Abstract
Compliance with vaccination is linked to its safety. In Italy, a plan to identify people who could be at an increased risk of adverse events (AEs) was defined so they could be vaccinated in a protected setting. We conducted an audit to describe the process of AE risk assessment and occurrence in the Reggio Emilia Province in Italy in people who received any of the four COVID-19 vaccines currently used in Italy. Incidence of AEs was calculated by dose and type of vaccine and type of setting (standard vs. protected). After 182,056 first doses were administered, 521 (0.3%) AEs were reported. Most of the AEs were non-serious (91.4%) and non-allergic (92.7%). The percentage of AEs was similar in both settings: 0.3% in the standard setting and 0.2% in the protected setting. However, the incidence of AEs was higher among those who had an allergist visit than among those who did not (IR 666.7 vs. 124.9). All deaths (1.6/100.000) occurred in standard settings and after the Pfizer and Moderna vaccines. The incidence of AEs was lower after the second dose (IR 286.2 vs. 190.3), except for mRNA vaccines, for which it was higher after the second dose (IR 169.8 vs. 251.8). Although vaccination in a protected medical setting could reassure patients with a history of allergies to be vaccinated, allergy history and other anamnestic information is not useful in predicting the risk of COVID-19 vaccine-related AEs in the general population.
1. Introduction
Despite initial hesitancy and mistrust in vaccine safety in Italy [1], the majority of the population has been vaccinated. As of the end of December 2021, 9.02 billion doses have been administered globally, with 57.4% of the world population receiving at least one dose of a COVID-19 vaccine [2]. Almost 90% of the Italian population has received at least one dose of the vaccine, with 84% of the eligible population fully vaccinated [3].
The first vaccine against SARS-CoV-2 approved by the European Medicines Agency and the Italian Medicines Agency (AIFA) and made available in Italy was the Comirnaty vaccine, developed by Pfizer-BioNTech. On 28 December 2020, the first doses were administered to healthcare workers. In January 2021, Moderna and Oxford-AstraZeneca vaccines were approved, followed by the approval of the Janssen vaccine on 11 March 2021.
The first reports of adverse events after vaccination were those identified by randomized control trials assessing their effectiveness and safety [4,5,6,7]. However, these studies only included healthy volunteers, excluding participants with a history of an allergic reactions or immunocompromising conditions.
Post-marketing surveillance of the vaccine effects in the real world is fundamental to ensure safety, to inform policy concerning mass vaccination, and to guide clinicians in individual vaccine recommendations. Data from real-world surveillance have been reported [8,9,10], confirming the overall safety profile but also identifying some excess risk of rare adverse events, in particular anaphylaxis, thrombosis with thrombocytopenia syndrome, stroke, some immune-mediated disorders such as Guillain-Barré Syndrome [11], and hormonal disorders [12]. Nevertheless, data from AE surveillance systems are affected by several biases and cannot quantify the real burden of mild adverse events and those for whom adverse events may be more common. There are several studies to date reporting adverse events from different perspectives or populations, neurological AEs [13], AEs requiring admissions to EDs [14], and AEs among HCWs [15,16,17]. However, there are few comprehensive population-based reports, especially stratified according to the risk of allergic reactions.
Compliance with vaccination is linked to its safety. The Centers for Disease Control and Prevention (CDC) published different recommendations regarding the safety of these vaccines for patients with a history of allergic reactions [18]. The Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC) recommends that people with known allergies and a history of anaphylaxis should not be excluded from vaccination; instead, they require a more specific and individualised management, such as prolonged observation, premedication, or stabilisation of the underlying disease. In addition, they should be vaccinated in an appropriately staffed setting with immediately available aids to deal with serious anaphylactic emergencies [19]. To ensure the highest level of safety possible and to facilitate a greater response to vaccination, Italy defined a plan to identify people who could be at an increased risk of adverse effects and to vaccinate them in a safe setting. Although the risk stratification was developed mainly for the purpose of assessing history of allergy, people with non-allergy conditions, such as malignancy, obesity, or frailty, were vaccinated in the protected setting if deemed necessary.
This audit report aims to describe the process of adverse effect risk assessment and the occurrence of allergic and non-allergic adverse effects in the population who underwent vaccination in the Reggio Emilia Province.
2. Materials and Methods
2.1. Study Design
Data on adverse events reported to AIFA were used to describe the characteristics and calculate the incidence of events in the cohort of people who received the first dose between 27 December 2020 and 12 September 2021 in the Reggio Emilia Province. Persons included were those who had a suggested exemption from a GP or an appointment or presented voluntarily for the first dose of any of the COVID-19 vaccines currently authorised and used in Italy: Pfizer–BioNTech’s Comirnaty (BNT162b2), Spikevax Moderna COVID-19 vaccine (mRNA-1273), Vaxzeveria AstraZeneca AZD1222 (ChAdOc1-S nCoV-19) and Johnson & Johnson’s Janssen (J&J/Janssen) COVID-19 vaccine (JNJ-78436735).
We report AEs following the first and second dose of the COVID-19 vaccine, both as a proportion of AEs out of the total AEs reported and from the total patients vaccinated.
2.2. Triage and Risk Stratification
According to the local vaccination protocol at the time, persons with low risk of AEs and persons with temporary exemption due to non-allergy indications (quarantine, current or previous COVID-19 infection, ongoing influenza, and pregnancy) were vaccinated in a standard setting, such as a fair hall, following standard procedures that included waiting for 15 min after vaccination, with all the necessary aids and drugs available and medical staff able to recognise and manage possible allergic reactions. Persons with suspected allergies, persons with a history of allergies that have never resulted in anaphylaxis (to food, pollen, insect bites and stings) with chronic urticarial or cutaneous reactions to non-official drugs, persons with a positive family history of reactions to drugs or patients with a history of severe allergic reactions (e.g., anaphylaxis) to any antigen except injectable drugs and vaccines were considered to be at moderate/high risk and were referred to an allergy evaluation. Those without confirmed cutaneous allergic reactions to drugs that contain PEG were vaccinated in a protected setting. The protected setting could be either an outpatient or a hospital setting, with easy access to the recovery room and with the immediate availability of medical devices to deal with serious anaphylactic emergencies and allowing a stay of 30 min for observation after vaccination.
A general questionnaire was sent to all persons scheduled for vaccination via phone message or email, either before the vaccination session or when arriving at the vaccination site. Those who answered positively to one of the allergy-related questions were asked four more specific questions once they presented for the vaccination session. They were asked by the vaccinating doctor: (1) if they suffer from severe anaphylaxis (involvement of the cardiovascular and/or respiratory system) from any cause or an unknown cause; (2) if they suffer from uncontrolled asthma; (3) if they were diagnosed with mastocytosis; (4) if they have had allergic reactions to any drug or previous vaccinations or to polyethylene glycol (PEG) and polysorbates. If the answer was “no” to all four questions, the individual was considered “low risk” and proceeded to the vaccination under the usual protocol with a 15 min observation period. Patients with at least one of these conditions were considered at moderate/high risk and were referred to an allergy appointment to carry out specific diagnostics. The public health department of the local health authority receives these requests first and evaluates whether they could be vaccinated or should be referred to an allergy evaluation. In addition to doctors from the vaccination centre, the public health department can also receive and evaluate requests from general practitioners, paediatricians, and dermatologists. Furthermore, general practitioners and specialists could notify the public health department of patients with conditions or diseases that may qualify them for inclusion in the high-risk pathway or exempt them from vaccination temporarily or permanently. Therefore, some persons with non-allergy conditions, such as malignancy, obesity, or frailty, were vaccinated in the protected setting if deemed necessary. The public health department examined all reports and made decisions on whether an allergy appointment is needed and if and where people could receive the vaccination.
Allergy evaluations were done before the first dose, and in the case of suspected reactions after the first dose, they could be done before the second dose. They included cutaneous tests if indicated to evaluate possible COVID-19 vaccine hypersensitivity, such as COVID-19 vaccine excipients, specifically polyethylene glycol (PEG), polysorbate 80, and polysorbate 20.
Persons with allergies to drugs that contain PEG (Depomedrol, Depoprovera, SonoVue, Herzeptin/Herzuma/Trastuzumab, Arcalyst, Definity, Zirtec, Taxotere, Neulasta, Mircrea/Epoetin beta, etc.) or polysorbate (Amiodarone, Taxotere, Lantus, Alpidra, Trulicity, Xolari, Herceptin/Herzuma, Invega, Humira, Tremfya, Remsima, monoclonal antibodies, Kenakort, growth factors, etc.), confirmed by skin tests were exempted from vaccination.
2.3. Triage for the Second Dose
In the case of a reaction to the first dose of the COVID-19 vaccine, the vaccination with the second dose depended on the extent of the reaction. In the case of local or mild general symptoms, the vaccine could be administered in a standard setting. If the patient had vaso-vagal crisis, tremor, tingle, or anxiety after the first dose, the observation period could be 30 min in the standard setting. In the case of the presence of local symptoms that were particularly important, such as a wheal larger than 10 cm or a late urticarial reaction that started 60 min after the first dose, the vaccine could be administered in the standard setting but with Loratidin or Deltacortene 1 h before the second dose. If the reaction after the first dose was an immediate allergic reaction occurring within 60 min of vaccination, such as generalised urticaria, angioedema of larynx, eyes, or lips, dyspnea, chest tightness, hypotension, gastrointestinal symptoms, or unconsciousness, the vaccination was suspended, and an allergy evaluation was considered. In the case of anaphylaxis after the first dose of mRNA vaccine or any of its components, or in the case of infection with SARS-CoV-2 after the first dose, the second dose was not administered.
2.4. Reporting of AEs
Adverse drug reactions (ADRs) were reported to the Pharmacovigilance Service in the form of Individual Case Safety Reports (ICSRs) by the general practitioner/family doctor, the Vaccination Centre, the pharmacist, or the Local Health Authority (ASL) or directly by the patient by filling in the form available on the AIFA portal or through the VigiFarmaco application. The severity category of AEs is selected by the reporter during the compilation of the safety form following the indications available on the portal. Cases that do not comply with the criteria for AE or contain missing information are controlled by the regional health authority and AIFA.
2.5. Data Sources
Data about vaccination appointments, vaccines administered, and persons exonerated were retrieved from the local health authority vaccine information system. Data about allergy testing and test results were obtained from the allergy database of the dermatology unit. Adverse events data were obtained from the pharmaceutical department of the local health authority.
2.6. Definition of Adverse Events
Serious AEs considered 75 different clinically serious conditions divided into five categories according to the recommendations of AIFA [20]: hospitalisation or prolonged hospitalisation, severe or permanent invalidity, life-threatening conditions, death, and other clinically relevant conditions.
2.7. Statistical Analysis
Descriptive statistics were used, and data are presented as frequency and percentages. Incidence rate (IR) was calculated as the number of AEs per 100,000 persons vaccinated. Analyses were stratified by vaccine dose, risk pathway, and type of vaccine. STATA 16.1 (Stata Corporation, College Station, TX, USA) was used for all analyses.
3. Results
3.1. First Dose
Out of 182,214 persons who presented for vaccination, 180,305 persons were vaccinated in a standard setting (green pathway), 1751 were vaccinated in the protected setting, 1 person was exonerated, and 157 did not get vaccinated (Figure 1).
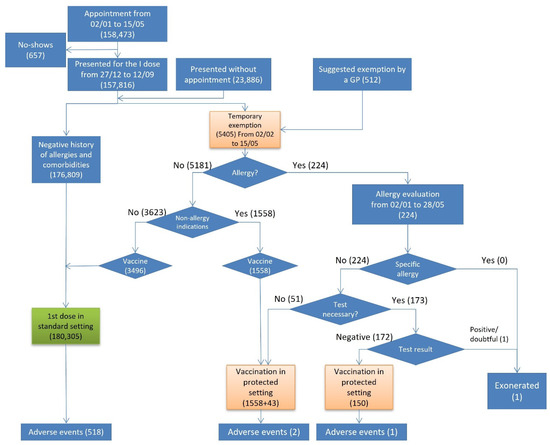
Figure 1.
Flowchart of the first dose vaccinations, exonerations, and adverse events by risk pathway.
Out of 5405 who were temporarily exempted, 224 reported histories of an allergy and were referred to an allergy evaluation. While no one had a vaccine-specific allergy, 173 undertook an allergy test. Only one person was exonerated from the vaccination, since he demonstrated inconclusive skin test results for COVID-19 vaccine excipients. Out of 5181 persons who were temporarily exempted due to non-allergy indications, 1558 were vaccinated in the protected setting.
Overall, 521 (0.3%) AEs were reported after 182,056 first doses administered, out of which 281 were self-reported (53.9%) and 240 reported by a health worker (46.1%) (Table 1). Out of 180,305 persons vaccinated in the standard setting, 518 (0.3%) had an adverse event (Figure 1). In the protected setting, 3 (0.2%) out of 1751 vaccinated persons developed an adverse event, all of which were non-severe, and one occurred in a person with a history of allergy. The incidence of AEs was higher among those who had been referred to an allergy evaluation than among those who did not (IR 666.7 vs. 124.9) (Table 2).

Table 1.
Total number of AEs after first and second dose.

Table 2.
Characteristics of adverse events after the first dose of COVID-19 vaccine, by risk pathway.
Although 85.4% (445/521) of AEs were systemic, most of them were non-serious (91.4%) (476/521) and non-allergic (92.7%) (483/521) (Table 1). All serious AEs happened in the standard setting. The list of specific conditions reported as severe and categorized by organ system and dose is summarized in Supplementary Table S1. Death occurred in 1.6/100.000 persons. All deaths occurred in the standard setting and after the Pfizer and Moderna vaccines (Supplementary Table S2).
AEs were mostly reported by females (73.1%, IR 369.3 vs. 177.5) during the first 24 h after vaccination (78.5%) in the age category 50–59 years (IR 418.6) (Table 1) and after administration of the AZ/JJ vaccine (59.3%, IR 540.2 vs. 169.8) (Supplementary Table S3).
3.2. Second Dose
Out of 180,305 persons vaccinated with the first dose in the standard setting, 4340 (2.4%) were temporarily exempted due to various reasons including positivity to COVID-19 or being isolated as a contact of a positive person (Figure 2). Out of 68 persons who had an adverse reaction after the first dose and were eligible for the second dose, 32 were vaccinated in the protected setting and seven in the standard setting. Out of 1751 persons vaccinated with the first dose in the protected setting, 29 (1.7%) were exonerated due to positivity to COVID-19. One person who had an adverse event after the first dose was vaccinated in the protected setting with no adverse events after the second dose.
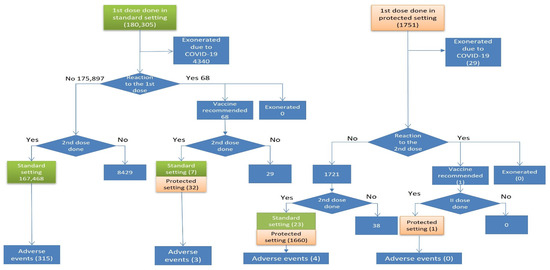
Figure 2.
Flowchart of the second dose vaccinations, exonerations, and adverse events by risk pathway.
Overall, 169,191 persons were vaccinated with the second dose: 167,498 in the standard setting and 1693 in the protected setting. After the second dose, 322 (0.2%) AEs were reported: 133 (41.3%) self-reported and 189 (58.7%) reported by a healthcare worker (Table 1). Out of 322 AEs reported, 315 (97.8%) occurred in the standard setting and 7 (2.2%) in the protected setting (Table 3). The incidence of AEs was lower after the second dose (IR 286.2 vs. 190.3), while the incidence of death was higher after the second dose (2.4/100,000 vs. 1.6/100,000) if the period after vaccination was unrestricted. Restricting the period after vaccination to 21 days, the incidence of death became similar for both doses (1.6 vs. 1.8). All four persons who died after the second dose didn’t have any AE after the first dose.

Table 3.
Characteristics of adverse events after the second dose of COVID-19 vaccine, by risk pathway.
Although the overall incidence of AEs after the second dose was the highest in persons who had a reaction after the first dose but were vaccinated in the standard setting (IR 7692.3), all 27 severe AEs after the second dose occurred in people who did not report an AE after the first dose (Table 3). In percentages, there were 8.6% of severe AEs after the first dose, 8.4% after the send dose overall, and 8.5% after the second and not after the first dose.
Similar to the first dose, most of the AEs were not severe (91.6%), non-allergic (93.8%), immediate (84.8%), and systemic (88.2%). All severe and 99% of non-severe AEs after the second dose occurred in people who had not had an AE after the first dose. In contrast to the first dose, a substantially higher incidence of AEs was associated with the second dose of Pfizer/Moderna than with AZ/JJ (IR 151.8 vs. 53.4) (Supplementary Tables S4 and S5).
4. Discussion
The occurrence of AEs in our study was similar in both settings, suggesting that the process put in place to identify a person’s increased risk of AEs was not effective. Furthermore, the specialist allergy evaluations identified only one person who should be exonerated due to a confirmed allergy to PEG. The only group that showed an increased risk of AEs was that including those who had a reaction to the first dose. However, these reactions were mostly non-severe and non-systemic; almost all non-severe and severe AEs (including death) after the second dose occurred in persons who did not have an adverse reaction to the first dose.
There are still many uncertainties regarding whether or not persons with a history of allergies or anaphylaxis should be vaccinated with a COVID-19 vaccine. When considering only allergic AEs, we found 0.001% of allergic reactions after the first dose and 0.002% after the second dose. The Israeli study on immunisation with the BNT162b2, the only study so far to evaluate allergic reactions in highly allergic individuals, reported nearly 2% of allergic reactions (1.4% after the first dose and 1.8% after the second dose) [21]. However, they included only 429 persons with a high risk of allergies who were referred to the allergy evaluation and vaccinated in supervised medical settings, with no comparison to the population vaccinated in standard settings. Thus, the small sample size might be a reason for a much higher rate of allergic AEs in their study. Another reason could be the fact that in our study persons with non-allergy indications (n = 1558 for the first dose) were vaccinated in the protected setting as well, which could have diluted the rate of allergic AEs.
Our results, in terms of share of non-serious AEs, death, sex distribution, HCW-reported AEs, and occurrence within 48 h, are very similar to the results of the Italian Medicines Agency (Agenzia Italiana del farmaco-AIFA) report for the entirety of Italy [22]. However, overall percentage and incidence were lower in the AIFA report than in our study for both doses (dose I: 158 vs. 286.2–100,000 doses; dose II: 77 vs. 190.3/100,000 doses). According to their estimation, the majority were in the category “other clinically relevant conditions”, and for only around one-third (36%) of the reported severe AEs a causal link with the vaccine could be confirmed.
The overall incidence of AEs and serious AEs in our study was lower after the second dose, but for mRNA vaccines Pfizer and Moderna, it was higher after the second dose. Similar findings were found by the CDC’s studies and in clinical trials in which higher reactogenicity was reported after the second dose of Pfizer-BioNTech [23,24]. However, the incidence of death was similar after both doses in our study. All seven deaths reported (three after the first dose and four after the second dose) in our study were reported after the Comirnaty vaccine. It is unlikely that these deaths were a consequence of anaphylaxis but it is more likely that they were due to the worsening of an underlying condition [22]. This is also supported by the fact that they all occurred in persons vaccinated in the standard setting among persons with no reactions reported after the first dose.
A common finding to date is that an AE is disproportionately reported by women. This was also the case in our study (73.1 and 74.5% of AEs after the first and second dose, respectively, were reported by women) and in previous reports and vaccines [8,23,25]. Although behavioural factors such as lower reporting by men due to perceived self-rated health probably play an important role in disproportional AE incidence between sexes, various genetic, hormonal, and biological determinants are considered fundamental for the robustness of female immune response to both their own and foreign antibodies [26,27,28]. Further studies are needed to define exact social, demographic, and clinical risk factors for allergic AEs following COVID-19 vaccination.
The major limitation of our study is that the AEs for the second dose were self-reported or reported by healthcare workers and not actively and systematically collected, while for the first dose, an active collection of all reactions was done when people presented for the second dose. Individual case reports by the multidisciplinary expert team and standardized causality assessment algorithms should be applied for both doses in order to improve AE reporting. Due to the descriptive design of the study and lack of official test to estimate the effectiveness of the risk stratification, it is not possible to make any causal inference. However, this is the first population-based study to evaluate the risk of various AEs according to the allergy risk stratification; we defined a cohort that included all the people who presented for vaccination, and we collected all the referrals and procedures for assessing AE risk. Although it might be generalizable only to the settings with similar organizational and epidemiological contexts, this audit might be useful to inform future vaccination strategies in patients at risk of AEs or those who have already experienced an AE.
5. Conclusions
The percentage and incidence of AEs after all COVID-19 vaccines currently approved and used in Italy is very low, and most AEs have a local, non-severe character. Despite a large effort in screening and assessing the risk of AEs, the occurrence of AEs was similar in all risk-stratified groups. Only reactions after the first dose identified a higher risk of AEs, but this stratification only identified a small proportion of AEs in second doses, which were mostly non-severe cases. Although vaccination in a protected, medical setting could calm and reassure patients to be vaccinated, a history of allergies and non-allergic comorbidities is not useful in predicting the risk of COVID-19 vaccine-related AEs in the general population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines10071111/s1, Table S1: List of severe AEs by organ system and dose; Table S2: Characteristics of adverse events after the first dose of Pfizer/Moderna COVID-19 vaccine; Table S3: Characteristics of adverse events after the first dose of AstraZeneca and J&J/Janssen COVID-19 vaccine; Table S4: Characteristics of adverse events after the second dose of Pfizer/Moderna COVID-19 vaccine; Table S5: Characteristics of adverse events after the second dose of AstraZeneca and J&J/Janssen COVID-19 vaccine.
Author Contributions
Conceptualization, A.M. (Alberico Motolese), L.F., M.V. and P.G.R.; methodology, L.B. and E.B.; validation, L.B., R.B. (Raffaele Brancaccio). and A.M. (Alberico Motolese); investigation, L.F., L.B., A.C., R.B. (Raffaele Brancaccio) and A.M. (Alberico Motolese); data curation, L.B., P.M. and M.O.; formal analysis, P.M. and M.O.; project administration, O.D.; literature search, O.D., A.M. and R.B. (Rostyslav Boyko); writing—original draft preparation, O.D.; writing—review and editing, R.B. (Raffaele Brancaccio) and P.G.R.; visualization, A.M. (Alfonso Motolese) and R.B. (Rostyslav Boyko); supervision, A.M. (Alberico Motolese), M.V. and P.G.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This report is the result of the monitoring activity that is a part of the audit system built for the evaluation of the implementation of the new organizational pathway of vaccination for COVID-19. Ethics approval and consent to participate were not required, as this is an audit based on data routinely collected by the Reggio Emilia Local Health Authority, a public body of the National Health Service. Among the institutional functions of the Local Health Authority is the surveillance of infectious diseases, the implementation and monitoring of vaccination campaigns, and the collecting of adverse effects data. Dissemination of COVID-19 surveillance data was authorised by the Italian Presidency of the Council of Ministers on 27 February 2020 (ordinance No 640).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available upon reasonable request. According to Italian law, anonymized data can only be made publicly available if there is no potential for the re-identification of individuals (https://www.garanteprivacy.it; accessed on 12 July 2022). Thus, the data underlying this study are available on request to researchers who meet the criteria for access to confidential data. In order to obtain data, approval must be obtained from the Area Vasta Emilia Nord (AVEN) Ethics Committee, who would then authorize us to provide aggregated or anonymized data. Data access requests should be addressed to the Ethics Committee at CEReggioemilia@ausl.re.it as well as to the authors at the Epidemiology unit of AUSL–IRCCS of Reggio Emilia at info.epi@ausl.re.it, who are the data guardians.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Palamenghi, L.; Barello, S.; Boccia, S.; Graffigna, G. Mistrust in biomedical research and vaccine hesitancy: The forefront challenge in the battle against COVID-19 in Italy. Eur. J. Epidemiol. 2020, 35, 785–788. [Google Scholar] [CrossRef] [PubMed]
- OurWorldInData. Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 28 December 2021).
- Ministry of Health of Italy. COVID-19 Vaccines Report. Available online: https://www.governo.it/it/cscovid19/report-vaccini/ (accessed on 22 December 2021).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Beatty, A.L.; Peyser, N.D.; Butcher, X.E.; Cocohoba, J.M.; Lin, F.; Olgin, J.E.; Pletcher, M.J.; Marcus, G.M. Analysis of COVID-19 Vaccine Type and Adverse Effects Following Vaccination. JAMA Netw. Open 2021, 4, e2140364. [Google Scholar] [CrossRef] [PubMed]
- Almohaya, A.M.; Qari, F.; Zubaidi, G.A.; Alnajim, N.; Moustafa, K.; Alshabi, M.M.; Alsubaie, F.M.; Almutairi, I.; Alwazna, Q.; Al-Tawfiq, J.A.; et al. Early solicited adverse events following the BNT162b2 mRNA vaccination, a population survey from Saudi Arabia. Prev. Med. Rep. 2021, 24, 101595. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Park, K.; Kim, T.E.; Kwon, Y.; Lee, Y.K. COVID-19 vaccine safety monitoring in Republic of Korea from February 26, 2021 to October 31, 2021. Osong Public Health Res. Perspect. 2021, 12, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Meeting Highlights from the Pharmacovigilance Risk Assessment Committee (PRAC) 7–10 February 2022. Available online: https://www.ema.europa.eu/en/news/meeting-highlights-pharmacovigilance-risk-assessment-committee-prac-7-10-february-2022 (accessed on 1 March 2022).
- García-Grimshaw, M.; Ceballos-Liceaga, S.E.; Hernández-Vanegas, L.E.; Núñez, I.; Hernández-Valdivia, N.; Carrillo-García, D.A.; Michel-Chávez, A.; Galnares-Olalde, J.A.; Carbajal-Sandoval, G.; Del Mar Saniger-Alba, M.; et al. Neurologic adverse events among 704,003 first-dose recipients of the BNT162b2 mRNA COVID-19 vaccine in Mexico: A nationwide descriptive study. Clin. Immunol. 2021, 229, 108786. [Google Scholar] [CrossRef] [PubMed]
- Kewan, T.; Flores, M.; Mushtaq, K.; Alwakeel, M.; Burton, R.; Campbell, J.; Perry, H.; Al-Jaghbeer, M.; Abi Fadel, F. Characteristics and outcomes of adverse events after COVID-19 vaccination. J. Am. Coll. Emerg. Physicians Open 2021, 2, e12565. [Google Scholar] [CrossRef] [PubMed]
- Ughi, N.; Del Gaudio, F.; Dicuonzo, A.; Orso, M.; Micheloni, G.; Puoti, M.; Pani, A.; Scaglione, F.; Zoppini, L.; Rossetti, C.; et al. Host factors and history of SARS-CoV-2 infection impact the reactogenicity of BNT162b2 mRNA vaccine: Results from a cross-sectional survey on 7,014 workers in healthcare. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7985–7996. [Google Scholar] [PubMed]
- Valera-Rubio, M.M.; Sierra-Torres, M.I.M.; Castillejo García, R.R.; Cordero-Ramos, J.J.; López-Márquez, M.R.M.; Cruz-Salgado, Ó.O.; Calleja-Hernández, M.Á.M. Adverse events reported after administration of BNT162b2 and mRNA-1273 COVID-19 vaccines among hospital workers: A cross-sectional survey-based study in a Spanish hospital. Expert Rev. Vaccines 2022, 21, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Choi, S.H.; Kim, B.; Kim, J.Y.; Ji, Y.S.; Kim, S.H.; Kim, C.K.; Kim, T.; Choo, E.J.; Moon, J.E.; et al. Serum Antibody Response Comparison and Adverse Reaction Analysis in Healthcare Workers Vaccinated with the BNT162b2 or ChAdOx1 COVID-19 Vaccine. Vaccines 2021, 9, 1379. [Google Scholar] [CrossRef] [PubMed]
- CDC. Interim Clinical Considerations for Use of mRNA COVID-19 Vaccines Currently Authorized in the United States—Appendix B. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html?CDC (accessed on 28 December 2021).
- The Italian Society of Allergy, Asthma and Clinical Immunology (SIAAIC). COVID-19 Vaccines: Guidelines for the Management of Patients at Risk of Allergic Reactions. Available online: https://www.siaaic.org/?p=5949 (accessed on 1 March 2022).
- Italian Medicines Agency. Procedura Operativa AIFA per I Responsabili Locali di Farmacovigilanza: Gestione Delle Segnalazioni Nella Rete Nazionale di Farmacovigilanza. (AIFA Operative Procedure for the Local Persons Responsible for Pharmacovigilance: Management of the Reporting in the National Network of Pharmacovigilance). Available online: https://www.aifa.gov.it/documents/20142/0/Procedura_Operativa_RLFV_ottobre-2018.pdf (accessed on 14 March 2022).
- Shavit, R.; Maoz-Segal, R.; Iancovici-Kidon, M.; Offengenden, I.; Haj Yahia, S.; Machnes Maayan, D.; Lifshitz-Tunitsky, Y.; Niznik, S.; Frizinsky, S.; Deutch, M.; et al. Prevalence of Allergic Reactions After Pfizer-BioNTech COVID-19 Vaccination Among Adults with High Allergy Risk. JAMA Netw. Open 2021, 4, e2122255. [Google Scholar] [CrossRef] [PubMed]
- Italian Agency for Drugs Authorization. COVID-19 Vaccine Safety Annual Report. Available online: https://www.aifa.gov.it/documents/20142/1315190/Rapporto_annuale_su_sicurezza_vaccini%20anti-COVID-19.pdf (accessed on 14 March 2022). (In Italian)
- Gee, J.; Marquez, P.; Su, J.; Calvert, G.M.; Liu, R.; Myers, T.; Nair, N.; Martin, S.; Clark, T.; Markowitz, L.; et al. First Month of COVID-19 Vaccine Safety Monitoring—United States, 14 December 2020–13 January 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Chapin-Bardales, J.; Gee, J.; Myers, T. Reactogenicity Following Receipt of mRNA-Based COVID-19 Vaccines. JAMA 2021, 325, 2201–2202. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.; Nair, N. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA 2021, 325, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Potluri, T.; Fink, A.L.; Sylvia, K.E.; Dhakal, S.; Vermillion, M.S.; Vom Steeg, L.; Deshpande, S.; Narasimhan, H.; Klein, S.L. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccines 2019, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Hejblum, B.P.; Simon, N.; Jojic, V.; Dekker, C.L.; Thiébaut, R.; Tibshirani, R.J.; Davis, M.M. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 869–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovats, S.; Carreras, E.; Agrawal, H. Sex Steroid Receptors in Immune Cells. In Sex Hormones and Immunity to Infection; Klein, S., Roberts, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 53–91. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).