Neutralization Activity against SARS-CoV-2 Variants after Booster Vaccination in Populations without COVID-19: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Protocol
2.2. Eligibility Criteria
- Population—participants received two doses of homologous COVID-19 vaccines and without history of laboratory-confirmed COVID-19;
- Intervention—booster dose of the COVID-19 vaccines;
- Comparison—before (day 0) and after (day 14/28) the booster vaccination;
- Outcomes—Neutralization activity against different types of SARS-CoV-2 variants after a booster dose was evaluated by comparing the change of neutralization titers. Long-term immunogenicity post prime vaccination and the final concentration of neutralization antibody were also analyzed.
- Study designs—Before–after studies were eligible for inclusion. Animal studies, case reports, reviews, editorials and conference abstracts were excluded.
2.3. Data Extraction and Quality Assessment
2.4. Outcomes
2.5. Data Synthesis and Statistical Analysis
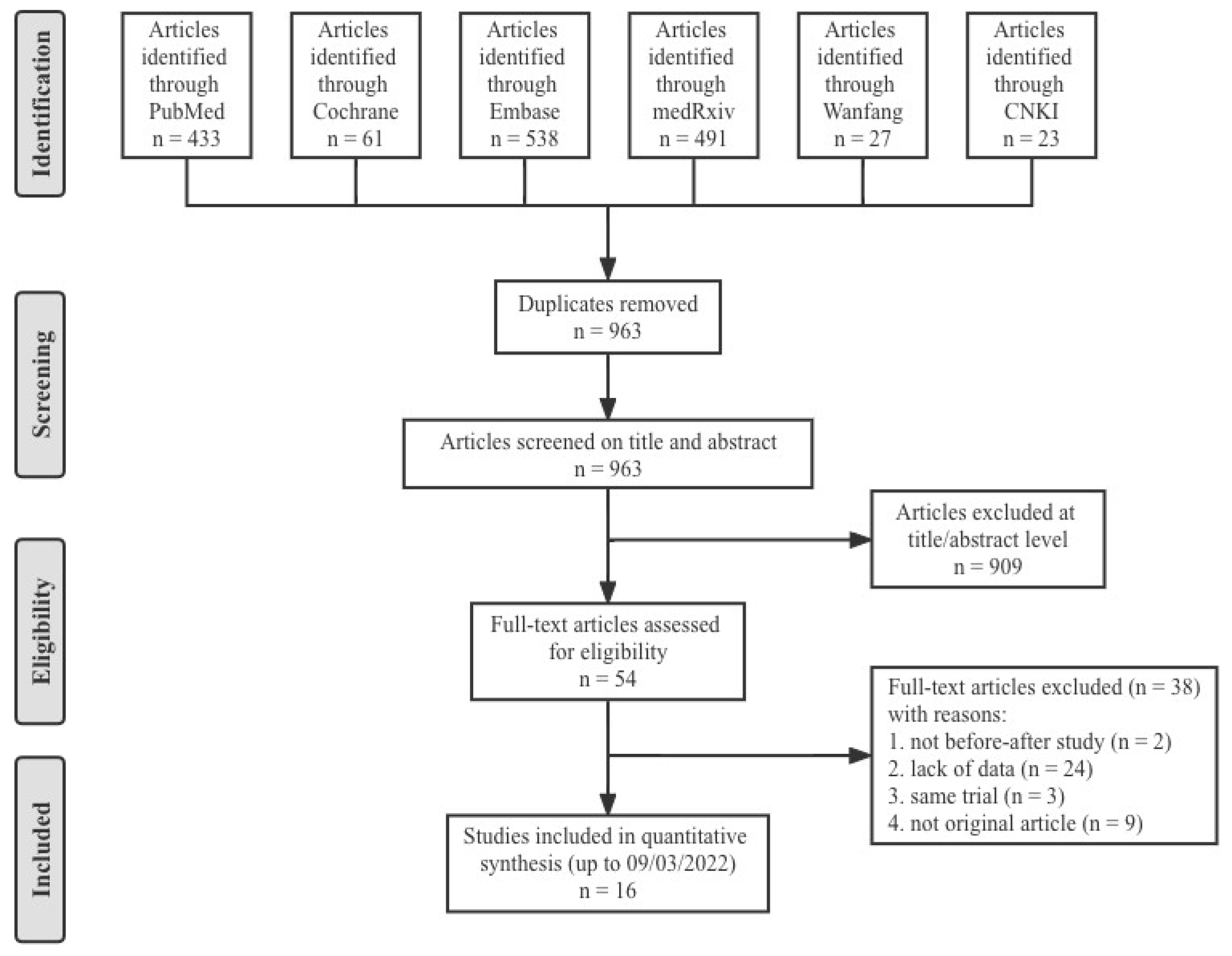
3. Results
3.1. Characteristics of the Studies
3.2. Long-Term Neutralization Activity against SARS-CoV-2 Variants Post Prime Immunization
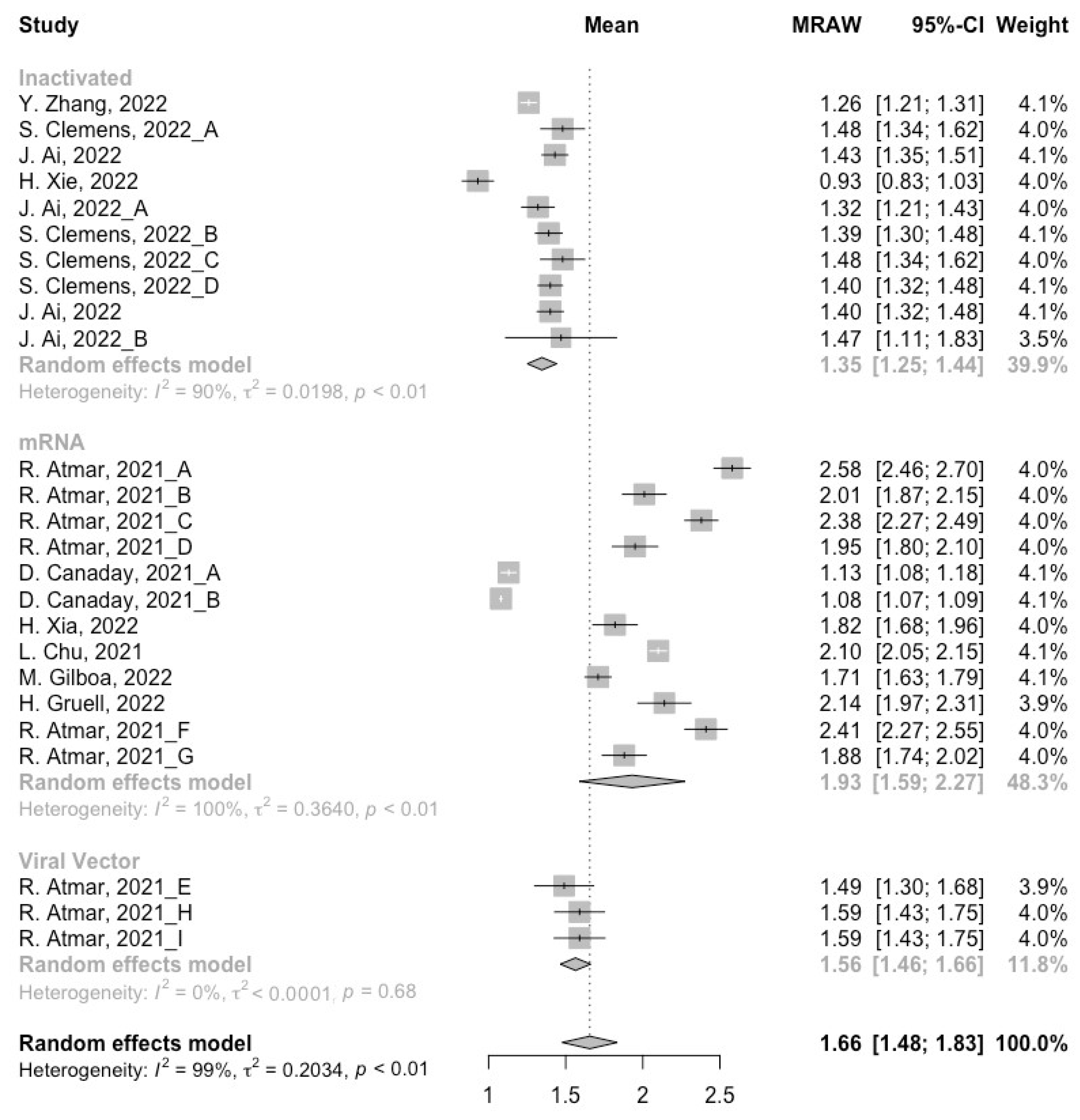
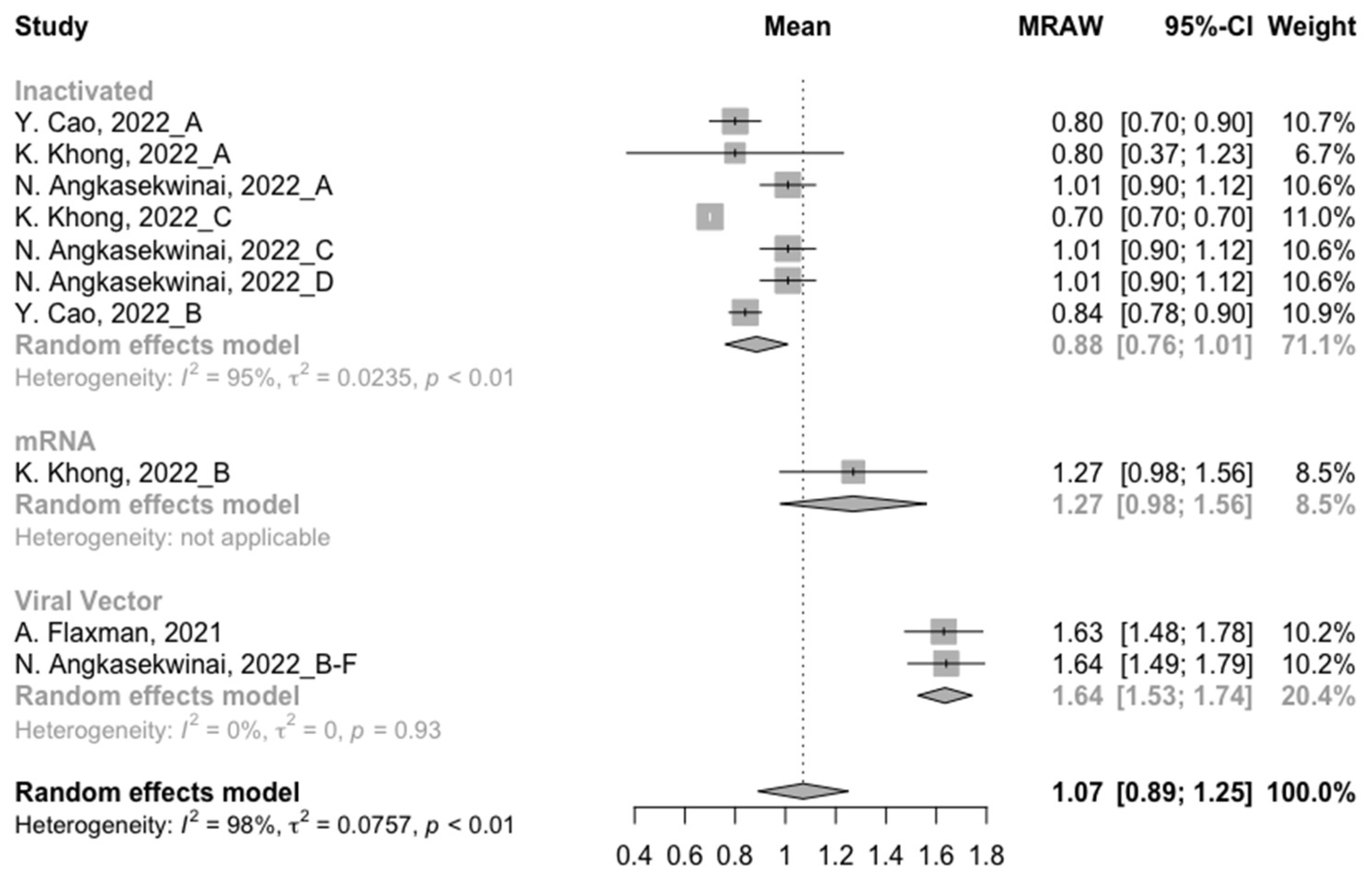
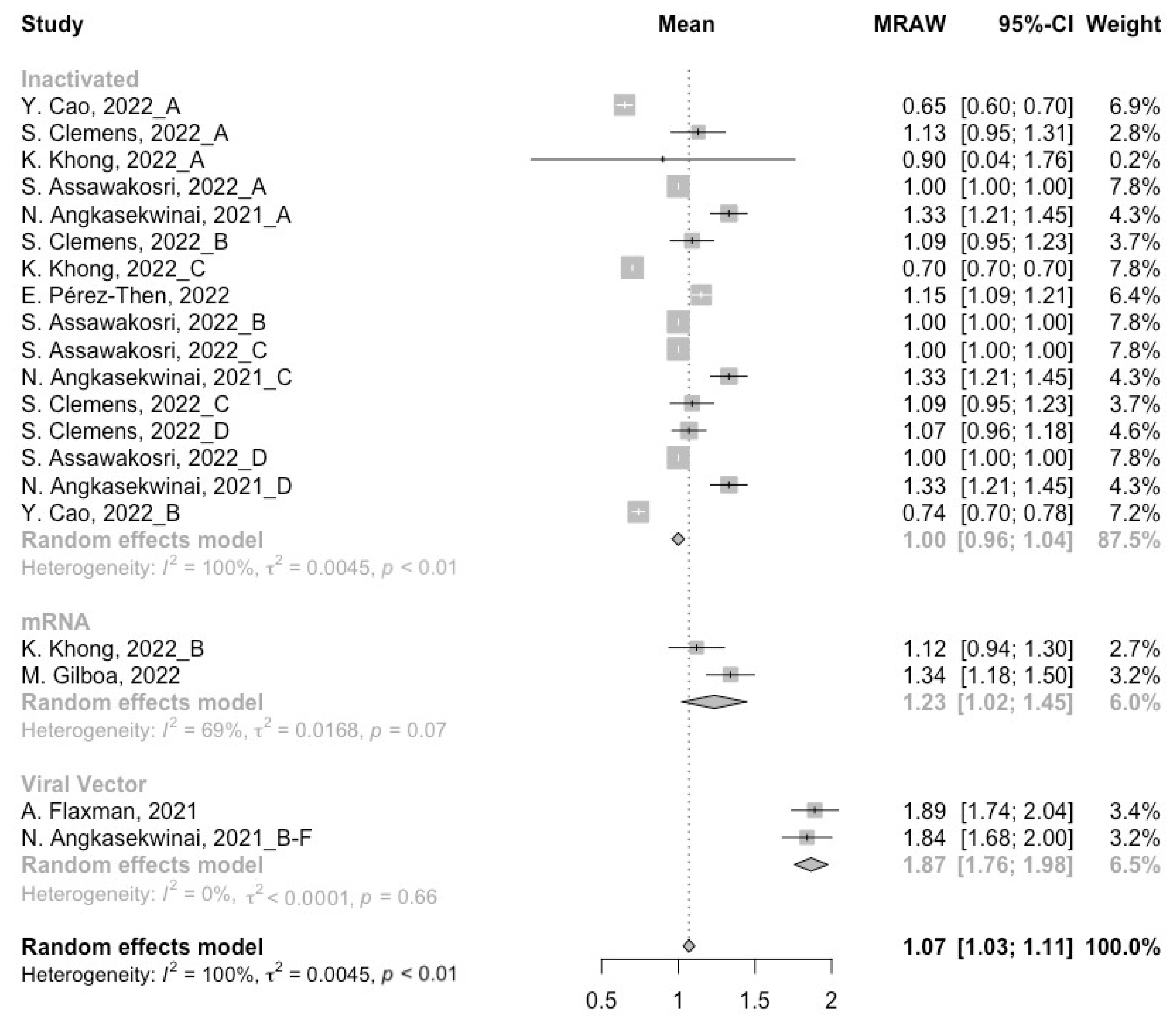
3.3. Neutralization Activity against SARS-CoV-2 Variants Post Homologous Boosters
3.4. Neutralization Activity against SARS-CoV-2 Variants Post Heterologous Boosters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 21 April 2022).
- Ai, J.; Zhang, H.; Zhang, Y.; Lin, K.; Zhang, Y.; Wu, J.; Wan, Y.; Huang, Y.; Song, J.; Fu, Z.; et al. Omicron variant showed lower neutralizing sensitivity than other SARS-CoV-2 variants to immune sera elicited by vaccines after boost. Emerg. Microbes Infect. 2022, 11, 337–343. [Google Scholar] [CrossRef]
- McCallum, M.; Czudnochowski, N.; Rosen, L.E.; Zepeda, S.K.; Bowen, J.E.; Walls, A.C.; Hauser, K.; Joshi, A.; Stewart, C.; Dillen, J.R.; et al. Structural basis of SARS-CoV-2 Omicron immune evasion and receptor engagement. Science 2022, 375, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Khandia, R.; Singhal, S.; Alqahtani, T.; Kamal, M.A.; El-Shall, N.A.; Nainu, F.; Desingu, P.A.; Dhama, K. Emergence of SARS-CoV-2 Omicron (B.1.1.529) variant, salient features, high global health concerns and strategies to counter it amid ongoing COVID-19 pandemic. Environ. Res. 2022, 209, 112816. [Google Scholar] [CrossRef] [PubMed]
- Plante, J.A.; Mitchell, B.M.; Plante, K.S.; Debbink, K.; Weaver, S.C.; Menachery, V.D. The variant gambit: COVID-19’s next move. Cell Host Microbe 2021, 29, 508–515. [Google Scholar] [CrossRef]
- Baraniuk, C. COVID-19: How effective are vaccines against the delta variant? BMJ (Clin. Res. Ed.) 2021, 374, n1960. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. SARS-CoV-2 Variant Classifications and Definitions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-classifications.html#concern (accessed on 21 April 2022).
- Nemet, I.; Kliker, L.; Lustig, Y.; Zuckerman, N.; Erster, O.; Cohen, C.; Kreiss, Y.; Alroy-Preis, S.; Regev-Yochay, G.; Mendelson, E.; et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 386, 492–494. [Google Scholar] [CrossRef]
- Levine-Tiefenbrun, M.; Yelin, I.; Alapi, H.; Katz, R.; Herzel, E.; Kuint, J.; Chodick, G.; Gazit, S.; Patalon, T.; Kishony, R. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat. Med. 2021, 27, 2108–2110. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, G.R.; Yadav, P.D.; Abraham, P.; Nyayanit, D.A.; Sapkal, G.N.; Shete, A.M.; Gupta, N.; Vadrevu, K.M.; Ella, R.; Panda, S.; et al. Booster dose of the inactivated COVID-19 vaccine BBV152 (Covaxin) enhances the neutralizing antibody response against alpha, Beta, Delta and omicron variants of concern. J. Travel Med. 2022, 29, taac039. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Al-Thani, H.; El-Menyar, A. The emergence of new SARS-CoV-2 variant (Omicron) and increasing calls for COVID-19 vaccine boosters-The debate continues. Travel Med. Infect. Dis. 2022, 45, 102246. [Google Scholar] [CrossRef]
- Au, W.Y.; Cheung, P.P. Effectiveness of heterologous and homologous COVID-19 vaccine regimens: Living systematic review with network meta-analysis. BMJ (Clin. Res. Ed.) 2022, 377, e069989. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; Zubizarreta, J.R.; González, C.; Pizarro, A.; Acevedo, J.; Leo, K.; Paredes, F.; Bralic, T.; Vergara, V.; et al. Effectiveness of homologous and heterologous booster doses for an inactivated SARS-CoV-2 vaccine: A large-scale prospective cohort study. Lancet Glob. Health 2022, 10, e798–e806. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. (Engl. Ed.) 2021, 74, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.W. Popping the (PICO) question in research and evidence-based practice. Appl. Nurs. Res. 2002, 15, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; Ottawa Hospital Research Institute: Ottawa, ON, Canada, 2000. [Google Scholar]
- Atmar, R.L.; Lyke, K.E.; Deming, M.E.; Jackson, L.A.; Branche, A.R.; El Sahly, H.M.; Rostad, C.A.; Martin, J.M.; Johnston, C.; Rupp, R.E.; et al. Homologous and Heterologous Covid-19 Booster Vaccinations. N. Engl. J. Med. 2022, 386, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, A.; Marchevsky, N.G.; Jenkin, D.; Aboagye, J.; Aley, P.K.; Angus, B.; Belij-Rammerstorfer, S.; Bibi, S.; Bittaye, M.; Cappuccini, F.; et al. Reactogenicity and immunogenicity after a late second dose or a third dose of ChAdOx1 nCoV-19 in the UK: A substudy of two randomised controlled trials (COV001 and COV002). Lancet 2021, 398, 981–990. [Google Scholar] [CrossRef]
- Cao, Y.; Hao, X.; Wang, X.; Wu, Q.; Song, R.; Zhao, D.; Song, W.; Wang, Y.; Yisimayi, A.; Wang, W.; et al. Humoral immunogenicity and reactogenicity of CoronaVac or ZF2001 booster after two doses of inactivated vaccine. Cell Res. 2022, 32, 107–109. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, H.; Zhang, Q.; Zhang, Y.; Lin, K.; Fu, Z.; Song, J.; Zhao, Y.; Fan, M.; Wang, H.; et al. Recombinant protein subunit vaccine booster following two-dose inactivated vaccines dramatically enhanced anti-RBD responses and neutralizing titers against SARS-CoV-2 and Variants of Concern. Cell Res. 2022, 32, 103–106. [Google Scholar] [CrossRef]
- Costa Clemens, S.A.; Weckx, L.; Clemens, R.; Almeida Mendes, A.V.; Ramos Souza, A.; Silveira, M.B.V.; da Guarda, S.N.F.; de Nobrega, M.M.; de Moraes Pinto, M.I.; Gonzalez, I.G.S.; et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): A phase 4, non-inferiority, single blind, randomised study. Lancet 2022, 399, 521–529. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, Y.; Zhang, H.; Zhang, Q.; Fu, Z.; Lin, K.; Song, J.; Zhao, Y.; Fan, M.; Wang, H.; et al. Safety and immunogenicity of a third-dose homologous BBIBP-CorV boosting vaccination: Interim results from a prospective open-label study. Emerg. Microbes Infect. 2022, 11, 639–647. [Google Scholar] [CrossRef]
- Khong, K.W.; Liu, D.; Leung, K.Y.; Lu, L.; Lam, H.Y.; Chen, L.; Chan, P.C.; Lam, H.M.; Xie, X.; Zhang, R.; et al. Antibody Response of Combination of BNT162b2 and CoronaVac Platforms of COVID-19 Vaccines against Omicron Variant. Vaccines 2022, 10, 160. [Google Scholar] [CrossRef]
- Xie, H.; Wen, X.; Li, J.; Chen, W.; Chen, M.; Zhang, L.; Lv, M.; Zhou, S.; Bai, S.; Zhao, W.; et al. Evaluation of Immunogenicity by Pseudovirus Neutralization Assays for SARS-CoV-2 Variants after Primary and Booster Immunization. Int. J. Infect. Dis. 2022, 117, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Zou, J.; Kurhade, C.; Cai, H.; Yang, Q.; Cutler, M.; Cooper, D.; Muik, A.; Jansen, K.U.; Xie, X.; et al. Neutralization and durability of 2 or 3 doses of the BNT162b2 vaccine against Omicron SARS-CoV-2. Cell Host Microbe 2022, 30, 485–488.e3. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Montefiori, D.; Huang, W.; Nestorova, B.; Chang, Y.; Carfi, A.; Edwards, D.K.; Oestreicher, J.; Legault, H.; Girard, B.; et al. Immune Memory Response After a Booster Injection of mRNA-1273 for Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2). medRxiv 2021. [Google Scholar] [CrossRef]
- Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Lustig, Y.; Cohen, C.; Rahav, G.; Asraf, K.; Amit, S.; Jaber, H.; Nemet, I.; et al. Early Immunogenicity and Safety of the Third Dose of BNT162b2 Messenger RNA Coronavirus Disease 2019 Vaccine Among Adults Older than 60 Years: Real-World Experience. J. Infect. Dis. 2022, 225, 785–792. [Google Scholar] [CrossRef]
- Gruell, H.; Vanshylla, K.; Tober-Lau, P.; Hillus, D.; Schommers, P.; Lehmann, C.; Kurth, F.; Sander, L.E.; Klein, F. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat. Med. 2022, 28, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Then, E.; Lucas, C.; Monteiro, V.S.; Miric, M.; Brache, V.; Cochon, L.; Vogels, C.B.F.; Malik, A.A.; De la Cruz, E.; Jorge, A.; et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat. Med. 2022, 28, 481–485. [Google Scholar] [CrossRef]
- Assawakosri, S.; Kanokudom, S.; Suntronwong, N.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Duangchinda, T.; Chantima, W.; Pakchotanon, P.; et al. Neutralizing Activities against the Omicron Variant after a Heterologous Booster in Healthy Adults Receiving Two Doses of CoronaVac Vaccination. J. Infect. Dis. 2022, jiac092. [Google Scholar] [CrossRef] [PubMed]
- Angkasekwinai, N.; Niyomnaitham, S.; Sewatanon, J.; Phumiamorn, S.; Sukapirom, K.; Senawong, S.; Toh, Z.Q.; Umrod, P.; Somporn, T.; Chumpol, S.; et al. The immunogenicity and reactogenicity of four COVID-19 booster vaccinations against SARS-CoV-2 variants of concerns (Delta, Beta, and Omicron) following CoronaVac or ChAdOx1 nCoV-19 primary series. medRxiv 2022. [Google Scholar] [CrossRef]
- Fernandes, Q.; Inchakalody, V.P.; Merhi, M.; Mestiri, S.; Taib, N.; Moustafa Abo El-Ella, D.; Bedhiafi, T.; Raza, A.; Al-Zaidan, L.; Mohsen, M.O.; et al. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann. Med. 2022, 54, 524–540. [Google Scholar] [CrossRef]
- Gardner, B.J.; Kilpatrick, A.M. Estimates of reduced vaccine effectiveness against hospitalization, infection, transmission and symptomatic disease of a new SARS-CoV-2 variant, Omicron (B.1.1.529), using neutralizing antibody titers. medRxiv 2021. [Google Scholar] [CrossRef]
- Kirby, T. New variant of SARS-CoV-2 in UK causes surge of COVID-19. Lancet Respir. Med. 2021, 9, e20–e21. [Google Scholar] [CrossRef]
- Duan, L.; Zheng, Q.; Zhang, H.; Niu, Y.; Lou, Y.; Wang, H. The SARS-CoV-2 Spike Glycoprotein Biosynthesis, Structure, Function, and Antigenicity: Implications for the Design of Spike-Based Vaccine Immunogens. Front. Immunol. 2020, 11, 576622. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Gong, W. Will Mutations in the Spike Protein of SARS-CoV-2 Lead to the Failure of COVID-19 Vaccines? J. Korean Med. Sci. 2021, 36, e124. [Google Scholar] [CrossRef]
- Koehler, M.; Ray, A.; Moreira, R.A.; Juniku, B.; Poma, A.B.; Alsteens, D. Molecular insights into receptor binding energetics and neutralization of SARS-CoV-2 variants. Nat. Commun. 2021, 12, 6977. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Zia, T.; Suleman, M.; Khan, T.; Ali, S.S.; Abbasi, A.A.; Mohammad, A.; Wei, D.-Q. Higher infectivity of the SARS-CoV-2 new variants is associated with K417N/T, E484K, and N501Y mutants: An insight from structural data. J. Cell. Physiol. 2021, 236, 7045–7057. [Google Scholar] [CrossRef] [PubMed]
- Burki, T.K. Omicron variant and booster COVID-19 vaccines. Lancet Respir. Med. 2022, 10, e17. [Google Scholar] [CrossRef]
- Loubet, P.; Laureillard, D.; Martin, A.; Larcher, R.; Sotto, A. Why promoting a COVID-19 vaccine booster dose? Anaesth. Crit. Care Pain Med. 2021, 40, 100967. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Quach, T.H.T.; Tran, T.M.; Phuoc, H.N.; Nguyen, H.T.; Vo, T.K.; Vo, G.V. Reactogenicity and immunogenicity of heterologous prime-boost immunization with COVID-19 vaccine. Biomed. Pharmacother. 2022, 147, 112650. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef]
- Liu, J.; Ewald, B.A.; Lynch, D.M.; Denholtz, M.; Abbink, P.; Lemckert, A.A.; Carville, A.; Mansfield, K.G.; Havenga, M.J.; Goudsmit, J.; et al. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J. Virol. 2008, 82, 4844–4852. [Google Scholar] [CrossRef] [Green Version]
- Sadarangani, M.; Marchant, A.; Kollmann, T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021, 21, 475–484. [Google Scholar] [CrossRef]
- Zhu, F.-C.; Guan, X.-H.; Li, Y.-H.; Huang, J.-Y.; Jiang, T.; Hou, L.-H.; Li, J.-X.; Yang, B.-F.; Wang, L.; Wang, W.-J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Buchbinder, S.P.; Mehrotra, D.V.; Duerr, A.; Fitzgerald, D.W.; Mogg, R.; Li, D.; Gilbert, P.B.; Lama, J.R.; Marmor, M.; Del Rio, C.; et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008, 372, 1881–1893. [Google Scholar] [CrossRef] [Green Version]
- Chiu, N.-C.; Chi, H.; Tu, Y.-K.; Huang, Y.-N.; Tai, Y.-L.; Weng, S.-L.; Chang, L.; Huang, D.T.-N.; Huang, F.-Y.; Lin, C.-Y. To mix or not to mix? A rapid systematic review of heterologous prime-boost covid-19 vaccination. Expert Rev. Vaccines 2021, 20, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Sholukh, A.M.; Fiore-Gartland, A.; Ford, E.S.; Miner, M.D.; Hou, Y.J.; Tse, L.V.; Kaiser, H.; Zhu, H.; Lu, J.; Madarampalli, B.; et al. Evaluation of Cell-Based and Surrogate SARS-CoV-2 Neutralization Assays. J. Clin. Microbiol. 2021, 59, e0052721. [Google Scholar] [CrossRef]
- Pfizer. Pfizer and BioNTech Initiate Study to Evaluate Omicron-Based COVID-19 Vaccine in Adults 18 to 55 Years of Age. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-initiate-study-evaluate-omicron-based (accessed on 13 June 2022).
- Sinovac. SINOVAC Omicron-Based Inactivated Vaccine Approved for Clinical Use in Hong Kong, China. Available online: http://www.sinovac.com.cn/news/shownews.php?id=1447 (accessed on 13 June 2022).
- Torres Jonathan, L.; Ozorowski, G.; Andreano, E.; Liu, H.; Copps, J.; Piccini, G.; Donnici, L.; Conti, M.; Planchais, C.; Planas, D.; et al. Structural insights of a highly potent pan-neutralizing SARS-CoV-2 human monoclonal antibody. Proc. Natl. Acad. Sci. USA 2022, 119, e2120976119. [Google Scholar] [CrossRef]
- Jaworski, J.P. Neutralizing monoclonal antibodies for COVID-19 treatment and prevention. Biomed. J. 2021, 44, 7–17. [Google Scholar] [CrossRef]

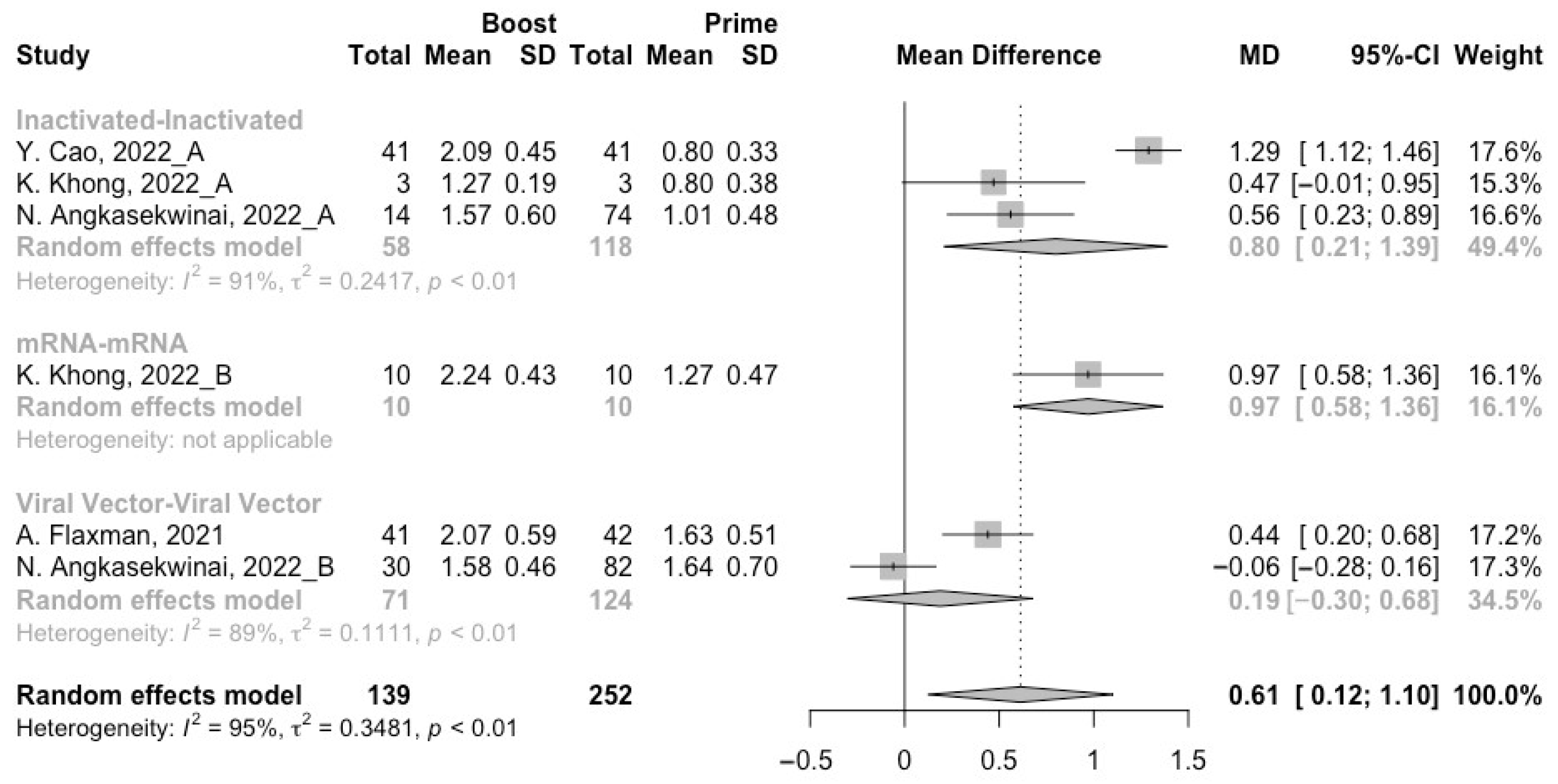
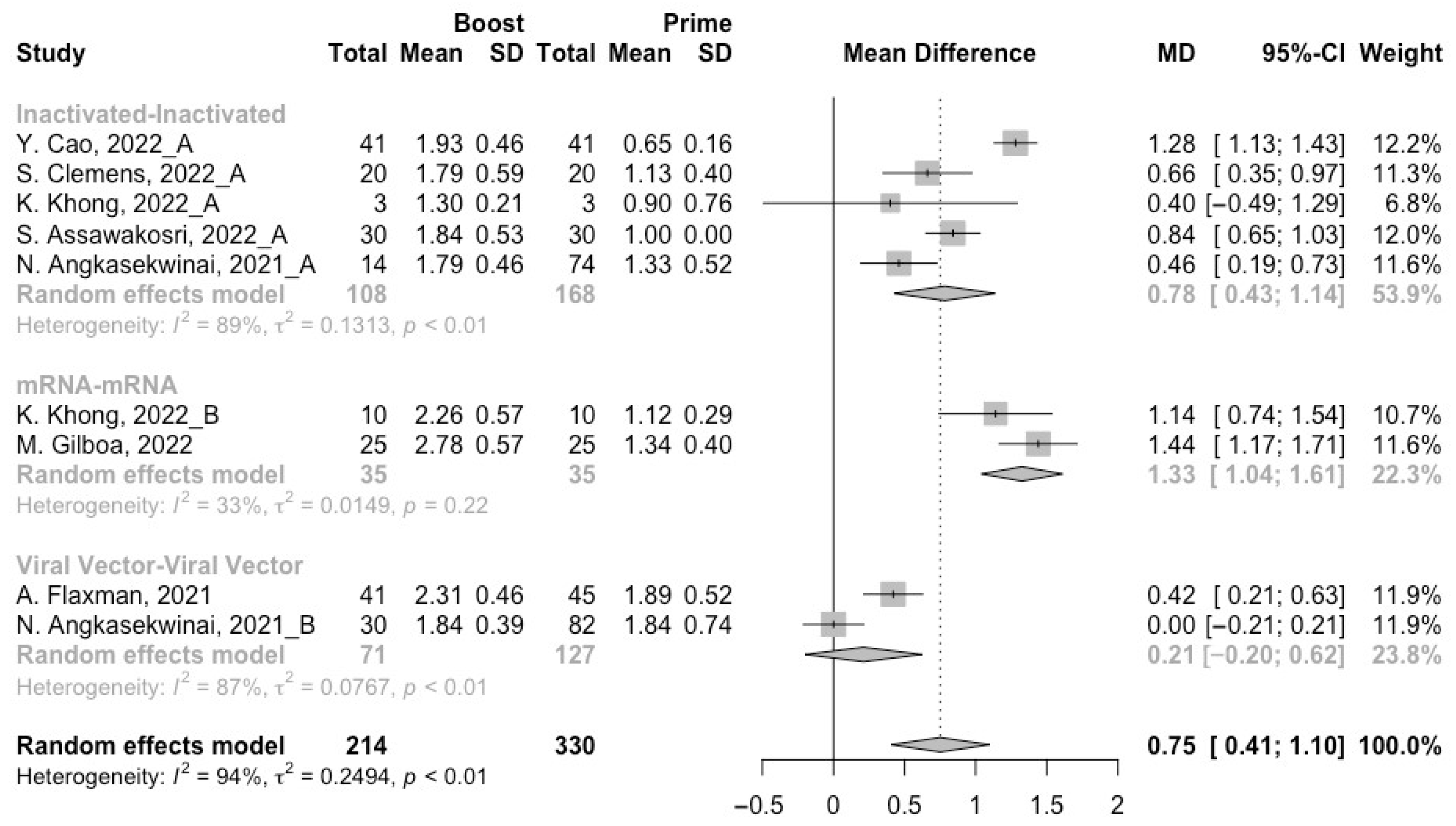

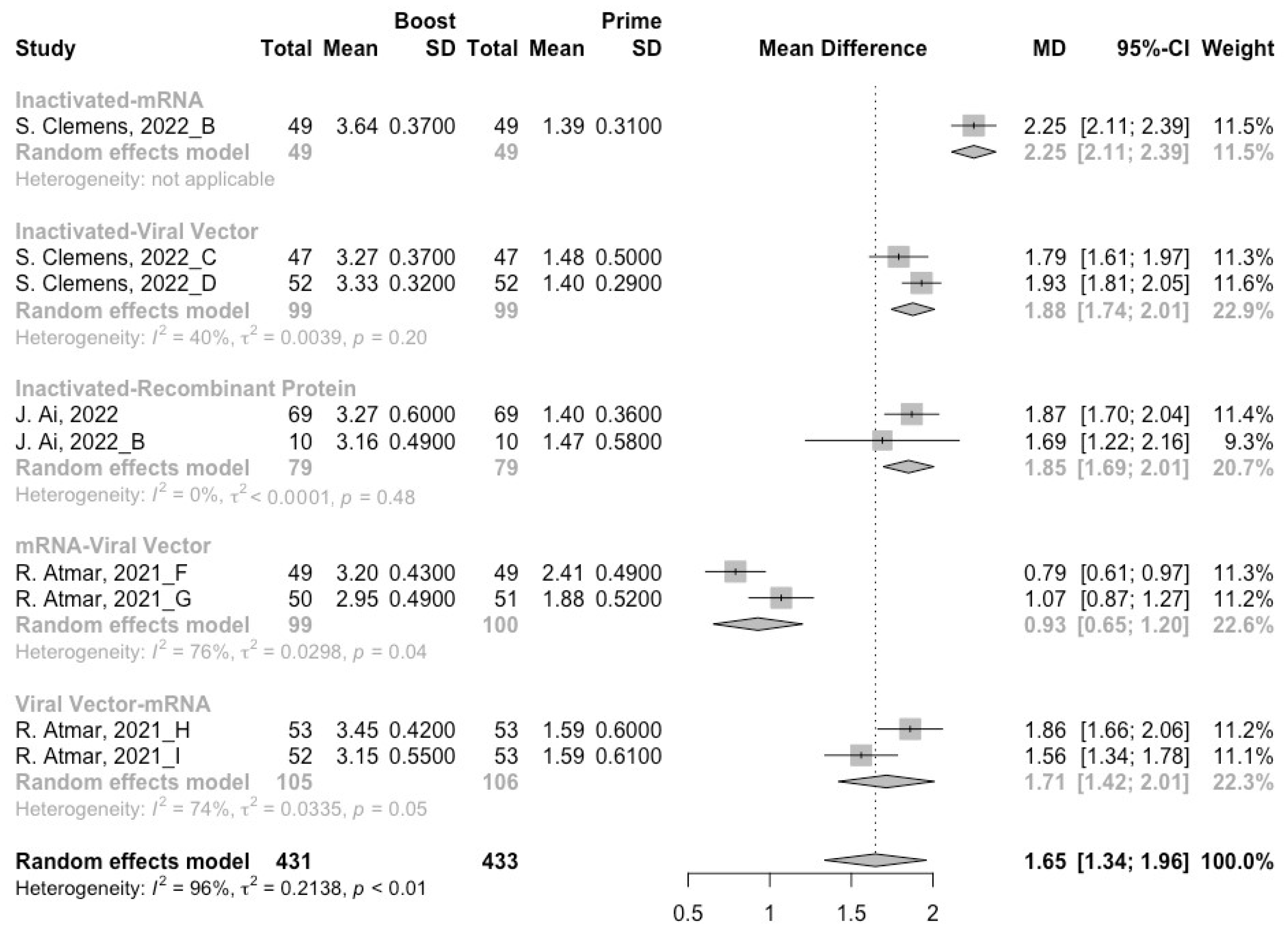

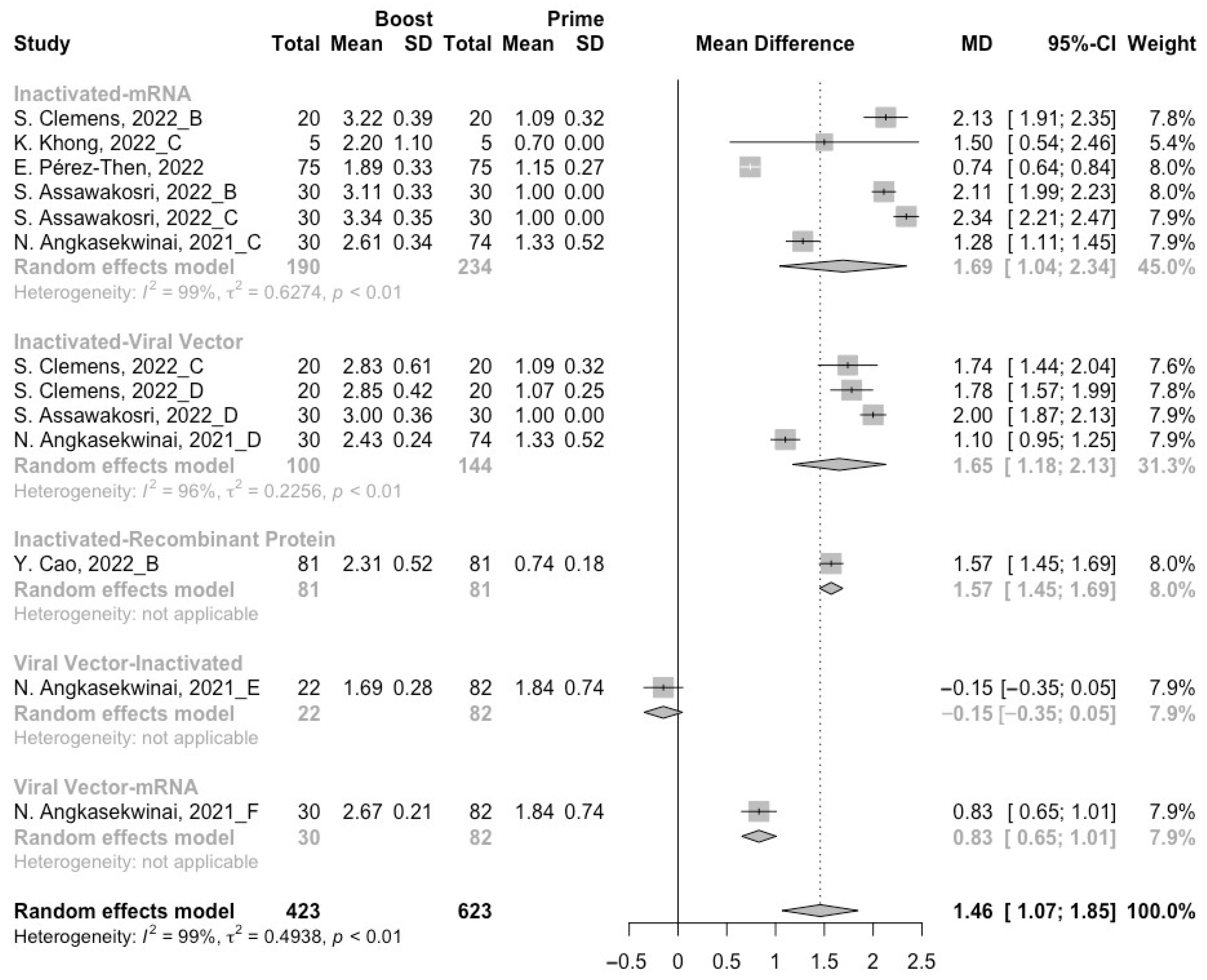

| Study and Year | Country | Number of Groups | Participants (N) | Characteristics of the Participants 1 | Age (Mean/Median) | Male (%) | COVID-19 Vaccines (Prime/Boost) 2 | Interval of Boost | SARS-CoV-2 Variants | NOS Score |
|---|---|---|---|---|---|---|---|---|---|---|
| Atmar et al., 2021 [2] | USA | 9 | 51; 50; 51; 50; 50; 49; 51; 53; 53 | Healthy adults | 53.1; 54.8; 54.3; 50.4; 50.1; 49.9; 50.3; 56.8; 47.7 | 37.3; 42; 49; 54; 54; 67.3; 54.9; 50.9; 45.3 | mRNA-1273/mRNA-1273; BNT/mRNA-1273; mRNA-1273/BNT; BNT/BNT; Ad26/Ad26; mRNA-1273/Ad26; BNT/Ad26; Ad26/mRNA-1273; Ad26/BNT | at least 12 weeks | wild type, Beta, Delta | 8 |
| Flaxman et al., 2021 [18] | UK | 1 | 75 | Healthy adults | 37 | 60 | ChAd/ChAd | 20–38 weeks | wild type, Alpha, Beta, Delta | 6 |
| Cao et al., 2022 [19] | China | 2 | 41; 81 | Healthy adults | 38.1; 40.7 | 24.4; 30.9 | CoronaVac/CoronaVac; CoronaVac/ZF2001 | 4–8 months | wild type, Beta, Delta | 9 |
| Ai et al., 2022 [20] | China | 1 | 69 | Healthy adults | 28 | 43.7 | BBIBP/ZF2001 | 4–8 months | wild type, Alpha, Beta, Delta | 9 |
| Clemens et al., 2022 [21] | Brazil | 4 | 281; 333; 295; 296 | Healthy adults | 60 | 39.5 | CoronaVac/CoronaVac; CoronaVac/BNT; CoronaVac/Ad26; CoronaVac/ChAd | 6 months | wild type, Delta, Omicron | 9 |
| Ai et al., 2022 [22] | China | 1 | 63 | Healthy adults | 28 | 42.9 | BBIBP/BBIBP | 4–8 months | wild type, Alpha, Beta, Delta | 9 |
| Khong et al., 2022 [23] | China | 3 | 3; 10; 5 | Healthy adults | 58; 53; 58.5 | 55.6; 46.7; 37.5 | CoronaVac/CoronaVac; BNT/BNT; CoronaVac/BNT | at least 6 months | wild type, Beta, Delta, Omicron | 6 |
| Xie et al., 2022 [24] | China | 1 | 46 | Healthy adults aged 18–59 years | NA | NA | CoronaVac/CoronaVac | at least 12 months | wild type, Alpha, Beta, Delta | 8 |
| Xia et al., 2022 [25] | USA | 1 | 24 | Healthy adults | 52.9 | 37.5 | BNT/BNT | NA | wild type, Omicron | 8 |
| Chu et al., 2021 [26] | USA | 1 | 295 | Healthy adults | 52 | 33.7 | mRNA-1273/mRNA-1273 | 7.2 ± 0.6 months | wild type, Delta | 9 |
| Gilboa et al., 2022 [27] | Israel | 1 | 159 | Healthy adults aged 60 years and older | 66 | 35 | BNT/BNT | NA | wild type, Delta | 8 |
| Gruell et al., 2022 [28] | Germany | 1 | 30 | Healthy adults | 49 | 43 | BNT/BNT | 26–41 weeks | wild type, Omicron | 7 |
| Ai et al., 2022 [29] | China | 2 | 10; 10 | Healthy adults | 27; 24.5 | 60; 60 | BBIBP/BBIBP; BBIBP/ZF2001 | 4–8 months | wild type, Beta, Delta, Omicron | 8 |
| Pérez-Then et al., 2022 [30] | The Dominican Republic | 1 | 75 | Healthy adults | 40.4 | 30 | CoronaVac/BNT | 109.5 ± 34.9 days | wild type, Delta, Omicron | 8 |
| Assawakosri et al., 2022 [31] | Thailand | 4 | 57; 54; 58; 55 | Healthy adults | 41.9; 41.6; 37; 44.1 | 40.4; 59.3; 47.8; 43.6 | CoronaVac/BBIBP; CoronaVac/BNT; CoronaVac/mRNA-1273; CoronaVac/ChAd | 5–7 months | wild type, Delta, Omicron | 9 |
| Angkasekwinai et al., 2021 [32] | Thailand | 6 | 14; 50; 50; 65; 23; 49 | Healthy adults | 31; 45.5; 32; 36.6; 51; 34 | 14.3; 6; 20; 21.5; 8.7; 26 | CoronaVac/BBIBP; ChAd/ChAd; CoronaVac/BNT; CoronaVac/ChAd; ChAd/BBIBP; ChAd/BNT | 8–12 weeks | wild type, Beta, Delta | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, H.; Peng, Z.; Si, S.; Alifu, X.; Zhou, H.; Chi, P.; Zhuang, Y.; Mo, M.; Yu, Y. Neutralization Activity against SARS-CoV-2 Variants after Booster Vaccination in Populations without COVID-19: A Meta-Analysis. Vaccines 2022, 10, 1101. https://doi.org/10.3390/vaccines10071101
Cheng H, Peng Z, Si S, Alifu X, Zhou H, Chi P, Zhuang Y, Mo M, Yu Y. Neutralization Activity against SARS-CoV-2 Variants after Booster Vaccination in Populations without COVID-19: A Meta-Analysis. Vaccines. 2022; 10(7):1101. https://doi.org/10.3390/vaccines10071101
Chicago/Turabian StyleCheng, Haoyue, Zhicheng Peng, Shuting Si, Xialidan Alifu, Haibo Zhou, Peihan Chi, Yan Zhuang, Minjia Mo, and Yunxian Yu. 2022. "Neutralization Activity against SARS-CoV-2 Variants after Booster Vaccination in Populations without COVID-19: A Meta-Analysis" Vaccines 10, no. 7: 1101. https://doi.org/10.3390/vaccines10071101
APA StyleCheng, H., Peng, Z., Si, S., Alifu, X., Zhou, H., Chi, P., Zhuang, Y., Mo, M., & Yu, Y. (2022). Neutralization Activity against SARS-CoV-2 Variants after Booster Vaccination in Populations without COVID-19: A Meta-Analysis. Vaccines, 10(7), 1101. https://doi.org/10.3390/vaccines10071101







