaccine: 10 Years Later [27] | THERATOPE® | Patients with metastatic breast cancer receiving cyclophosphamide (n = 1030) | After initial dose, THERATOPE® was given monthly for 4 months, and then quarterly until disease progression | Week-12 antibody titers showed high IgG in the treatment group and undetectable levels in the control group. Overall median survival between the treatment and control groups were not statistically significant (23.1 months vs. 22.3 months, respectively). |
| 2006 | Rotavirus Vaccine: Early Introduction in Latin America—Risks and Benefits [28] | Rotarix vaccine against attenuated serotype G1 rotavirus strain | Infants and young children | N/A | The efficacy of the vaccine against severe rotavirus gastroenteritis and associated hospitalization was 85% (p < 0.001vs. placebo) and even amounted to 100% against more severe cases of gastroenteritis. It also reduced the hospitalizations of diarrhea by 42% (95% confidence interval). The overall efficacy of Rotarix against the G1 rotavirus strain was seen to be 91%. |
| Publication Date | Study Name | Fast-Tracked Vaccine | Patient Population | Intervention | Results |
|---|---|---|---|---|---|
| 2019 | The Candidate Blood-stage Malaria Vaccine P27A Induces a Robust Humoral Response in a Fast Track to the Field Phase 1 Trial in Exposed and Nonexposed Volunteers [17] | P27A peptide vaccine against malaria | Phase 1a: 16 healthy European volunteers not previously exposed to malaria (8 volunteers per group) Phase 1b: 40 malaria exposed African volunteers (4 groups: 8 people getting vaccine, 2 people getting control) | P27A antigen (10 or 50 μg), adjuvanted with Alhydrogel or GLA-SE (2.5 or 5 μg), or control rabies vaccine (Verorab) were administered intramuscularly to 16 malaria-nonexposed and 40 malaria-exposed subjects on days 0, 28, and 56. | Phase 1a: Most common systemic adverse effects were tiredness (48.5%) and headache (29.2%). There were no significant abnormal vital signs or other severe effects. Phase 1b: 64.1% experienced fatigue and headache. There were no significant abnormal vital signs. |
| 2017 | Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1-DiCo malaria vaccine adjuvanted with GLA-SE or Alhydrogel® in European and African adults: A phase 1a/1b, randomized, double-blind multi-center trial [18] | Recombinant Plasmodium falciparum AMA1-DiCo malaria vaccine adjuvanted with GLA-SE or Alhydrogel® | Cohort A: 30 European adults Cohort B: 36 African adults | Cohort A: Participants received AMA1-DiCo with either Alhydrogel® (n = 15) or GLA-SE (n = 15) Cohort B: Participants received either AMA1-DiCo/GLA-SE (n = 18) or placebo (n = 18) | Cohort A: Local reactions (injection site pain, limited arm abduction at the shoulder) were seen with 93% of the Alhydrogel® group and 100% of the GLA-SE group. 1/30 participants experienced epilepsy unrelated to the vaccination. There were no further systemic reactions reported. Cohort B: Local reactions were experienced by 33% of the GLA-SE group and 17% of the placebo group. Systemic reactions were seen in 22% of the GLA-SE group and 22% of the placebo group. |
| 2003 | Cancer Vaccine THERATOPE®—Biomira [26] | THERAPTOPE® | 30 patients with ovarian cancer | The 30 participants of the phase II study were randomized to receive either 10 or 100 units of THERATOPE® administered subcutaneously at weeks 0, 2, and 5, and then every 4 weeks. Total of 6 doses. | The preliminary results in the R&D profile indicated that the vaccine was well-tolerated. All patients experienced mild flu-like syndrome for 2 to 5 days after vaccination. Only one patient discontinued treatment due to adverse events (dyspnea and hypoxia after the fourth dose). |
| 2012 | The failed Theratope vaccine: 10 years later [27] | THERAPTOPE® | women with metastatic breast cancer | N/A | Phase II trials reported minimal toxic effects, primarily mild injection-site reactions and flu-like symptoms. |
| 2006 | Rotavirus Vaccine: Early Introduction in Latin America—Risks and Benefits [28] | Rotarix and RotaShield vaccines | Infants and young children | N/A | Shortly after RotaShield became available in the US (October 1998), there were about 100 cases of intussusception associated with vaccine administration. In 1999, RotaShield was quickly withdrawn from the market. The preliminary clinical data on Rotarix was not sufficient to make conclusions about safety. |
| 2006 | Sipuleucel-T: APC 8015, APC-8015, Prostate Cancer Vaccine—Dendreon [29] | Sipuleucel-T | Men with advanced prostate cancer D9901: 127 men D9902A: 98 men | D9901: Patients were randomized to receive either Sipuleucel-T (n = 82) or placebo (n = 45) D9902A: Patients were randomized to receive Sipuleucel-T (n = 65) or placebo (n = 33) | For both D9901 and D9902A trials, Sipuleucel-T was well-tolerated among the vaccine recipients. |
| 2009 | Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer [30] | Sipuleucel-T | Men with advanced prostate cancer D9901: 127 men D9902A: 98 men | D9901: Patients were randomized to receive either Sipuleucel-T (n = 82) or placebo (n = 45) D9902A: Patients were randomized to receive Sipuleucel-T (n = 65) or placebo (n = 33) | Less than 3% of patients in D9901 and D9902A had treatment-related adverse events that prevented them from receiving all 3 infusions. Integrated data showed that the adverse reactions occurring at a higher rate (p ≤ 0.05) in the Sipuleucel-T group compared to the placebo group were chills, fever, headache, asthenia, dyspnea, vomiting and tremor. Additionally, these adverse reactions were primarily mild (grade 1 and 2, duration 1 to 2 days). |
| 2010 | Sipuleucel-T immunotherapy for castration-resistant prostate cancer [31] | Sipuleucel-T | 512 men with metastatic castration-resistant prostate cancer | 341 patients received Sipuleucel-T, and 171 patients received a placebo | Sipuleucel-T was generally well-tolerated in terms of adverse events and also prolonged survival in study patients |
| 2014 | Pandemic influenza A H1N1 vaccines and narcolepsy: vaccine safety surveillance in action [32] | H1N1-AS03-P vaccine | Adolescents in Finland, Sweden, Ireland, and the Netherlands, who received the vaccine | N/A | In 2010, reports of narcolepsy associated with vaccination in adolescents from Sweden and Finland prompted an investigation by the ECDC and VAESCO. The results of the investigation and other studies supported an increased risk of post-vaccination narcolepsy. |
| 2011 | Safety of pandemic H1N1 vaccines in children and adolescents [33] | Pandemic H1N1 vaccines (adjuvanted vs. non-adjuvanted) | Children and adolescents | N/A | Studies found that the adjuvanted vaccines were more reactogenic than non-adjuvanted vaccines. In terms of adverse events, they were both generally well-tolerated. Differing methodology between various studies made it difficult to make conclusions about the safety profile of these vaccines. |
| Publication Date | Study Name | Fast-Tracked Vaccine | Patient Population | Lessons Learned |
|---|---|---|---|---|
| 2013 | Pandemic influenza A (H1N1) 2009 vaccination in children: A UK perspective [19] | Adjuvanted vaccine AS03 for H1N1 | 6 months to 12 years old <10 years old 10–24 years old 30–60 years old | The substantial developments in understanding influenza epidemiology, pandemic policy planning, and vaccinology. As well as the discovery that using vaccines with oil in water adjuvant systems results in great immunogenicity in the younger population |
| 2011 | Panvax®: a monovalent inactivated unadjuvanted vaccine against pandemic influenza A (H1N1) 2009 [20] | Panvax® vaccine | Adults and children (6 months to 64 years old) | The future of pandemic vaccines lies in developing more broadly cross-protective preparations capable of preventing infection with both seasonal and pandemic strains if rapid containment of emerging viruses is to be achieved |
| 2003 | Cancer Vaccine THERATOPE®—Biomira [26] | THERAPTOPE® | 95 patients with ovarian cancer in 12 different US sites | The vaccine did not advance past the checkpoints in clinical trials, so it was not put on the market. |
| 2012 | The failed Theratope vaccine: 10 years later [27] | THERAPTOPE® | 1030 patients with metastatic breast cancer from 120 sites in 10 countries | THERAPTOPE® did not meet pre-determined statistical endpoints in clinical trials, so it was not released to the market. |
| 2006 | Rotavirus Vaccine: Early Introduction in Latin America—Risks and Benefits [28] | Rotavirus vaccines | 1000+ people in trials performed in 11 Latin American countries | Rotarix vaccine was safe and efficacious. Lessons learned from this study are that fast-tracking such vaccines would be of great importance to the safety of children in countries where deadly rotavirus is commonly found. |
| 2003 | HIV gp120 vaccine—VaxGen: AIDSVAX, AIDSVAX B/B, AIDSVAX B/E, HIV gp120 vaccine—Genentech, HIV gp120 vaccine AIDSVAX—VaxGen, HIV vaccine AIDSVAX—VaxGen [34] | AIDSVAX | 5108 men who have sex with men and 309 at-risk women | When organizations collaborate and combine their resources, remarkable goals can be achieved. |
| 2003 | Understanding the Results of the AIDSVAX Trial|AVAC [35] | AIDSVAX | Patients at high-risk for HIV infection received vaccine (n = 3330) vs. placebo (n = 1679) | The trial provided important information on the logistics of conducting AIDS vaccine efficacy trials and development of future HIV/AIDS vaccines. |
| 2009 | Antiviral role of toll-like receptor-3 agonists against seasonal and avian influenza viruses [36] | Not a vaccine but was studied for H5N1 | Mice | The study confirms the justification for regulatory agencies to consider fast-track development of drugs for prophylaxis and potentially the treatment of H5N1. |
| 2015 | Emergency treatment for exposure to Ebola virus: the need to fast-track promising vaccines [37] | VSVΔGZEBOV | One physician who had a needlestick in an Ebola treatment unit | It is important to have a sufficient supply of safe and effective vaccines that can be rapidly deployed in emergency situations. |
| 2018 | EBOVAC-Salone: Lessons learned from implementing an Ebola vaccine trial in an Ebola-affected country [38] | Ebola vaccine | Stage 1: 40 participants Stage 2: 730 participants | Research should be more closely incorporated into outbreak response planning, expediting timely and appropriate research projects. |
| 2018 | Ebola: Lessons on Vaccine Development [39] | Ebola vaccine | Many different species of animals such as lab mice, humanized mouse, hamster, guinea pig, ferret, and non-human primate. | Studying other EBOV antigens so that time, money, and better results can be achieved earlier rather than having extra expenses and reduced optimization. The epidemic demonstrated the lack of preparation and limitations in our public health response. Since then, better planning and preparation have been discussed and implicated. |
| 2018 | Ebola vaccines—Where are we? [40] | Ebola vaccine | N/A | Good coordination and collaboration during future epidemics are necessary when it comes to developing a vaccine safely and effectively. Encouraging the world health organization to improve current structures so that response and preparation will be improved when a future epidemic occurs. |
| 2020 | Post-marketing studies: can they provide a safety net for COVID-19 vaccines in the UK? [41] | COVID-19 vaccines | Adults in the UK | COVID-19 vaccines are quickly progressing through clinical development with fast-tracking, and post-marketing surveillance and observational studies can help bridge gaps in clinical trial data, especially in regard to safety. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, J.C.; Lao, C.T.; Yousif, M.M.; Luga, J.M. Fast Tracking—Vaccine Safety, Efficacy, and Lessons Learned: A Narrative Review. Vaccines 2022, 10, 1256. https://doi.org/10.3390/vaccines10081256
Wong JC, Lao CT, Yousif MM, Luga JM. Fast Tracking—Vaccine Safety, Efficacy, and Lessons Learned: A Narrative Review. Vaccines. 2022; 10(8):1256. https://doi.org/10.3390/vaccines10081256
Chicago/Turabian StyleWong, Jason C., Crystal T. Lao, Melanie M. Yousif, and Jacqueline M. Luga. 2022. "Fast Tracking—Vaccine Safety, Efficacy, and Lessons Learned: A Narrative Review" Vaccines 10, no. 8: 1256. https://doi.org/10.3390/vaccines10081256
APA StyleWong, J. C., Lao, C. T., Yousif, M. M., & Luga, J. M. (2022). Fast Tracking—Vaccine Safety, Efficacy, and Lessons Learned: A Narrative Review. Vaccines, 10(8), 1256. https://doi.org/10.3390/vaccines10081256







