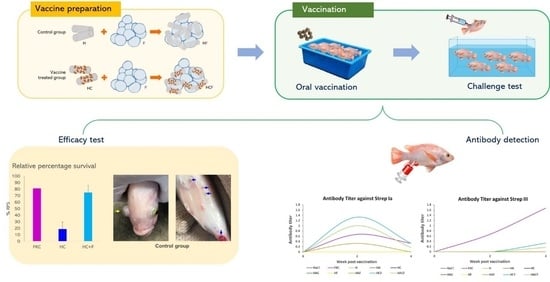
A Novel Efficient Piscine Oral Nano-Vaccine Delivery System: Modified Halloysite Nanotubes (HNTs) Preventing Streptococcosis Disease in Tilapia (Oreochromis sp.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the StrepKU-1 Vaccine
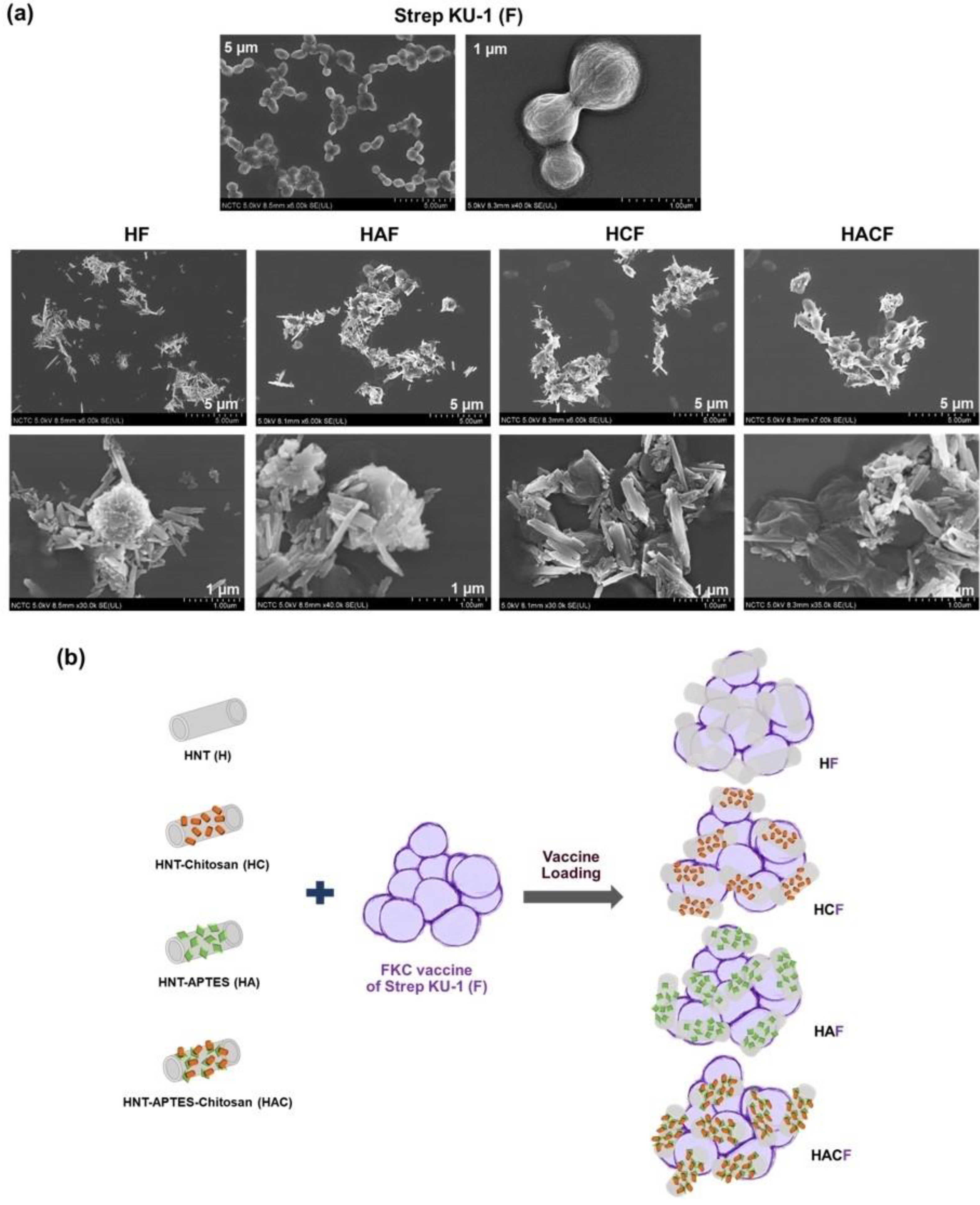
2.3. Preparation of StrepKU-1-Loaded HNTs
2.4. Physical Characterization by Scanning Electron Microscopy (SEM)
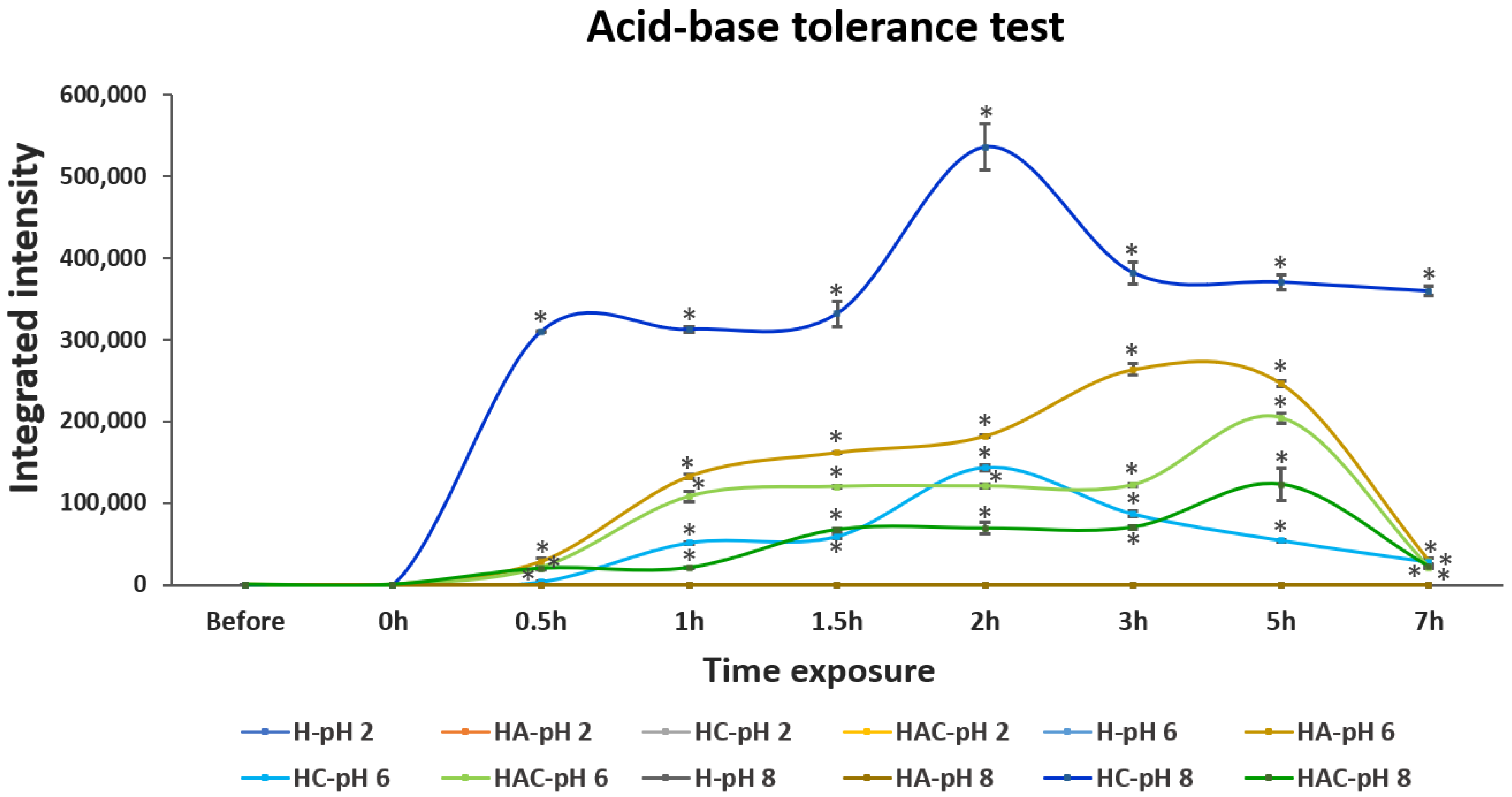
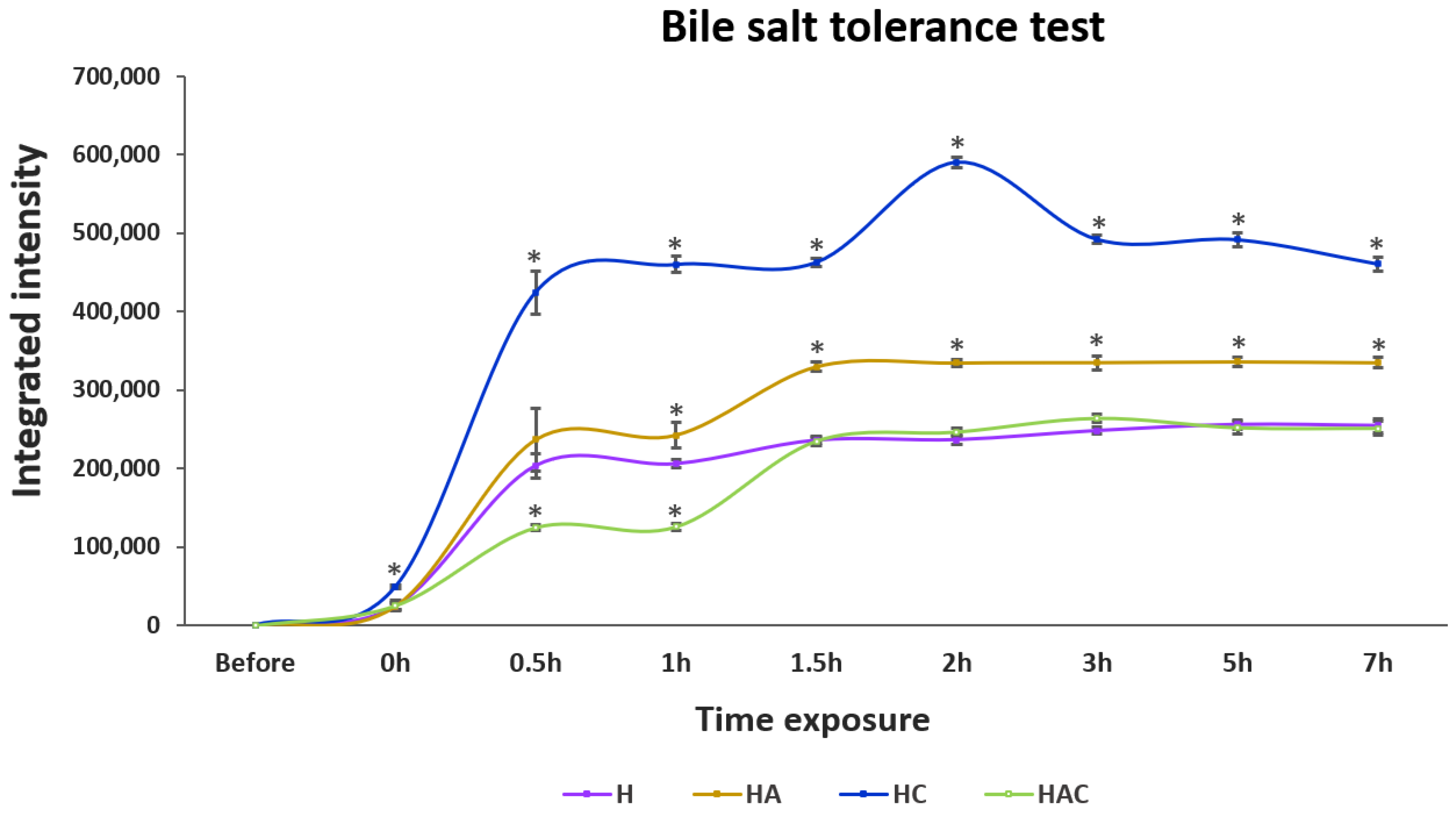
2.5. Acid, Base and Bile Salt Tolerance Profile of StrepKU-1-Loaded HNTs
2.6. In Vivo Efficacy Analysis of the StrepKU-1 Loaded HNTs
2.6.1. Vaccination and Challenge Test
2.6.2. Antibody Titer Assay
3. Results
3.1. Physical Observation of StrepKU-1-Loaded HNT Complexes by SEM Images
3.2. Acid, Base and Bile Salt Tolerance
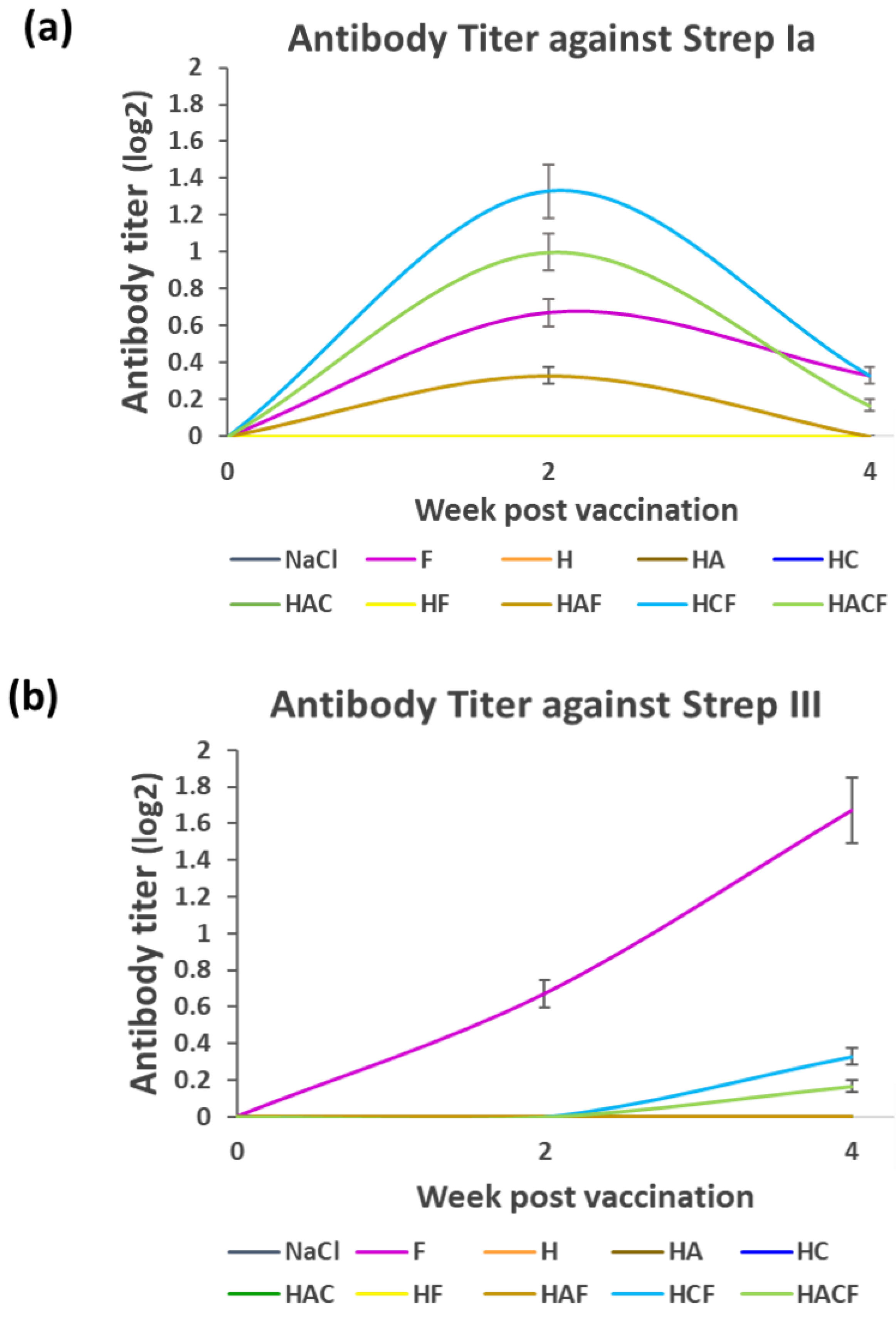
3.3. Specific Immunity of Fish Fed with the HNT Nano-Delivery System
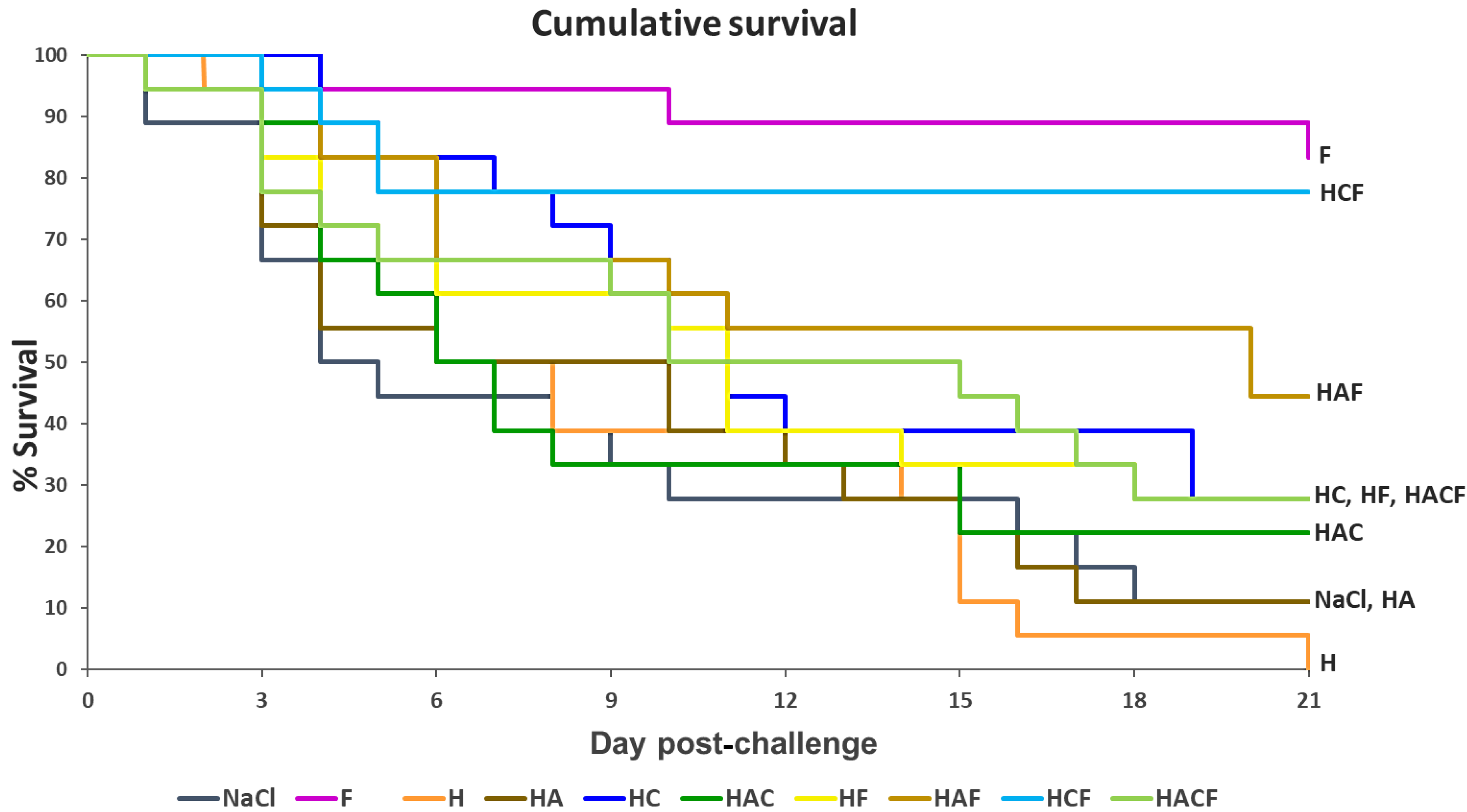
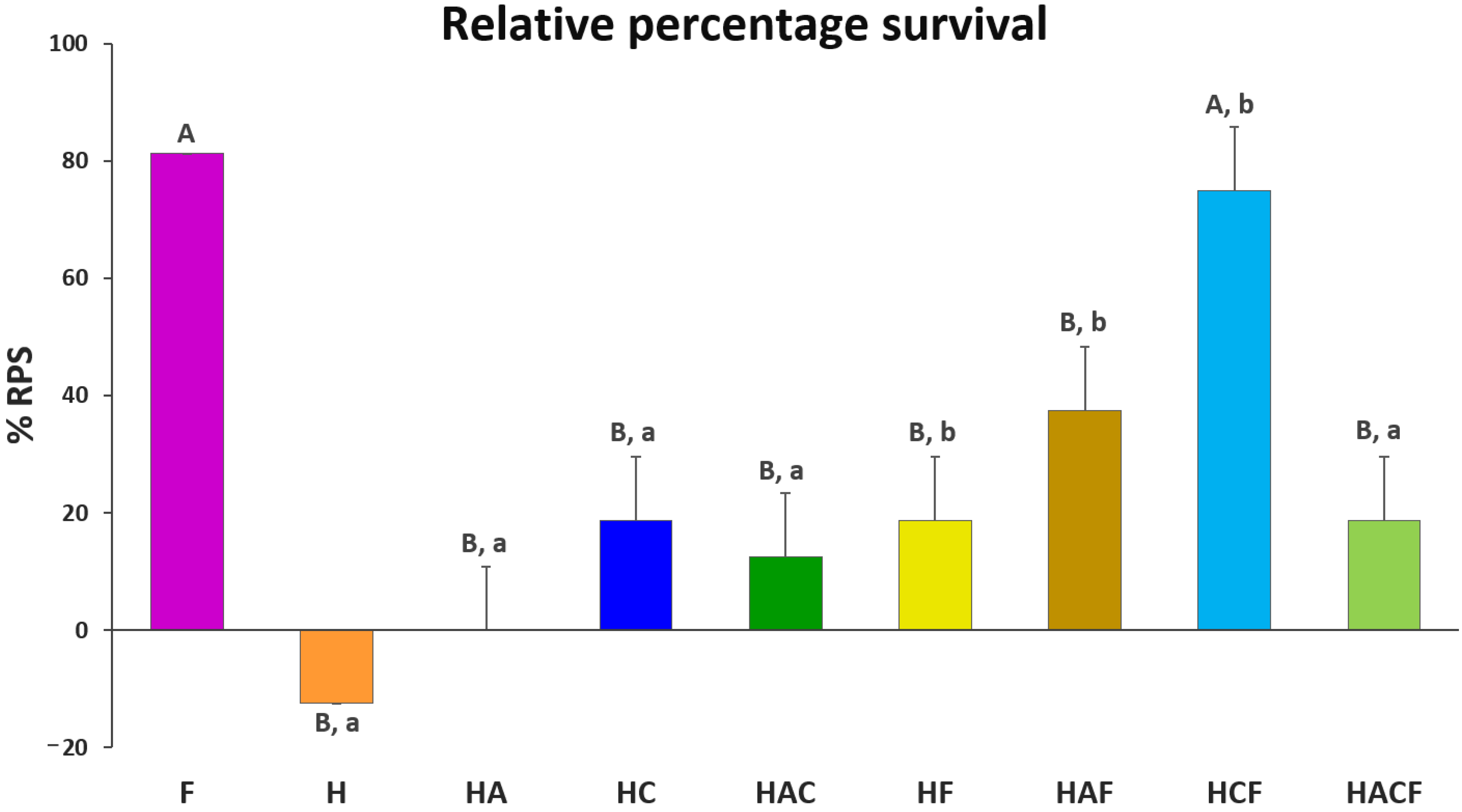
3.4. Efficacy Analysis of the StrepKU-1-Loaded HNTs against Streptococcosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, J.; Bruce, T.J.; Jones, E.M.; Cain, K.D. A review of fish vaccine development strategies: Conventional methods and modern biotechnological approaches. Microorganisms 2019, 7, 569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, A. Progress, challenges and opportunities in fish vaccine development. Fish Shellfish Immunol. 2019, 90, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K. Current prospects and challenges in fish vaccine development in India with special reference to Aeromonas hydrophila vaccine. Fish Shellfish Immunol. 2020, 100, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Bedekar, M.; Kole, S. Fundamentals of Fish Vaccination. In Vaccine Design. Methods in Molecular Biology; Thomas, S., Ed.; Humana: New York, NY, USA, 2021; Volume 2411. [Google Scholar] [CrossRef]
- Bogwald, J.; Dalmo, R.A. Review on Immersion Vaccines for Fish: An Update 2019. Microorganisms 2019, 7, 627. [Google Scholar] [CrossRef] [Green Version]
- Munang’andu, H.M.; Mutoloki, S.; Evensen, Ø. An overview of challenges limiting the design of protective mucosal vaccines for finfish. Front. Immunol. 2015, 6, 542. [Google Scholar] [CrossRef] [Green Version]
- Gravningen, K.; Sakai, M.; Mishiba, T.; Fujimoto, T. The efficacy and safety of an oil-based vaccine against Photobacterium damsela subsp. piscicida in yellowtail (Seriola quinqueradiata): A field study. Fish Shellfish Immunol. 2008, 24, 523–529. [Google Scholar] [CrossRef]
- Duff, D.C.B. The oral immunization of trout against Bacterium salmonicida. J. Immunol. 1942, 44, 87. [Google Scholar]
- Kang, S.H.; Hong, S.J.; Lee, Y.K.; Cho, S. Oral vaccine delivery for intestinal immunity-biological basis, barriers, delivery system, and M cell targeting. Polymers 2018, 10, 948. [Google Scholar] [CrossRef] [Green Version]
- Embregts, C.; Forlenza, M. Oral vaccination of fish: Lessons from humans and veterinary species. Dev. Comp. Immunol. 2016, 64, 118–137. [Google Scholar] [CrossRef] [Green Version]
- Sommerset, I.K.; Biering, B.E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2014, 4, 89–101. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Rodriguez-Torres, M.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, R.; Wu, L.; Zhang, C.; Davis, R.M.; Xu, B. Natural product-based nanomedicine: Recent advances and issues. Int. J. Nanomed. 2015, 10, 6055. [Google Scholar]
- McNamara, K.; Tofail, S.A. Nanosystems: The use of nanoalloys, metallic, bimetallic, and magnetic nanoparticles in biomedical applications. Phys. Chem. Chem. Phys. 2015, 17, 27981–27995. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Wu, F.; Yu, B.; Wang, W.; Cai, X. Folate-PG modified halloysite nanotube for enhancing tumor targeting and anticancer efficacy. Appl. Clay Sci. 2020, 193, 105664. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Layered composite based on halloysite and natural polymers: A carrier for the pH controlled release of drugs. New J. Chem. 2019, 43, 10887–10893. [Google Scholar] [CrossRef] [Green Version]
- Shutava, T.; Fakhrullin, R.; Lvov, Y. Spherical and tubule nanocarriers for sustained drug release. Curr. Opin. Pharmacol. 2014, 18, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husain, T.; Shoaib, M.H.; Ahmed, F.R.; Yousuf, R.I.; Farooqi, S.; Siddiqui, F.; Imtiaz, M.S.; Maboos, M.; Jabeen, S. Investigating halloysite nanotubes as a potential platform for oral modified delivery of different BCS class drugs: Characterization, optimization, and evaluation of drug release kinetics. Int. J. Nanomed. 2021, 16, 1725–1741. [Google Scholar] [CrossRef]
- Veerabadran, N.G.; Price, R.R.; Lvov, Y.M. Clay nanotubes for encapsulation and sustained release of drugs. Nano 2007, 2, 115–120. [Google Scholar] [CrossRef]
- Ahmed, F.R.; Shoaib, M.H.; Azhar, M.; Um, S.H.; Yousuf, R.I.; Hashmi, S.; Dar, A. In-vitro assessment of cytotoxicity of halloysite nanotubes against HepG2, HCT116 and human peripheral blood lymphocytes. Colloids Surf. B Biointerfaces 2015, 135, 50–55. [Google Scholar] [CrossRef]
- Yendluri, R.; Otto, D.P.; De Villiers, M.M.; Vinokurov, V.; Lvov, Y.M. Application of halloysite clay nanotubes as a pharmaceutical excipient. Int. J. Pharm. 2017, 521, 267–273. [Google Scholar] [CrossRef]
- Lvov, Y.; Wang, W.; Zhang, L.; Fakhrullin, R. Halloysite clay nanotubes for loading and sustained release of functional compounds. Adv. Mater. 2016, 28, 1227–1250. [Google Scholar] [CrossRef]
- Abdullayev, E.; Lvov, Y. Halloysite clay nanotubes as a ceramic “skeleton” for functional biopolymer composites with sustained drug release. J. Mater. Chem. B 2013, 1, 2894–20903. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, J.; Li, X.; Sun, Y.; Huang, S.; Li, Y.; Liu, H.; Xu, J.; Zhong, S. Multifunctional halloysite nanotubes for targeted delivery and controlled release of doxorubicin in-vitro and in-vivo studies. Nanotechnology 2017, 28, 375101. [Google Scholar] [CrossRef] [PubMed]
- Kayansamruaj, P.; Areechon, P.; Unajak, S. Development of fish vaccine in Southeast Asia: A challenge for the sustainability of SE Asia aquaculture. Fish Shellfish Immunol. 2020, 103, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Li, L.P.; Wang, R.; Liang, W.W.; Huang, T.; Huang, Y.; Luo, F.G.; Lei, A.Y.; Chen, M.; Gan, X. Development of live attenuated Streptococcus agalactiae vaccine for tilapia via continuous passage in vitro. Fish Shellfish Immunol. 2015, 45, 955–963. [Google Scholar] [CrossRef]
- Liu, G.; Chen, Z.K.; Gao, T.; Yao, H.; Liu, Y.; Zhang, W.; Lu, C. Development of Streptococcus agalactiae vaccines for tilapia. Dis. Aquat. Organ. 2016, 122, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Espinoza, F.C.; Cueva-Quiroz, V.A.; Yunis-Aguinaga, J.; de Moraes, J.R.E. A comparison of novel inactivation methods for production of a vaccine against Streptococcus agalactiae in Nile tilapia Oreochromis niloticus. Aquaculture 2020, 528, 735484. [Google Scholar] [CrossRef]
- Sukenda, S.; Rahman, R.; Nisaa, K.; Hidayatullah, D.; Vinasyiam, A. The efficacy of Streptococcus agalactiae vaccine preparations, administered to tilapia broodstock, in preventing streptococcosis in their offspring, via transfer of maternal immunity. Aquac. Int. 2018, 26, 785–798. [Google Scholar] [CrossRef]
- Ma, Y.; Hao, L.; Liang, Z.; Ma, J.; Ke, H.; Kang, H.; Yang, H.; Wu, J.; Feng, G.; Liu, Z. Characterization of novel antigenic vaccine candidates for Nile tilapia (Oreochromis niloticus) against Streptococcus agalactiae infection. Fish Shellfish Immunol. 2020, 105, 405–414. [Google Scholar] [CrossRef]
- Zhang, Z. Research advances on Tilapia streptococcosis. Pathogens 2021, 10, 558. [Google Scholar] [CrossRef]
- Pumchan, A.; Krobthong, S.; Roytrakul, S.; Sawatdichaikul, O.; Kondo, H.; Hirono, I.; Areechon, N.; Unajak, S. Novel chimeric multiepitope vaccine for Streptococcosis disease in Nile tilapia (Oreochromis niloticus Linn.). Sci. Rep. 2020, 10, 603. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, Q.; Huang, L.; Wang, K.; Wang, X.; Chen, D.; Geng, Y.; Huang, X.; Ouyang, P.; Lai, W. Effectivity of oral recombinant DNA vaccine against Streptococcus agalactiae in Nile tilapia. Dev. Comp. Immunol. 2017, 77, 77–87. [Google Scholar] [CrossRef]
- Pumchan, A.; Cheycharoen, O.; Unajak, S.; Prasittichai, C. An oral biologics carrier from modified halloysite nanotubes. New J. Chem. 2021, 45, 9130–9136. [Google Scholar] [CrossRef]
- Shan, Y.; Yang, N.; Teng, D.; Wang, X.; Mao, R.; Hao, Y.; Ma, H.; Fan, H.; Wang, J. Recombinant of the Staphylococcal Bacteriophage lysin CHAPk and its elimination against Streptococcus agalactiae biofilms. Microorganisms 2020, 8, 216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannika, K.; Pisuttharachai, D.; Srisapoome, P.; Wongtavatchai, J.; Kondo, H.; Hirono, I.; Unajak, S.; Areechon, N. Molecular serotyping, virulence gene profiling and pathogenicity of Streptococcus agalactiae isolated from tilapia farms in Thailand by multiplex PCR. J. Appl. Microbiol. 2017, 122, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Miccoli, A.; Manni, M.; Picchietti, S.; Scapigliati, G. State-of-the-art vaccine research for aquaculture use: The case of three economically relevant fish species. Vaccines 2021, 9, 140. [Google Scholar] [CrossRef]
- Munang’andu, H.M.; Paul, J.; Evensen, O. An overview of vaccination strategies and antigen delivery systems for Streptococcus agalactiae vaccines in Nile tilapia (Oreochromis niloticus). Vaccines 2016, 4, 48. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.Z.; Liu, Z.G.; Lu, M.X.; Ke, X.L.; Zhang, D.F.; Gao, F.Y.; Cao, J.M.; Wang, M.; Yi, M.M. Oral immunization with surface immunogenic protein from Streptococcus agalactiae expressed in Lactococcus lactis induces protective immune responses of tilapia Oreochromis niloticus. Aquaculture 2020, 18, 100538. [Google Scholar] [CrossRef]
- Yao, Y.Y.; Chen, D.D.; Cui, Z.W.; Zhang, X.Y.; Zhou, Y.Y.; Guo, X.; Li, A.H.; Zhang, Y.A. Oral vaccination of tilapia against Streptococcus agalactiae using Bacillus subtilis spores expressing Sip. Fish Shellfish Immunol. 2019, 86, 999–1008. [Google Scholar] [CrossRef]
- Bugatti, V.; Viscusi, G.; Naddeo, C.; Gorrasi, G. Nanocomposites based on PCL and halloysite nanotubes filled with lysozyme: Effect of draw ratio on the physical properties and release analysis. Nanomaterials 2017, 7, 213. [Google Scholar] [CrossRef] [Green Version]
- Gorrasi, G. Dispersion of halloysite loaded with natural antimicrobials into pectins: Characterization and controlled release analysis. Carbohydr. Polym. 2015, 127, 47–53. [Google Scholar] [CrossRef]
- Jiang, W.T.; Chang, P.H.; Tsai, Y.; Li, Z. Halloysite nanotubes as a carrier for the uptake of selected pharmaceuticals. Microporous Mesoporous. Mater. 2016, 220, 298–307. [Google Scholar] [CrossRef]
- Fizir, M.; Dramou, P.; Dahiru, N.S.; Ruya, W.; Huang, T.; He, H. Halloysite nanotubes in analytical sciences and in drug delivery: A review. Microchim. Acta 2018, 185, 389. [Google Scholar] [CrossRef] [PubMed]
- Hanif, M.; Jabbar, F.; Sharif, S.; Abbas, G.; Farooq, A.; Aziz, M. Halloysite nanotubes as a new drug-delivery system: A review. Clay. Miner. 2016, 51, 469–477. [Google Scholar] [CrossRef]
- Tharmavaram, M.; Pandey, G.; Rawtani, D. Surface modified halloysite nanotubes: A flexible interface for biological, environmental and catalytic applications. Adv. Colloid Interface Sci. 2018, 261, 82–101. [Google Scholar] [CrossRef]
- Setter, O.P.; Segal, E. Halloysite nanotubes–the nano-bio interface. Nanoscale 2020, 12, 23444–23460. [Google Scholar] [CrossRef]
- Yang, J.H.; Lee, J.H.; Ryu, H.J.; Elzatahry, A.A.; Alothman, Z.A.; Choy, J.H. Drug–clay nanohybrids as sustained delivery systems. Appl. Clay Sci. 2016, 130, 20–23. [Google Scholar] [CrossRef]
- Long, Z.; Zhang, J.; Shen, Y.; Zhou, C.; Liu, M. Polyethyleneimine grafted short halloysite nanotubes for gene delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 81, 224–235. [Google Scholar] [CrossRef]
- Kerdsakundee, N.; Li, W.; Martins, J.P.; Liu, Z.; Zhang, F.; Kemell, M.; Correia, A.; Ding, Y.; Airavaara, M.; Hirvonen, J.; et al. Multifunctional nanotube-mucoadhesive poly(methyl vinyl ether-co-maleic acid)@hydroxypropyl methylcellulose acetate succinate composite for site-specific oral drug delivery. Adv. Healthc. Mater. 2017, 6, 1–20. [Google Scholar] [CrossRef]
- Massaro, M.; Riela, S.; Baiamonte, C.; Blanco, J.L.J.; Giordano, C.; Lo Meo, P.; Milioto, S.; Noto, R.; Parisi, F.; Pizzolanti, G.; et al. Dual drug-loaded halloysite hybrid-based glycocluster for sustained release of hydrophobic molecules. RSC Adv. 2016, 6, 87935–87944. [Google Scholar] [CrossRef]
- Kurczewska, J.; Cegłowski, M.; Messyasz, B.; Schroeder, G. Dendrimer-functionalized halloysite nanotubes for effective drug delivery. Appl. Clay. Sci. 2018, 153, 134–143. [Google Scholar] [CrossRef]
- Plant, K.P.; LaPatra, S.; Call, D.R.; Cain, K. Attempts at validating a recombinant Flavobacterium Psychrophilum gliding motility protein N as a vaccine candidate in rainbow trout, Oncorhynchus mykiss (Walbaum) against bacterial cold-water disease. FEMS Microbiol. Lett. 2014, 358, 14–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arshadi, N.; Mousavi, S.; Amani, J.; Nazarian, S. Immunogenic potency of formalin and heat inactivated E. coli O157:H7 in mouse model administered by different routes. Avicenna J. Med. Biotechnol. 2020, 12, 194–200. [Google Scholar] [PubMed]
- Konnova, S.A.; Sharipova, I.R.; Demina, T.A.; Osin, Y.N.; Yarullina, D.R.; Ilinskaya, O.N.; Lvov, Y.M.; Fakhrullin, R.F. Biomimetic cell-mediated three-dimensional assembly of halloysite nanotubes. Chem. Commun. 2013, 49, 4208. [Google Scholar] [CrossRef]
- Yasumaru, F.; Lemos, D. Species specific in vitro protein digestion (pH-stat) for fish: Method development and application for juvenile rainbow trout (Oncorhynchus mykiss), cobia (Rachycentron canadum), and Nile tilapia Oreochromis niloticus. Aquaculture 2014, 426–427, 74–84. [Google Scholar] [CrossRef]
- Liu, M.; Yang, J.; You, Y.; He, R.; Chen, T.; Zhou, C. Functionalized halloysite nanotube by chitosan grafting for drug delivery of curcumin to achieve enhanced anticancer efficacy. J. Mater. Chem. B 2016, 4, 2253–2263. [Google Scholar] [CrossRef]
- Li, X.; Yang, Q.; Ouyang, J.; Yang, H.; Chang, S. Chitosan modified halloysite nanotubes as emerging porous microspheres for drug carrier. Appl. Clay Sci. 2016, 126, 306–312. [Google Scholar] [CrossRef]
- Hayat, M.; Yusoff, M.S.M.; Samad, M.J.; Abdul Razak, I.S.; Md Yasin, I.S.; Thompson, K.D.; Hasni, K. Efficacy of feed-based formalin-killed vaccine of Streptococcus iniae stimulates the gut-associated lymphoid tissues and immune response of red hybrid tilapia. Vaccines 2021, 9, 51. [Google Scholar] [CrossRef]
- Yan, S.; Zhu, J.; Wang, Z.; Yin, J.; Zheng, Y.; Chen, X. Layer-by-layer assembly of poly(L-glutamic acid)/chitosan microcapsules for high loading and sustained release of 5-fluorouracil. Eur. J. Pharm. Biopharm. 2011, 78, 336–345. [Google Scholar] [CrossRef]
- Tohidi, S.; Ghaee, A.; Barzin, J. Preparation and characterization of poly(lactic-co-glycolic acid)/chitosan electrospun membrane containing amoxicillin-loaded halloysite nanoclay. Polym. Adv. Technol. 2016, 27, 1020–1028. [Google Scholar] [CrossRef]
- Ahmed, F.; Soliman, F.M.; Adly, M.A.; Soliman, H.A.M.; Matbouli, M.E.; Saleh, M. Recent progress in biomedical applications of chitosan and its nanocomposites in aquaculture: A review. Res. Vet. Sci. 2019, 126, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Sun, F.; Liu, Q.; Wang, Q.; Zhang, Y.; Liu, X. An oral vaccine based on chitosan/aluminum adjuvant induces both local and systemic immune responses in turbot (Scophthalmus maximus). Vaccine 2021, 39, 7477–7484. [Google Scholar] [CrossRef] [PubMed]
- Naggar, M.E.; Medhat, F.; Taha, A. Applications of chitosan and chitosan nanoparticles in fish aquaculture. Egypt. J. Aquat. Biol. Fish. 2022, 26, 23–43. [Google Scholar]
- Nasr-Eldahan, S.; Nabil-Adam, A.; Shreadah, M.A.; Maher, A.M.; Ali, T.E.S. A review article on nanotechnology in aquaculture sustainability as a novel tool in fish disease control. Aquac. Int. 2021, 29, 1459–1480. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, G.; Chiappisi, L.; Gradzielski, M.; Lazzara, G. Effect of the supramolecular interactions on the nanostructure of halloysite/biopolymer hybrids: A comprehensive study by SANS, fluorescence correlation spectroscopy and electric birefringence. Phys. Chem. Chem. Phys. 2020, 22, 8193–8202. [Google Scholar] [CrossRef] [PubMed]
- Vikulina, A.; Voronin, D.; Fakhrullin, R.; Vinokurov, V.; Volodkin, D. Naturally derived nano- and micro-drug delivery vehicles: Halloysite, vaterite and nanocellulose. New J. Chem. 2020, 44, 5638–5655. [Google Scholar] [CrossRef] [Green Version]
- Fakhruddin, K.; Hassan, R.; Khan, M.U.A.; Allisha, S.N.; Razak, S.L.A.; Zreaqat, M.H.; Latip, H.F.M.; Jamaludin, M.N.; Hassan, A. Halloysite nanotubes and halloysite-based composites for biomedical applications. Arab. J. Chem. 2021, 14, 103294. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Reis, S.; Veiga, F.; Saleg, M.; Lvov, Y. Biomedical potential of clay nanotube formulations and their toxicity assessment. Exp. Opin. Drug Deliv. 2019, 16, 1169–1182. [Google Scholar] [CrossRef]
- Hu, D.; Zhang, Z.; Liu, M.; Lin, J.; Chen, X.; Ma, W. Multifunctional UV-shielding nanocellulose films modified with halloysite nanotubes-zinc oxide nanohybrid. Cellulose 2020, 27, 401–413. [Google Scholar] [CrossRef]
- Long, Z.; Wu, Y.P.; Gao, H.Y.; Zhang, J.; Ou, X.; He, R.R.; Liu, M. In vitro and in vivo toxicity evaluation of halloysite nanotubes. J. Mater. Chem. B 2018, 6, 7204–7216. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pumchan, A.; Sae-Ueng, U.; Prasittichai, C.; Sirisuay, S.; Areechon, N.; Unajak, S. A Novel Efficient Piscine Oral Nano-Vaccine Delivery System: Modified Halloysite Nanotubes (HNTs) Preventing Streptococcosis Disease in Tilapia (Oreochromis sp.). Vaccines 2022, 10, 1180. https://doi.org/10.3390/vaccines10081180
Pumchan A, Sae-Ueng U, Prasittichai C, Sirisuay S, Areechon N, Unajak S. A Novel Efficient Piscine Oral Nano-Vaccine Delivery System: Modified Halloysite Nanotubes (HNTs) Preventing Streptococcosis Disease in Tilapia (Oreochromis sp.). Vaccines. 2022; 10(8):1180. https://doi.org/10.3390/vaccines10081180
Chicago/Turabian StylePumchan, Ansaya, Udom Sae-Ueng, Chaiya Prasittichai, Soranuth Sirisuay, Nontawith Areechon, and Sasimanas Unajak. 2022. "A Novel Efficient Piscine Oral Nano-Vaccine Delivery System: Modified Halloysite Nanotubes (HNTs) Preventing Streptococcosis Disease in Tilapia (Oreochromis sp.)" Vaccines 10, no. 8: 1180. https://doi.org/10.3390/vaccines10081180
APA StylePumchan, A., Sae-Ueng, U., Prasittichai, C., Sirisuay, S., Areechon, N., & Unajak, S. (2022). A Novel Efficient Piscine Oral Nano-Vaccine Delivery System: Modified Halloysite Nanotubes (HNTs) Preventing Streptococcosis Disease in Tilapia (Oreochromis sp.). Vaccines, 10(8), 1180. https://doi.org/10.3390/vaccines10081180