Past, Present and Future of Bacillus Calmette-Guérin Vaccine Use in China
Abstract
1. Introduction
2. Introduction of the BCG to China
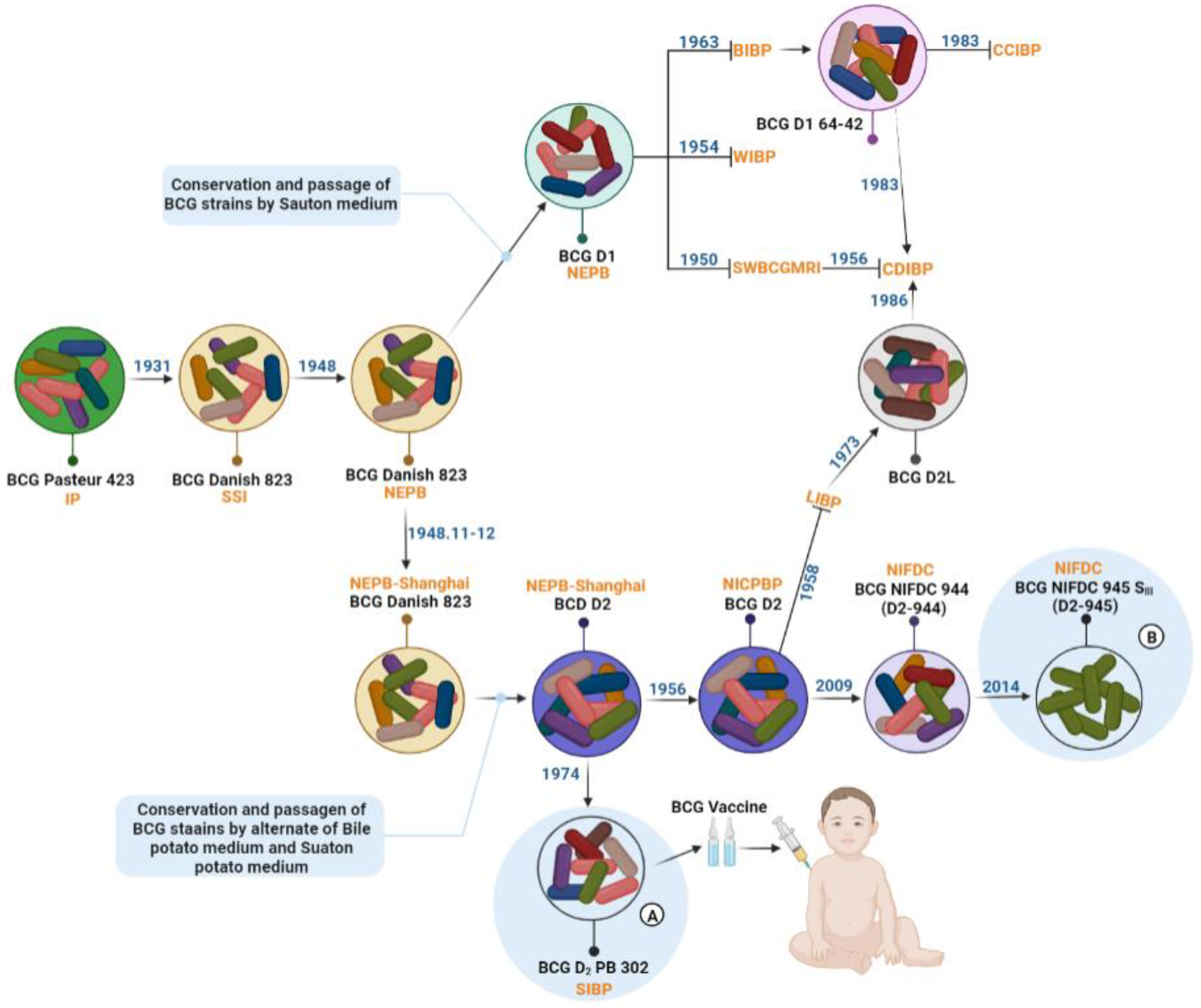
3. BCG Strains in China
3.1. BCG Strains in Modern Production
3.2. BCG Single-Cell Clone Strains
4. BCG Production, Inspection and Vaccination in China
5. Prospects for Future BCG Use in China
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calmette, A.; Guérin, C. Recherches expérimentales sur la défense de l’organisme contre l’infection tuberculeuse. Ann. Inst. Pasteur. 1911, 25, 625–641. [Google Scholar]
- Calmette, A.; Bocquet, A.; Negre, L. Contribution a l’etude du bacilli tuberculuex bilie. Ann. Inst. Pasteur. 1921, 9, 651–670. [Google Scholar]
- Calmette, A.; Guerin, C.; Bouquet, A. La Vaccination Préventive Contre la Tuberculose par le “BCG”; Libraries de L’academie de Medicine: Paris, France, 1927. [Google Scholar]
- Rosenthal, S.R. BCG vaccination against tuberculosis. Am. Rev. Respir. Dis. 1983, 128, 776. [Google Scholar] [PubMed]
- Behr, M.A.; Small, P.M. A historical and molecular phylogeny of BCG strains. Vaccine 1999, 17, 915–922. [Google Scholar] [CrossRef]
- Tran, V.; Liu, J.; Behr, M.A. BCG Vaccines. Microbiol. Spectr. 2014, 2, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Calmette, A.; Guerin, C.; Weill-Halle, B. Essai d’immunisation contre l’infection tuberculeuse. Bull. Acad. Med. 1924, 91, 787–796. [Google Scholar]
- Calmette, A.; Guerin, C.; Negre, L. Sur la vaccination préventive des enfants nouveau-nés contre la tuberculose par le BCG. Ann. Inst. Pasteur. 1927, 41, 201–232. [Google Scholar]
- McShane, H. Tuberculosis vaccines: Beyond bacille Calmette-Guerin. Philos. Trans. R Soc. Lond B Biol. Sci. 2011, 366, 2782–2789. [Google Scholar] [CrossRef]
- Ottenhoff, T.H.; Kaufmann, S.H. Vaccines against tuberculosis: Where are we and where do we need to go? PLoS Pathog. 2012, 8, e1002607. [Google Scholar] [CrossRef]
- Milstien, J.B.; Gibson, J.J. Quality control of BCG vaccine by WHO: A review of factors that may influence vaccine effectiveness and safety. Bull. World Health Organ. 1990, 68, 93–108. [Google Scholar]
- Zhang, Y.; Wallace, R.J., Jr.; Mazurek, G.H. Genetic differences between BCG substrains. Tuber. Lung Dis. 1995, 76, 43–50. [Google Scholar] [CrossRef]
- Mostowy, S.; Tsolaki, A.G.; Small, P.M.; Behr, M.A. The in vitro evolution of BCG vaccines. Vaccine 2003, 21, 4270–4274. [Google Scholar] [CrossRef]
- World Health Organization. Information Sheet. Observed Rate of Vaccine Reactions. Bacille Calmette-Guerin (BCG) Vaccine. 2012. Available online: https://www.who.int/vaccine_safety/initiative/tools/BCG_Vaccine_rates_information_sheet.pdf (accessed on 13 April 2012).
- Chard, A.N.; Gacic-Dobo, M.; Diallo, M.S.; Sodha, S.V.; Wallace, A.S. Routine vaccination coverage-worldwide, 2019. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1706–1710. [Google Scholar] [CrossRef]
- Luca, S.; Mihaescu, T. History of BCG Vaccine. Maedica 2013, 8, 53–58. [Google Scholar] [PubMed]
- Lange, B. Weitere Untersuchungen zur Klärung der Ursachen der Unglücksfälle in Lübeck. Tuberk 1931, 62, 335–351. [Google Scholar]
- Lange, B. Nouvelles recherche sur les causes des accidents de Lubeck. Rev. Tuberc. Extrait 1931, 12, 1142–1170. [Google Scholar]
- Fox, G.J.; Orlova, M.; Schurr, E. Tuberculosis in newborns: The lessons of the “Lübeck Disaster” (1929–1933). PLoS Pathog. 2016, 12, e1005271. [Google Scholar] [CrossRef]
- Lieou, Y.C.; Kouo, C.C. BCG vaccination: 11 years of experience. Chin. Med. J. 1949, 67, 275–278. [Google Scholar]
- Tang, F.F. BCG cultures. Chin. Med. J. Suppl. I 1936, 186. [Google Scholar]
- Liu, Y.C. The progress of BCG in China. Chin. Med. J. 1948, 34, 341. (In Chinese) [Google Scholar]
- Li, J.G. Wang Liang’s 50 years of BCG vaccine research. Chin. J. Antituberc. 1983, 3, 2–5. (In Chinese) [Google Scholar]
- Houston, M. The white death: A history of tuberculosis. BMJ 1999, 318, 1705. [Google Scholar] [CrossRef] [PubMed]
- Dubos, R.; Dubos, J. The White Plague: Tuberculosis, Man and Society; Little, Brown And Company: Boston, MA, USA, 1952; pp. 166–167. [Google Scholar]
- He, L. The Research on the Introduction of BCG into China. J. Dialectics Nat. 2010, 32, 54–58. [Google Scholar]
- Chen, Z.R. The Whole Process of Chen Zhengren, Wei Xihua, Zhu Zongyao Studying BCG Abroad. In History of Chinese Antituberculosis; China Antituberculosis Association: Shanghai, China, 1983; Volume 1, pp. 34–35. (In Chinese) [Google Scholar]
- Chen, Z.R.; Wei, X.H.; Zhu, Z.Y. BCG in China. Chin. Med. J. 1982, 95, 437–442. [Google Scholar]
- Lei, Q.R.; Liu, C.Q.; Song, W.H.; Wu, G.M. Selection of BCG strains. China Antituberc. Commun. 1983, 3, 7–9. (In Chinese) [Google Scholar]
- Zou, B.Z. Achievements of BCG vaccination in the past ten years since the founding of the People’s Republic of China. China Antituberc. 1959, 5, 10–13. (In Chinese) [Google Scholar]
- Wang, L.; Zou, B.Z.; Zhu, Z.Y. Preliminary Report on the Comparative Study of Biological Characters and Immunity of Six BCG Strains from Different Sources, Academic Proceedings of the Institute for the Control of Biological Products; Ministry of Health: Beijing, China, 1958; pp. 46–58. (In Chinese) [Google Scholar]
- The Central People’s Government Ministry of Health’s Anti-Tuberculosis. Physician Training Course, Lecture Notes for Anti-Tuberculosis Physician Training; People’s Medical Publishing House: Beijing, China, 1958; p. 389. [Google Scholar]
- World Health Organization. Requirements for Dried BCG Vaccine; World Health Organization Technical Report Series, No. 329; WHO: Geneva, Switzerland, 1985. [Google Scholar]
- Ministry of Health of the People’s Republic of China. Notice on the Promotion and Use of 2 Danish Strain, Weiyaozhengfa 1992 No. 370; Ministry of Health of the People’s Republic of China: Beijing, China, 1992. (In Chinese) [Google Scholar]
- Zhao, A.H.; Jia, S.Z.; Kou, L.J.; Qiao, L.Y.; Wang, G.Z. Molecular Genetic Characteristics of BCG Strains Used for Vaccine Production in China and Other Countries. Chin. J. Biol. 2009, 22, 583–587. (In Chinese) [Google Scholar]
- Zhao, A.H.; Qiao, L.Y.; Jia, S.Z.; Wang, G.Z. Genetic Stability during Subculture of BCG for Vaccine Production in China. Chin. J. Biol. 2009, 22, 1102–1104+1112. (In Chinese) [Google Scholar]
- Ten Dam, H.G.; Toman, K.; Hitze, K.L.; Guld, J. Present knowledge of immunization against tuberculosis. Bull. World Health Organ. 1976, 54, 255–269. [Google Scholar]
- Osborn, T.W. Changes in BCG strains. Tubercle 1983, 64, 1–13. [Google Scholar] [CrossRef]
- Jing, H.; Jiang, J.Q.; Chen, H.; Yu, J.J.; Zhang, Y.; Ying, X.J.; Ying, M.M. Correlation analysis of key quality factors of BCG for intradermal injection. Int. J. Biol. 2020, 43, 171–175. (In Chinese) [Google Scholar]
- Jiang, J.Q.; Zhang, Y.; Yu, H.Y.; Xuan, S.F.; Jing, H. Quality comparability study on manufacturing site change of BCG vaccine. Int. J. Biol. 2019, 42, 73–77. (In Chinese) [Google Scholar]
- Chen, Z.W.; Wu, L.T.; Jing, H.; Yan, Z.H. The stability observation of domestic BCG vaccine for intradermal injection. Int. J. Biol. 2019, 42, 69–72. (In Chinese) [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People′s Republic of China; China Medical Science Press: Beijing, China, 2020; Volume Ⅲ, pp. 124–126. (In Chinese) [Google Scholar]
- Dierig, A.; Tebruegge, M.; Krivec, U.; Heininger, U.; Ritz, N.; Paediatric Tuberculosis Network European Trials group. Current status of Bacille Calmette Guérin (BCG) immunisation in Europe—A ptbnet survey and review of current guidelines. Vaccine 2015, 33, 4994–4999. [Google Scholar] [CrossRef]
- Zwerling, A.; Behr, M.A.; Verma, A.; Brewer, T.F.; Menzies, D.; Pai, M. The BCG World Atlas: A database of global BCG vaccination policies and practices. PLoS Med. 2011, 8, e1001012. [Google Scholar] [CrossRef]
- World Health Organization. Global tuberculosis program and global program on vaccines: Statement on BCG revaccination for the prevention of tuberculosis. Wkly. Epidemiol. Rec. 1995, 70, 229–236. [Google Scholar]
- Ministry of Health of the People’s Republic of China. Notice on Stopping BCG Revaccination. Dis. Control. Prev. 1997. (In Chinese) [Google Scholar]
- Eisenhut, M.; Paranjothy, S.; Abubakar, I.; Bracebridge, S.; Lilley, M.; Mulla, R.; Lack, K.; Chalkley, D.; McEvoy, M. BCG vaccination reduces risk of infection with Mycobacterium tuberculosis as detected by gamma interferon release assay. Vaccine 2009, 27, 6116–6120. [Google Scholar] [CrossRef]
- Liu, J.; Tran, V.; Leung, A.S.; Alexander, D.C.; Zhu, B. BCG vaccines: Their mechanisms of attenuation and impact on safety and protective efficacy. Hum. Vaccines 2009, 5, 70–78. [Google Scholar] [CrossRef]
- Chan, P.C.; Yang, C.H.; Chang, L.Y.; Wang, K.F.; Kuo, Y.C.; Lin, C.J.; Lee, S.-W.; Hsueh, P.-R.; Fang, C.-T.; Huang, L.-M. Lower prevalence of tuberculosis infection in BCG vaccinees: A cross-sectional study in adult prison inmates. Thorax 2013, 68, 263–268. [Google Scholar] [CrossRef][Green Version]
- Mangtani, P.; Abubakar, I.; Ariti, C.; Beynon, R.; Pimpin, L.; Fine, P.E.; Rodrigues, L.C.; Smith, P.G.; Lipman, M.; Whiting, P.F.; et al. Protection by BCG vaccine against tuberculosis: A systematic review of randomized controlled trials. Clin. Infect. Dis. 2014, 58, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Peng, J.; Hu, L.; Luo, Y.; Niu, H.; Bai, C.; Wang, Q.; Li, F.; Yu, H.; Wang, B.; et al. A multistage mycobacterium tuberculosis subunit vaccine LT70 including latency antigen Rv2626c induces long-term protection against tuberculosis. Hum. Vaccines Immunother. 2016, 12, 1670–1677. [Google Scholar] [CrossRef] [PubMed]
- Goscé, L.; Bitencourt, J.; Gupta, R.K.; Arruda, S.; Rodrigues, L.C.; Abubakar, I. BCG vaccination following latent TB treatment: Possible implications for different settings. Int. J. Infect. Dis. 2019, 80S, S17–S19. [Google Scholar] [CrossRef]
- Michelsen, S.W.; Soborg, B.; Koch, A.; Carstensen, L.; Hoff, S.T.; Agger, E.M.; Lillebaek, T.; Sorensen, H.C.F.; Wohlfahrt, J.; Melbye, M. The effectiveness of BCG vaccination in preventing Mycobacterium tuberculosis infection and disease in Greenland. Thorax 2014, 69, 851–856. [Google Scholar] [CrossRef]
- Nguipdop-Djomo, P.; Heldal, E.; Rodrigues, L.C.; Abubakar, I.; Mangtani, P. Duration of BCG protection against tuberculosis and change in effectiveness with time since vaccination in Norway: A retrospective population-based cohort study. Lancet Infect. Dis. 2016, 16, 219–226. [Google Scholar] [CrossRef]
- Mangtani, P.; Nguipdop-Djomo, P.; Keogh, R.H.; Trinder, L.; Smith, P.G.; Fine, P.E.; Sterne, J.; Abubakar, I.; Vynnycky, E.; Watson, J.; et al. Observational study to estimate the changes in the effectiveness of bacillus Calmette-Guérin (BCG) vaccination with time since vaccination for preventing tuberculosis in the UK. Health Technol. Assess. 2017, 21, 1–54. [Google Scholar] [CrossRef]
- Technical Guidance Group of the Fifth National TB Epidemiological Survey. The fifth national tuberculosis epidemiological survey in 2010. Chin. J. Antituberc. 2012, 34, 485–508. [Google Scholar]
- Gao, L.; Zhang, H.; Hu, M.G. Expert consensus on the estimation of the nationaL burden on Latent tuberculosis infection. Chin. J. Antituberc. 2022, 44, 4–8. [Google Scholar]
- Special Programme on AIDS and Expanded Programme on Immunization. Joint statement. Consultation on human immunodeficiency virus (HIV) and routine childhood immunization. Nurs. J. India 1988, 79, 93–94. [Google Scholar]
- Maertzdorf, J.; Repsilber, D.; Parida, S.K.; Stanley, K.; Roberts, T.; Black, G.; Walzl, G.; Kaufmann, S.H.E. Human gene expression profiles of susceptibility and resistance in tuberculosis. Genes Immun. 2011, 12, 15–22. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Zheng, H.; Pan, Y.; Liu, H.; Du, P.; Wan, L.; Liu, J.; Zhu, B.; Zhao, G. Genome sequencing and analysis of BCG vaccine strains. PLoS ONE 2013, 8, e71243. [Google Scholar]
- Joung, S.M.; Ryoo, S. BCG vaccine in Korea. Clin. Exp. Vaccine Res. 2013, 2, 83–91. [Google Scholar] [CrossRef]
- Nieuwenhuizen, N.E.; Kulkarni, P.S.; Shaligram, U.; Cotton, M.F.; Rentsch, C.A.; Eisele, B.; Grode, L.; Kaufmann, S.H. The Recombinant Bacille Calmette-Guérin Vaccine VPM1002: Ready for Clinical Efficacy Testing. Front. Immunol. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.E. Variation in protection by BCG: Implications of and for heterologous immunity. Lancet 1995, 346, 1339–1345. [Google Scholar] [CrossRef]
- Ritz, N.; Hanekom, W.A.; Robins-Browne, R.; Britton, W.J.; Curtis, N. Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol. Rev. 2008, 32, 821–841. [Google Scholar] [CrossRef]
- Rowland, R.; McShane, H. Tuberculosis vaccines in clinical trials. Expert Rev. Vaccines 2011, 10, 645–658. [Google Scholar] [CrossRef]
- Gao, L.; Lu, W.; Bai, L.; Wang, X.; Xu, J.; Catanzaro, A.; Cárdenas, V.; Li, X.; Yang, Y.; Du, J.; et al. Latent tuberculosis infection in rural China: Baseline results of a population-based, multicentre, prospective cohort study. Lancet Infect. Dis. 2015, 15, 309–310. [Google Scholar] [CrossRef]
- National Technic Steering Group of the Epidemiology Sampling Survey for Tuberculosis. Report on Fourth National Epidemiological Sampling Survey of Tuberculosis. Chin. J. Tuberc. Respir. Dis. 2002, 25, 3–7. [Google Scholar]
- National Technic Steering Group of the Epidemiology Sampling Survey for Tuberculosis. Report on nationwide random survey for the epidemiology of tuberculosis in 2000. Chin. J. Antituberc. 2002, 24, 65. [Google Scholar]
- Colditz, G.A.; Brewer, T.F.; Berkey, C.S.; Wilson, M.E.; Burdick, E.; Fineberg, H.V.; Mosteller, F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 1994, 271, 698–702. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Report on BCG Vaccine Use for Protection against Mycobacterial Infections Including Tuberculosis, Leprosy, and Other Nontuberculous Mycobacteria (NTM) Infections. Available online: https://www.who.int/immunization/sage/meetings/2017/october/1_BCG_report_revised_version_online.pdf?ua=1 (accessed on 20 June 2017).
- Trunz, B.B.; Fine, P.; Dye, C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: A meta-analysis and assessment of cost-effectiveness. Lancet 2006, 367, 1173–1180. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Lu, J.; Wang, G.; Zhao, A.; Xu, M. Past, Present and Future of Bacillus Calmette-Guérin Vaccine Use in China. Vaccines 2022, 10, 1157. https://doi.org/10.3390/vaccines10071157
Li J, Lu J, Wang G, Zhao A, Xu M. Past, Present and Future of Bacillus Calmette-Guérin Vaccine Use in China. Vaccines. 2022; 10(7):1157. https://doi.org/10.3390/vaccines10071157
Chicago/Turabian StyleLi, Junli, Jinbiao Lu, Guozhi Wang, Aihua Zhao, and Miao Xu. 2022. "Past, Present and Future of Bacillus Calmette-Guérin Vaccine Use in China" Vaccines 10, no. 7: 1157. https://doi.org/10.3390/vaccines10071157
APA StyleLi, J., Lu, J., Wang, G., Zhao, A., & Xu, M. (2022). Past, Present and Future of Bacillus Calmette-Guérin Vaccine Use in China. Vaccines, 10(7), 1157. https://doi.org/10.3390/vaccines10071157





