Exploring Sea Lice Vaccines against Early Stages of Infestation in Atlantic Salmon (Salmo salar)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antigens Selection and Purification of Recombinant Proteins
2.2. Fish Vaccination, Challenge Trial, and Ethics Statement
2.3. High-Throughput Transcriptome Sequencing
2.4. RNA-Seq Analysis
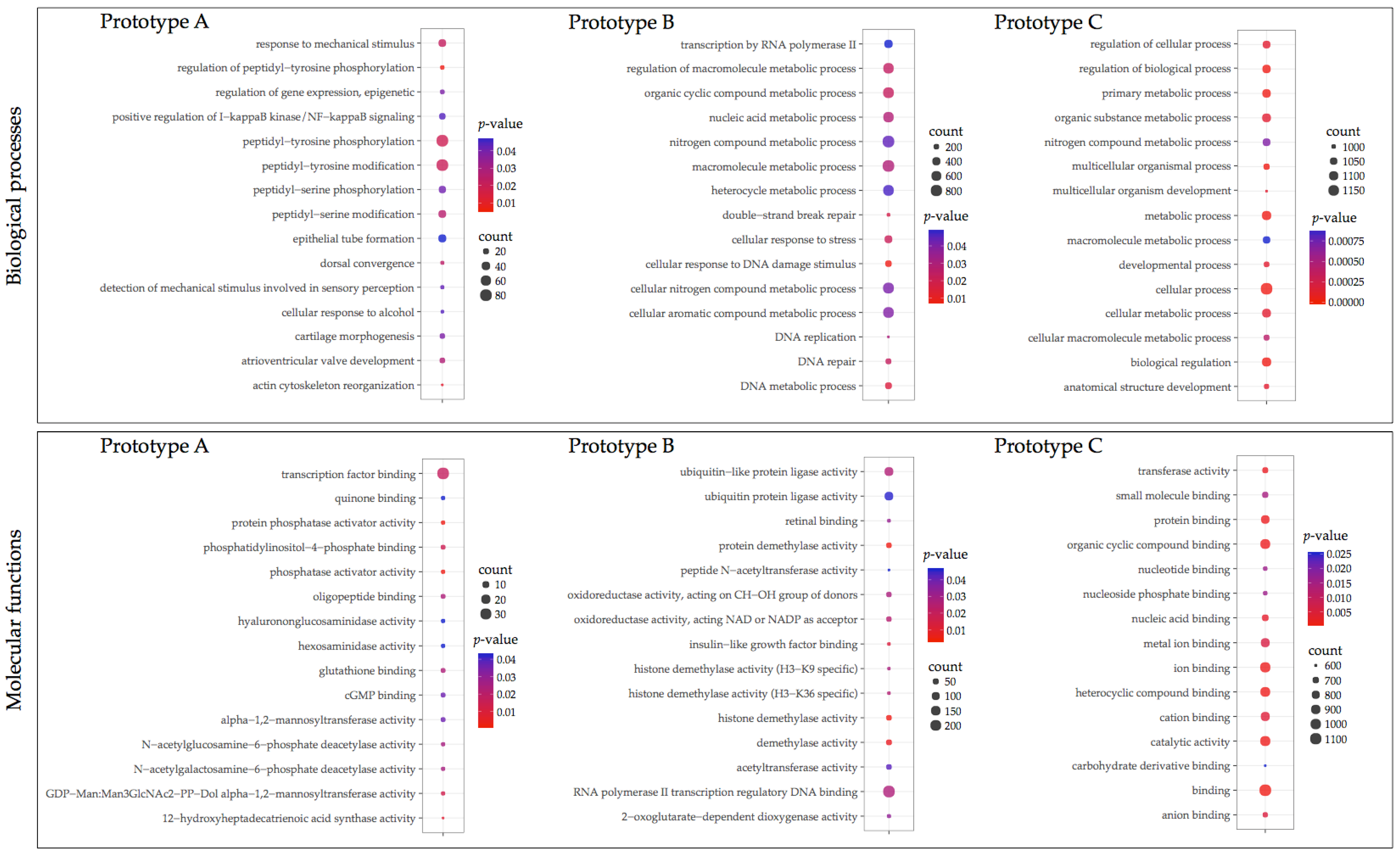
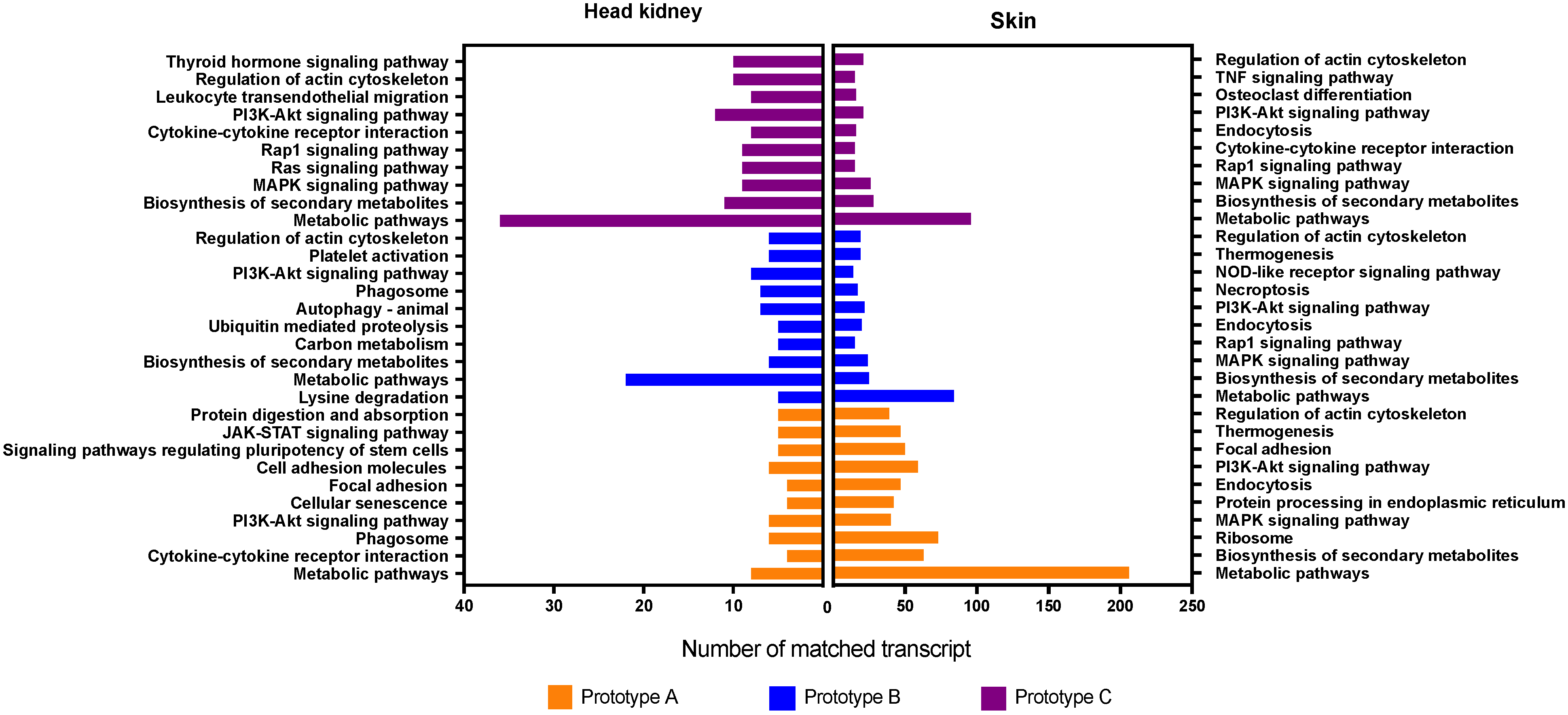
2.5. Gene Ontology and Pathway Enrichment Analyses
2.6. RNA Extraction and RT-qPCR
3. Results
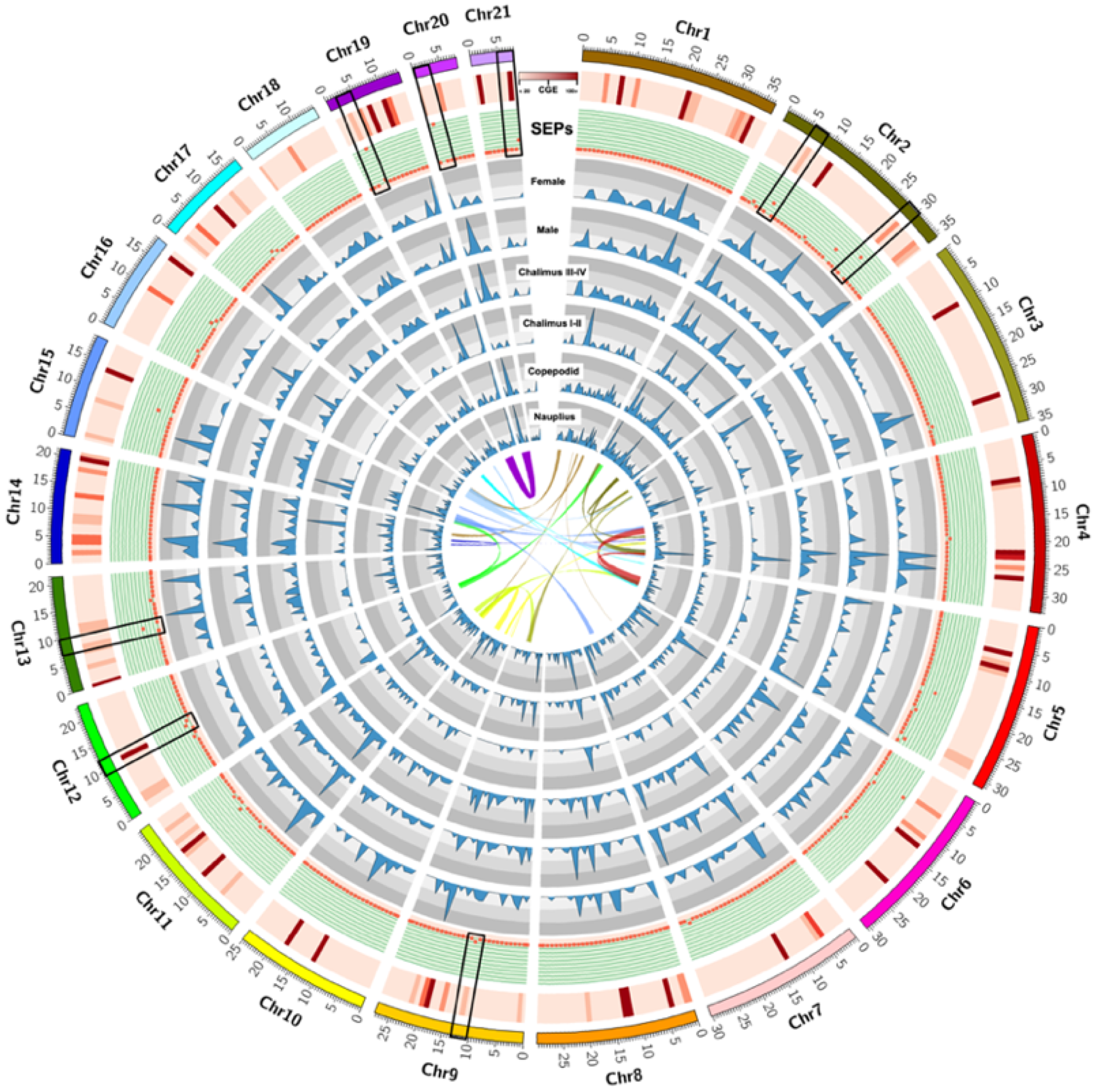
3.1. Identification of Excretory/Secretory Proteins (SEPs) during the Early-Developmental Stage of Sea Lice
3.2. Transcriptome Modulation in Head Kidney, and Skin
3.3. GO Enrichment and KEGG Pathway Analysis
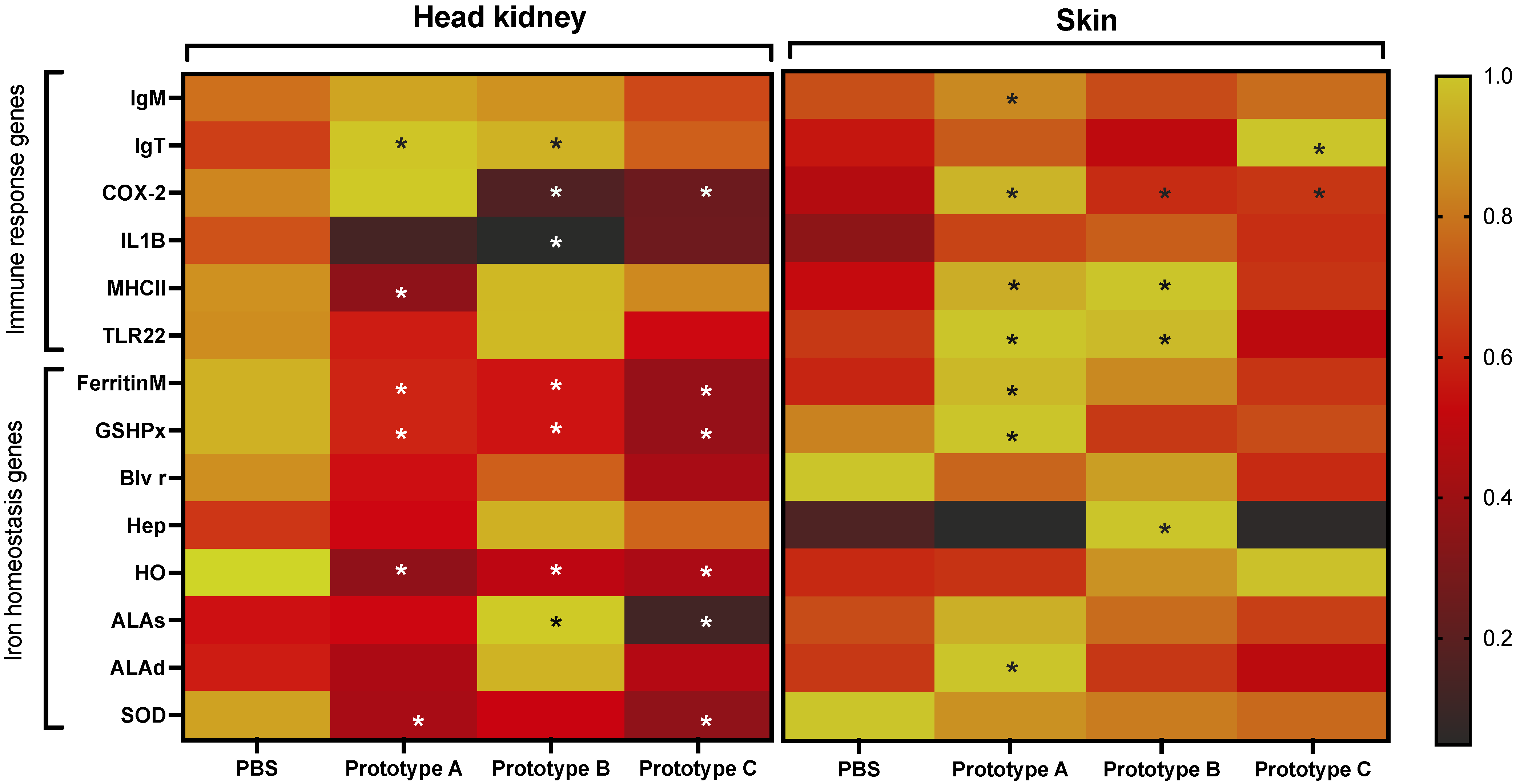
3.4. RT-qPCR Evaluation of Genes Associated with C. Rogercresseyi Infestation
3.5. Antigens’ Efficacy in Preventing Parasite Attachment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Barrett, L.T.; Oppedal, F.; Robinson, N.; Dempster, T. Prevention not cure: A review of methods to avoid sea lice infestations in salmon aquaculture. Rev. Aquac. 2020, 12, 2527–2543. [Google Scholar] [CrossRef]
- Sommerset, I.; Krossøy, B.; Biering, E.; Frost, P. Vaccines for fish in aquaculture. Expert Rev. Vaccines 2005, 4, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Caruffo, M.; Maturana, C.; Kambalapally, S.; Larenas, J.; Tobar, J.A. Protective oral vaccination against infectious salmon anaemia virus in Salmo salar. Fish Shellfish Immunol. 2016, 54, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Aravena, A.; Fuentes, Y.; Cartagena, J.; Brito, T.; Poggio, V.; La Torre, J.; Mendoza, H.; Gonzalez-Nilo, F.; Sandino, A.M.; Spencer, E. Development of a nanoparticle-based oral vaccine for Atlantic salmon against ISAV using an alphavirus replicon as adjuvant. Fish Shellfish Immunol. 2015, 45, 157–166. [Google Scholar] [CrossRef]
- von Gersdorff Jørgensen, L. The fish parasite Ichthyophthirius Multifiliis–Host immunology, vaccines and novel treatments. Fish Shellfish Immunol. 2017, 67, 586–595. [Google Scholar] [CrossRef]
- Gallardo-Escárate, C.; Arriagada, G.; Carrera, C.; Goncalves, A.T.; Nunez-Acuna, G.; Valenzuela-Miranda, D.; Valenzuela-Munoz, V. The race between host and sea lice in the Chilean salmon farming: A genomic approach. Rev. Aquac. 2019, 11, 325–339. [Google Scholar] [CrossRef]
- Stutzer, C.; Richards, S.A.; Ferreira, M.; Baron, S.; Maritz-Olivier, C. Metazoan parasite vaccines: Present status and future prospects. Front. Cell. Infect. Microbiol. 2018, 8, 67. [Google Scholar] [CrossRef] [Green Version]
- Rappuoli, R. Reverse vaccinology. Curr. Opin. Microbiol. 2000, 3, 445–450. [Google Scholar] [CrossRef]
- Heinson, A.I.; Woelk, C.H.; Newell, M.-L. The promise of reverse vaccinology. Int. Health 2015, 7, 85–89. [Google Scholar] [CrossRef]
- Tellam, R.L.; Vuocolo, T.; Eisemann, C.; Briscoe, S.; Riding, G.; Elvin, C.; Pearson, R. Identification of an immuno-protective mucin-like protein, peritrophin-55, from the peritrophic matrix of Lucilia cuprina larvae. Insect Biochem. Mol. Biol. 2003, 33, 239–252. [Google Scholar] [CrossRef]
- Bartley, K.; Huntley, J.F.; Wright, H.W.; Nath, M.; Nisbet, A.J. Assessment of cathepsin D and L-like proteinases of poultry red mite, Dermanyssus gallinae (De Geer), as potential vaccine antigens. Parasitology 2012, 139, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.; Dixit, P.; Sharma, R. Immunodiagnostic/protective role of cathepsin L cysteine proteinases secreted by Fasciola species. Vet. Parasitol. 2008, 154, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Franta, Z.; Frantová, H.; Konvičková, J.; Horn, M.; Sojka, D.; Mareš, M.; Kopáček, P. Dynamics of digestive proteolytic system during blood feeding of the hard tick Ixodes ricinus. Parasites Vectors 2010, 3, 119. [Google Scholar] [CrossRef] [Green Version]
- Horn, M.; Nussbaumerová, M.; Šanda, M.; Kovářová, Z.; Srba, J.; Franta, Z.; Sojka, D.; Bogyo, M.; Caffrey, C.R.; Kopáček, P. Hemoglobin digestion in blood-feeding ticks: Mapping a multipeptidase pathway by functional proteomics. Chem. Biol. 2009, 16, 1053–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghoneim, H.; Klinkert, M.-Q. Biochemical properties of purified cathepsin B from Schistosoma mansoni. Int. J. Parasitol. 1995, 25, 1515–1519. [Google Scholar] [CrossRef]
- Williamson, A.L.; Brindley, P.J.; Abbenante, G.; Datu, B.J.; Prociv, P.; Berry, C.; Girdwood, K.; Pritchard, D.I.; Fairlie, D.P.; Hotez, P.J. Hookworm aspartic protease, Na-APR-2, cleaves human hemoglobin and serum proteins in a host-specific fashion. J. Infect. Dis. 2003, 187, 484–494. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, E.; McCarthy, E.; Copley, L.; Jackson, D.; Johnson, D.; Dalton, J.P.; Mulcahy, G. Characterisation of cathepsin B-like cysteine protease of Lepeophtheirus salmonis. Aquaculture 2010, 310, 38–42. [Google Scholar] [CrossRef]
- Maldonado-Aguayo, W.; Chávez-Mardones, J.; Gonçalves, A.T.; Gallardo-Escárate, C. Cathepsin gene family reveals transcriptome patterns related to the infective stages of the salmon louse Caligus rogercresseyi. PLoS ONE 2015, 10, e0123954. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, E.; Cunningham, E.; Copley, L.; Jackson, D.; Johnston, D.; Dalton, J.P.; Mulcahy, G. Cathepsin L proteases of the parasitic copepod, Lepeophtheirus salmonis. Aquaculture 2012, 356, 264–271. [Google Scholar] [CrossRef]
- Elvin, C.M.; Vuocolo, T.; Pearson, R.D.; East, I.J.; Riding, G.A.; Eisemann, C.H.; Tellam, R.L. Characterization of a major peritrophic membrane protein, peritrophin-44, from the larvae of Lucilia cuprina: cDNA and deduced amino acid sequences. J. Biol. Chem. 1996, 271, 8925–8935. [Google Scholar] [CrossRef] [Green Version]
- Hegedus, D.; Erlandson, M.; Gillott, C.; Toprak, U. New insights into peritrophic matrix synthesis, architecture, and function. Annu. Rev. Entomol. 2009, 54, 285–302. [Google Scholar] [CrossRef]
- Du, X.-J.; Wang, J.-X.; Liu, N.; Zhao, X.-F.; Li, F.-H.; Xiang, J.-H. Identification and molecular characterization of a peritrophin-like protein from fleshy prawn (Fenneropenaeus chinensis). Mol. Immunol. 2006, 43, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ma, F.; Wang, W.; Ren, Q. Identification and molecular characterization of a peritrophin-like gene, involved in the antibacterial response in Chinese mitten crab, Eriocheir sinensis. Dev. Comp. Immunol. 2015, 50, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Devenport, M.; Alvarenga, P.H.; Shao, L.; Fujioka, H.; Bianconi, M.L.; Oliveira, P.L.; Jacobs-Lorena, M. Identification of the Aedes aegypti peritrophic matrix protein AeIMUCI as a heme-binding protein. Biochemistry 2006, 45, 9540–9549. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.H.; Fernandes, K.M.; Goncalves, W.G.; Zanuncio, J.C.; Serrao, J.E. A peritrophin mediates the peritrophic matrix permeability in the workers of the bees Melipona quadrifasciata and Apis mellifera. Arthropod Struct. Dev. 2019, 53, 100885. [Google Scholar] [CrossRef] [PubMed]
- Dinglasan, R.; Devenport, M.; Florens, L.; Johnson, J.; McHugh, C.; Donnelly-Doman, M.; Carucci, D.; Yates Iii, J.; Jacobs-Lorena, M. The Anopheles gambiae adult midgut peritrophic matrix proteome. Insect Biochem. Mol. Biol. 2009, 39, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Magalhaes, T. What is the association of heme aggregates with the peritrophic matrix of adult female mosquitoes? Parasites Vectors 2014, 7, 362. [Google Scholar] [CrossRef] [Green Version]
- Song, R.; Ge, T.; Fan, X.; Zhang, Y.; Zhai, X.; Li, M.; Zhang, W.; Wu, L.; Cheung, A.K.L.; Chahan, B. Recombinant cysteine proteinase as anti-tick targeting Hyalomma asiaticum infestation. Exp. Parasitol. 2022, 235, 108234. [Google Scholar] [CrossRef]
- Seixas, A.; Oliveira, P.; Termignoni, C.; Logullo, C.; Masuda, A.; da Silva Vaz, I. Rhipicephalus (Boophilus) microplus embryo proteins as target for tick vaccine. Vet. Immunol. Immunopathol. 2012, 148, 149–156. [Google Scholar] [CrossRef]
- Price, D.R.; Küster, T.; Øines, Ø.; Oliver, E.M.; Bartley, K.; Nunn, F.; Lima Barbero, J.F.; Pritchard, J.; Karp-Tatham, E.; Hauge, H. Evaluation of vaccine delivery systems for inducing long-lived antibody responses to Dermanyssus gallinae antigen in laying hens. Avian Pathol. 2019, 48, S60–S74. [Google Scholar] [CrossRef] [Green Version]
- Tellam, R.L.; Eisemann, C.; Vuocolo, T.; Casu, R.; Jarmey, J.; Bowles, V.; Pearson, R. Role of oligosaccharides in the immune response of sheep vaccinated with Lucilia cuprina larval glycoprotein, peritrophin-95. Int. J. Parasitol. 2001, 31, 798–809. [Google Scholar] [CrossRef]
- Nisbet, A.J.; Huntley, J.F. Progress and opportunities in the development of vaccines against mites, fleas and myiasis-causing flies of veterinary importance. Parasite Immunol. 2006, 28, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Carpio, Y.; Basabe, L.; Acosta, J.; Rodríguez, A.; Mendoza, A.; Lisperger, A.; Zamorano, E.; González, M.; Rivas, M.; Contreras, S.; et al. Novel gene isolated from Caligus rogercresseyi: A promising target for vaccine development against sea lice. Vaccine 2011, 29, 2810–2820. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.; Karlsen, M.; Villar, M.; Olsen, R.H.; Leknes, L.M.; Furevik, A.; Yttredal, K.L.; Tartor, H.; Grove, S.; Alberdi, P.; et al. Vaccination with ectoparasite proteins involved in midgut function and blood digestion reduces salmon louse infestations. Vaccines 2020, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Swain, J.K.; Carpio, Y.; Johansen, L.-H.; Velazquez, J.; Hernandez, L.; Leal, Y.; Kumar, A.; Estrada, M.P. Impact of a candidate vaccine on the dynamics of salmon lice (Lepeophtheirus salmonis) infestation and immune response in Atlantic salmon (Salmo salar L.). PLoS ONE 2020, 15, e0239827. [Google Scholar] [CrossRef]
- Martins, M.L.; Shoemaker, C.A.; Xu, D.; Klesius, P.H. Effect of parasitism on vaccine efficacy against Streptococcus iniae in Nile tilapia. Aquaculture 2011, 314, 18–23. [Google Scholar] [CrossRef]
- Raynard, R.S.; Bricknell, I.R.; Billingsley, P.F.; Nisbet, A.J.; Vigneau, A.; Sommerville, C. Development of vaccines against sea lice. Pest Manag. Sci. 2002, 58, 569–575. [Google Scholar] [CrossRef]
- Tadiso, T.M.; Krasnov, A.; Skugor, S.; Afanasyev, S.; Hordvik, I.; Nilsen, F. Gene expression analyses of immune responses in Atlantic salmon during early stages of infection by salmon louse (Lepeophtheirus salmonis) revealed bi-phasic responses coinciding with the copepod-chalimus transition. BMC Genom. 2011, 12, 141. [Google Scholar] [CrossRef] [Green Version]
- Miguel, R.-G.; Jens, S.; Kurt, B.; Richard, C.T. Immunization of rainbow trout Oncorhynchus mykiss against Discocotyle sagittata (Monogenea). Dis. Aquat. Org. 2003, 55, 23–30. [Google Scholar]
- Dan, X.-M.; Zhang, T.-W.; Li, Y.-W.; Li, A.-X. Immune responses and immune-related gene expression profile in orange-spotted grouper after immunization with Cryptocaryon irritans vaccine. Fish Shellfish Immunol. 2013, 34, 885–891. [Google Scholar] [CrossRef]
- Valenzuela-Muñoz, V.; Benavente, B.P.; Casuso, A.; Leal, Y.; Gallardo-Escárate, C. Chimeric protein IPath® with chelating activity improves Atlantic salmon’s immunity against infectious diseases. Vaccines 2021, 9, 361. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Muñoz, V.; Gallardo-Escárate, C. Iron metabolism modulation in Atlantic salmon infested with the sea lice Lepeophtheirus salmonis and Caligus rogercresseyi: A matter of nutritional immunity? Fish Shellfish Immunol. 2017, 60, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Fast, M.D. Fish immune responses to parasitic copepod (namely sea lice) infection. Dev. Comp. Immunol. 2014, 43, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Torrissen, O.; Jones, S.; Asche, F.; Guttormsen, A.; Skilbrei, O.T.; Nilsen, F.; Horsberg, T.E.; Jackson, D. Salmon lice–impact on wild salmonids and salmon aquaculture. J. Fish Dis. 2013, 36, 171–194. [Google Scholar] [CrossRef] [Green Version]
- Quinones, R.A.; Fuentes, M.; Montes, R.M.; Soto, D.; León-Muñoz, J. Environmental issues in Chilean salmon farming: A review. Rev. Aquac. 2019, 11, 375–402. [Google Scholar] [CrossRef]
- Kotob, M.H.; Menanteau-Ledouble, S.; Kumar, G.; Abdelzaher, M.; El-Matbouli, M. The impact of co-infections on fish: A review. Vet. Res. 2017, 47, 98. [Google Scholar] [CrossRef] [Green Version]
- González, L.; Carvajal, J. Life cycle of Caligus rogercresseyi, (Copepoda: Caligidae) parasite of Chilean reared salmonids. Aquaculture 2003, 220, 101–117. [Google Scholar] [CrossRef]
- Núñez-Acuña, G.; Marambio, J.P.; Valenzuela, T.; Wadsworth, S.; Gallardo-Escarate, C. Antimicrobial peptides from Salmon salar skin induce frontal filament development and olfactory/cuticle-related genes in the sea louse Caligus rogercresseyi. Aquaculture 2016, 464, 171–177. [Google Scholar] [CrossRef]
- Pike, A.W.; Wadsworth, S.L. Sealice on Salmonids: Their Biology and Control. In Advances in Parasitology; Baker, J.R., Muller, R., Rollinson, D., Eds.; Academic Press: New York, NY, USA, 1999; Volume 44, pp. 233–337. [Google Scholar]
- Valenzuela-Muñoz, V.; Boltaña, S.; Gallardo-Escárate, C. Uncovering iron regulation with species-specific transcriptome patterns in Atlantic and coho salmon during a Caligus rogercresseyi infestation. J. Fish Dis. 2017, 40, 1169–1184. [Google Scholar] [CrossRef]
- Sutherland, B.J.G.; Koczka, K.W.; Yasuike, M.; Jantzen, S.G.; Yazawa, R.; Koop, B.F.; Jones, S.R.M. Comparative transcriptomics of Atlantic Salmo salar, chum Oncorhynchus keta and pink salmon O. gorbuscha during infections with salmon lice Lepeophtheirus salmonis. BMC Genom. 2014, 15, 200. [Google Scholar] [CrossRef] [Green Version]
- Valenzuela-Muñoz, V.; Boltaña, S.; Gallardo-Escárate, C. Comparative immunity of Salmo salar and Oncorhynchus kisutch during infestation with the sea louse Caligus rogercresseyi: An enrichment transcriptome analysis. Fish Shellfish Immunol. 2016, 59, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Escárate, C.; Valenzuela-Muñoz, V.; Nuñez-Acuña, G.; Valenzuela-Miranda, D.; Gonçalves, A.T.; Escobar-Sepulveda, H.; Liachko, I.; Nelson, B.; Roberts, S.; Warren, W. Chromosome-scale genome assembly of the sea louse Caligus rogercresseyi by SMRT sequencing and Hi-C analysis. Sci. Data 2021, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.; McLean, K.; Monaghan, S.J.; McNair, C.; Inglis, N.F.; McDonald, H.; Adams, S.; Richards, R.; Roy, W.; Smith, P.; et al. Characterisation of proteins in excretory/secretory products collected from salmon lice, Lepeophtheirus salmonis. Parasites Vectors 2018, 11, 294. [Google Scholar] [CrossRef] [Green Version]
- Gallardo-Escárate, C.; Valenzuela-Muñoz, V.; Nuñez-Acuña, G. RNA-Seq analysis using de novo transcriptome assembly as a reference for the salmon louse Caligus rogercresseyi. PLoS ONE 2014, 9, e92239. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Muñoz, V.; Gallardo-Escárate, C.; Benavente, B.P.; Valenzuela-Miranda, D.; Núñez-Acuña, G.; Escobar-Sepulveda, H.; Váldes, J.A. Whole-genome transcript expression profiling reveals novel insights into transposon genes and non-coding RNAs during Atlantic salmon seawater adaptation. Biology 2022, 11, 1. [Google Scholar] [CrossRef]
- Moriya, Y.; Itoh, M.; Okuda, S.; Yoshizawa, A.C.; Kanehisa, M. KAAS: An automatic genome annotation and pathway reconstruction server. Nucleic Acids Res. 2007, 35, W182–W185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, S.; Wakem, M.; Dijkman, G.; Alsarraj, M.; Nguyen, M. A practical approach to RT-qPCR—Publishing data that conform to the MIQE guidelines. Methods 2010, 50, S1–S5. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Casuso, A.; Núñez-Acuña, G.; Valenzuela-Muñoz, V.; Leal, Y.; Gallardo-Escárate, C. Identification of peritrophins as potential vaccine candidates against sea lice: A reverse vaccinology approach. Fish Shellfish Immunol. 2019, 91, 393. [Google Scholar] [CrossRef]
- Gallardo-Escárate, C.; Valenzuela-Muñoz, V.; Núñez-Acuña, G.; Carrera, C.; Gonçalves, A.T.; Valenzuela-Miranda, D.; Benavente, B.P.; Roberts, S. Catching the complexity of salmon-louse interactions. Fish Shellfish Immunol. 2019, 90, 199–209. [Google Scholar] [CrossRef]
- Ford, L.; Zhang, J.; Liu, J.; Hashmi, S.; Fuhrman, J.A.; Oksov, Y.; Lustigman, S. Functional analysis of the cathepsin-like cysteine protease genes in adult Brugia malayi using RNA interference. PLoS Negl. Trop. Dis. 2009, 3, e377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashmi, S.; Britton, C.; Liu, J.; Guiliano, D.B.; Oksov, Y.; Lustigman, S. Cathepsin L is essential for embryogenesis and development of Caenorhabditis elegans. J. Biol. Chem. 2002, 277, 3477–3486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo, L.; Muhlia-Almazan, A.; Saborowski, R.; García-Carreño, F. Aspartic cathepsin D endopeptidase contributes to extracellular digestion in clawed lobsters Homarus americanus and Homarus gammarus. Mar. Biotechnol. 2010, 12, 696–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciardi, A.; Visitsunthorn, K.; Dalton, J.P.; Ndao, M. A vaccine consisting of Schistosoma mansoni cathepsin B formulated in Montanide ISA 720 VG induces high level protection against murine schistosomiasis. BMC Infect. Dis. 2016, 16, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Wang, C.; Huang, Y.; Zhang, S.; Yu, H.; Meng, J.; Pan, B. Evaluation of the vaccine efficacy of three digestive protease antigens from Dermanyssus gallinae using an in vivo rearing system. Vaccine 2020, 38, 7842–7849. [Google Scholar] [CrossRef] [PubMed]
- Shivam, S.; El-Matbouli, M.; Kumar, G. Development of fish parasite vaccines in the OMICs era: Progress and opportunities. Vaccines 2021, 9, 179. [Google Scholar] [CrossRef]
- Li, Z.; Li, F.; Han, Y.; Xu, L.; Yang, F. VP24 is a chitin-binding protein involved in white spot syndrome virus infection. J. Virol. 2016, 90, 842–850. [Google Scholar] [CrossRef] [Green Version]
- Binning, S.A.; Shaw, A.K.; Roche, D.G. Parasites and host performance: Incorporating infection into our understanding of animal movement. Integr. Comp. Biol. 2017, 57, 267–280. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Muñoz, J.L.P.; Hawes, C.; Oyarzún, R.; Pontigo, J.P.; Saravia, J.; González, M.P.; Mardones, O.; Labbé, B.S.; Morera, F.J.; et al. Ectoparasite Caligus rogercresseyi modifies the lactate response in Atlantic salmon (Salmo salar) and Coho salmon (Oncorhynchus kisutch). Vet. Parasitol. 2017, 243, 6–11. [Google Scholar] [CrossRef]
- Barber, I. Parasites, behaviour and welfare in fish. Appl. Anim. Behav. Sci. 2007, 104, 251–264. [Google Scholar] [CrossRef]
- Huntingford, F.A.; Adams, C.; Braithwaite, V.A.; Kadri, S.; Pottinger, T.G.; Sandøe, P.; Turnbull, J.F. Current issues in fish welfare. J. Fish Biol. 2006, 68, 332–372. [Google Scholar] [CrossRef] [Green Version]
- Dawood, M.A.; Koshio, S.; Ishikawa, M.; Yokoyama, S. Interaction effects of dietary supplementation of heat-killed Lactobacillus plantarum and β-glucan on growth performance, digestibility and immune response of juvenile red sea bream, Pagrus major. Fish Shellfish Immunol. 2015, 45, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Núñez-Acuña, G.; Détrée, C.; Gallardo-Escárate, C.; Gonçalves, A.T. Functional diets modulate lncRNA-coding RNAs and gene interactions in the intestine of rainbow trout Oncorhynchus mykiss. Mar. Biotechnol. 2017, 19, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, K.; Matthews, L.; Bron, J.; Roberts, R.; Tinch, A.; Stear, M. The control of sea lice in Atlantic salmon by selective breeding. J. R. Soc. Interface 2015, 12, 20150574. [Google Scholar] [CrossRef] [Green Version]
- Hemmingsen, W.; MacKenzie, K.; Sagerup, K.; Remen, M.; Bloch-Hansen, K.; Imsland, A.K.D. Caligus elongatus and other sea lice of the genus Caligus as parasites of farmed salmonids: A review. Aquaculture 2020, 522, 735160. [Google Scholar] [CrossRef]
- Sutherland, B.G.; Covello, J.M.; Friend, S.E.; Poley, J.D.; Koczka, K.W.; Purcell, S.L.; MacLeod, T.L.; Donovan, B.R.; Pino, J.; Gonzalez-Vecino, J.L.; et al. Host-parasite transcriptomics during immunostimulant-enhanced rejection of salmon lice (Lepeophtheirus salmonis) by Atlantic salmon (Salmo salar). Facets 2017, 2, 477–495. [Google Scholar] [CrossRef] [Green Version]
- Jensen, L.B.; Provan, F.; Larssen, E.; Bron, J.E.; Obach, A. Reducing sea lice (Lepeophtheirus salmonis) infestation of farmed Atlantic salmon (Salmo salar L.) through functional feeds. Aquac. Nutr. 2015, 21, 983–993. [Google Scholar] [CrossRef]
- Valenzuela-Muñoz, V.; Benavente, B.P.; Casuso, A.; Leal, Y.; Valenzuela-Miranda, D.; Núñez-Acuña, G.; Sáez-Vera, C.; Gallardo-Escárate, C. Transcriptome and morphological analysis in Caligus rogercresseyi uncover the effects of Atlantic salmon vaccination with IPath®. Fish Shellfish Immunol. 2021, 117, 169–178. [Google Scholar] [CrossRef]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 49. [Google Scholar] [CrossRef] [Green Version]
- Esteban, M. An overview of the immunological defenses in fish skin. Int. Sch. Res. Not. 2012, 2012, 853470. [Google Scholar]
- Costa, R.A.; Power, D.M. Skin and scale regeneration after mechanical damage in a teleost. Mol. Immunol. 2018, 95, 73–82. [Google Scholar] [CrossRef]
- Ceballos-Francisco, D.; Cordero, H.; Guardiola, F.A.; Cuesta, A.; Esteban, M.Á. Healing and mucosal immunity in the skin of experimentally wounded gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2017, 71, 210–219. [Google Scholar] [CrossRef]
- Cordero, H.; Brinchmann, M.F.; Cuesta, A.; Esteban, M.A. Chronic wounds alter the proteome profile in skin mucus of farmed gilthead seabream. BMC Genom. 2017, 18, 939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, M.; Abdel-Baki, A.A.; Dkhil, M.A.; El-Matbouli, M.; Al-Quraishy, S. Antiprotozoal effects of metal nanoparticles against Ichthyophthirius multifiliis. Parasitology 2017, 144, 1802–1810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncalves, R.; Zhang, X.; Cohen, H.; Debrabant, A.; Mosser, D.M. Platelet activation attracts a subpopulation of effector monocytes to sites of Leishmania major infection. J. Exp. Med. 2011, 208, 1253–1265. [Google Scholar] [CrossRef] [PubMed]
- Hundelshausen, P.V.; Weber, C. Platelets as immune cells. Circ. Res. 2007, 100, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, A.; Skugor, S.; Todorcevic, M.; Glover, K.A.; Nilsen, F. Gene expression in Atlantic salmon skin in response to infection with the parasitic copepod Lepeophtheirus salmonis, cortisol implant, and their combination. BMC Genom. 2012, 13, 130. [Google Scholar] [CrossRef] [Green Version]
- Boltaña, S.; Sanchez, M.; Valenzuela, V.; Gallardo-Escárate, C. Density-dependent effects of Caligus rogercresseyi infestation on the immune responses of Salmo salar. Fish Shellfish Immunol. 2016, 59, 365–374. [Google Scholar] [CrossRef]
- Fast, M.D.; Muise, D.M.; Easy, R.E.; Ross, N.W.; Johnson, S.C. The effects of Lepeophtheirus salmonis infections on the stress response and immunological status of Atlantic salmon (Salmo salar). Fish Shellfish Immunol. 2006, 21, 228–241. [Google Scholar] [CrossRef]
- Jones, S.R.; Fast, M.D.; Johnson, S.C.; Groman, D.B. Differential rejection of salmon lice by pink and chum salmon: Disease consequences and expression of proinflammatory genes. Dis. Aquat. Org. 2007, 75, 229–238. [Google Scholar] [CrossRef]
- Braden, L.M.; Barker, D.E.; Koop, B.F.; Jones, S.R.M. Comparative defense-associated responses in salmon skin elicited by the ectoparasite Lepeophtheirus salmonis. Comp. Biochem. Physiol. Part D Genom. Proteom. 2012, 7, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Holm, H.; Santi, N.; Kjøglum, S.; Perisic, N.; Skugor, S.; Evensen, Ø. Difference in skin immune responses to infection with salmon louse (Lepeophtheirus salmonis) in Atlantic salmon (Salmo salar L.) of families selected for resistance and susceptibility. Fish Shellfish Immunol. 2015, 42, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Pontigo, J.P.; Saravia, J.; Oyarzún, R.; González, M.P.; Hawes, C.; Morera, F.J.; Pino, J.; Wadsworth, S.; Muñoz, J.L.P.; Vargas-Chacoff, L. Modulation of the expression of immune-related gene in Atlantic and Coho salmon during infestation with the sea lice Caligus rogercresseyi. Fishes 2019, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Acuña, G.; Gonçalves, A.T.; Valenzuela-Muñoz, V.; Pino-Marambio, J.; Wadsworth, S.; Gallardo-Escárate, C. Transcriptome immunomodulation of in-feed additives in Atlantic salmon Salmo salar infested with sea lice Caligus rogercresseyi. Fish Shellfish Immunol. 2015, 47, 450–460. [Google Scholar] [CrossRef]
- Rauta, P.R.; Samanta, M.; Dash, H.R.; Nayak, B.; Das, S. Toll-like receptors (TLRs) in aquatic animals: Signaling pathways, expressions and immune responses. Immunol. Lett. 2014, 158, 14–24. [Google Scholar] [CrossRef]
- Ong, C.-l.Y.; Gillen, C.M.; Barnett, T.C.; Walker, M.J.; McEwan, A.G. An Antimicrobial role for zinc in innate immune defense against group a Streptococcus. J. Infect. Dis. 2014, 209, 1500–1508. [Google Scholar] [CrossRef] [Green Version]
- Subramanian Vignesh, K.; Deepe, G.S., Jr. Metallothioneins: Emerging modulators in immunity and infection. Int. J. Mol. Sci. 2017, 18, 2197. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Zhang, R.; Wei, X.; Lv, M.; Jiang, Z. Chapter Seven—Metalloimmunology: The metal ion-controlled immunity. In Advances in Immunology; Dong, C., Jiang, Z., Eds.; Academic Press: New York, NY, USA, 2020; Volume 145, pp. 187–241. [Google Scholar]
- Stafford, S.L.; Bokil, N.J.; Achard, M.E.S.; Kapetanovic, R.; Schembri, M.A.; McEwan, A.G.; Sweet, M.J. Metal ions in macrophage antimicrobial pathways: Emerging roles for zinc and copper. Biosci. Rep. 2013, 33, e00049. [Google Scholar] [CrossRef]
- Chaigne-Delalande, B.; Lenardo, M.J. Divalent cation signaling in immune cells. Trends Immunol. 2014, 35, 332–344. [Google Scholar] [CrossRef] [Green Version]
- Núñez, G.; Sakamoto, K.; Soares, M.P. Innate nutritional immunity. J. Immunol. 2018, 201, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Hennigar, S.R.; McClung, J.P. Nutritional immunity: Starving pathogens of trace minerals. Am. J. Lifestyle Med. 2016, 10, 170–173. [Google Scholar] [CrossRef] [PubMed]





| Prototype | Feature ID | Fold Change | Lowest E-Value | Annotation |
|---|---|---|---|---|
| A | contig_0004466 | ↑4960.596 | 2 | RNA-binding protein 44 [Danio rerio] |
| contig_0004597 | ↑25.248 | 4 | Manganese-dependent ADP-ribose/CDP-alcohol diphosphatase [Danio rerio] | |
| contig_0020187 | ↑182.493 | 1 | Huntingtin [Takifugu rubripes] | |
| contig_0025250 | ↑21.707 | 6 | Protein Jumonji [Danio rerio] | |
| contig_0034576 | ↑24.093 | 5 | Transmembrane protein 208 [Danio rerio] | |
| contig_0046089 | ↑18.726 | 4 | Glucocorticoid receptor [Oncorhynchus mykiss] | |
| contig_0053965 | ↑18.575 | 0.7 | Protein broad-minded [Danio rerio] | |
| contig_0055996 | ↓−32.997 | 0.4 | Heparan-sulfate 6-O-sulfotransferase 1-B [Danio rerio] | |
| contig_0056158 | ↑19.593 | 5 | Neurexin-1b [Danio rerio] | |
| contig_0071724 | ↑29.905 | 0.6 | Disks large homolog 1[Danio rerio] | |
| contig_0071725 | ↓−43.599 | 0.3 | Rab-like protein 3 [Danio rerio] | |
| contig_0080432 | ↑57.479 | 1 × 10−5 | POC1 centriolar protein homolog A [Danio rerio] | |
| contig_0082059 | ↓−19.149 | 0.2 | Protein tilB homolog [Danio rerio] | |
| contig_0129067 | ↓−20.274 | 0.6 | Nuclear receptor ROR-alpha A [Danio rerio] | |
| contig_0151557 | ↓−27.038 | 0.5 | Cyclic nucleotide-gated cation channel [Ictalurus punctatus] | |
| contig_0163106 | ↓−23.455 | 7 × 10−144 | Trimeric intracellular cation channel type A [Danio rerio] | |
| contig_0203229 | ↓−19.214 | 1 | Cell surface hyaluronidase [Danio rerio] | |
| contig_0278209 | ↓−19.172 | 2 × 10−7 | Striated muscle preferentially expressed protein kinase [Danio rerio] | |
| contig_0322455 | ↓−32.997 | 0.9 | Creatine kinase, testis isozyme [Oncorhynchus mykiss] | |
| contig_0425609 | ↑75.365 | 0.6 | U11/U12 small nuclear ribonucleoprotein 35 kDa protein [Danio rerio] | |
| contig_0685545 | ↓−47.84 | 7 × 10−46 | Myosin heavy chain, fast skeletal muscle [Cyprinus carpio] | |
| B | contig_0004420 | ↓−36.35 | 0.7 | Sodium channel protein type 4 subunit alpha A [Takifugu rubripes] |
| contig_0014672 | ↓−36.35 | 0.07 | Centrosomal protein kizuna [Danio rerio] | |
| contig_0015979 | ↓−25.976 | 0.5 | PR domain zinc finger protein [Danio rerio] | |
| contig_0050531 | ↑195.503 | 0.8 | DENN domain-containing protein 11 [Danio rerio] | |
| contig_0059800 | ↓−29.103 | 4 | Heat shock 70 kDa protein 1 [Oryzias latipes] | |
| contig_0071672 | ↓−28.198 | 0.5 | Transmembrane protein 53 [Danio rerio] | |
| contig_0075736 | ↓24.574 | 1 | Ubiquitin carboxyl-terminal hydrolase 16 [Danio rerio] | |
| contig_0078326 | ↓−28.198 | 0.03 | Protein tweety homolog 3 [Danio rerio] | |
| contig_0091637 | ↑34.302 | 5 | Rab9 effector protein with kelch motifs [Danio rerio] | |
| contig_0096326 | ↑50.499 | 3 | Transmembrane protein 116 [Danio rerio] | |
| contig_0099678 | ↑210.084 | 2 | RNA-binding protein PNO1 [Oryzias latipes] | |
| contig_0111985 | ↑45.465 | 1 | Ankyrin repeat and IBR domain-containing protein 1 [Danio rerio] | |
| contig_0122208 | ↓−20.951 | 4 × 10−94 | Putative deoxyribonuclease tatdn3 [Danio rerio] | |
| contig_0133151 | ↑527.634 | 0.2 | E3 ubiquitin-protein ligase MYCBP2 [Danio rerio] | |
| contig_0158353 | ↑31.508 | 2 | Interferon regulatory factor 2-binding protein 2-B [Danio rerio] | |
| contig_0252619 | ↑48.853 | 0.06 | Polycomb protein suz12-B [Danio rerio] | |
| contig_0328706 | ↓−47.906 | 5 | E3 ubiquitin-protein ligase MYCBP2 [Danio rerio] | |
| contig_0389545 | ↑137.058 | 7 | NADH-ubiquinone oxidoreductase chain 1 [Gadus morhua] | |
| contig_0473006 | ↓−84.357 | 5 | Guanine nucleotide exchange protein smcr8a [Danio rerio] | |
| C | contig_0004540 | ↑28.043 | 2 | Pyridoxal-dependent decarboxylase domain-containing protein 1 [Danio rerio] |
| contig_0022126 | ↓−34.603 | 0.2 | CD166 antigen homolog A [Danio rerio] | |
| contig_0047184 | ↓−19.98 | 0.2 | Calcium/calmodulin-dependent protein kinase type II delta 2 chain [Danio rerio] | |
| contig_0055363 | ↑18.009 | 3 | V(D)J recombination-activating protein 2 [Oncorhynchus mykiss] | |
| contig_0094127 | ↑20.876 | 5 | Poly(A)-specific ribonuclease PARN [Danio rerio] | |
| contig_0098235 | ↑18.725 | 1 | Neurexin-1b [Danio rerio] | |
| contig_0104613 | ↑18.725 | 0.2 | Src kinase-associated phosphoprotein 1 [Takifugu rubripes] | |
| contig_0105909 | ↑21.592 | 0.2 | RING finger protein 145 [Danio rerio] | |
| contig_0112666 | ↓−21.083 | 1 | Ribonucleoside-diphosphate reductase large subunit [Danio rerio] | |
| contig_0117074 | ↓−18.745 | 0.6 | Sodium channel protein type 4 subunit alpha B [Takifugu rubripes] | |
| contig_0134124 | ↓−19.98 | 0.8 | E3 ubiquitin-protein ligase TRIP12 [Danio rerio] | |
| contig_0137098 | ↓−18.878 | 1 | Threonine synthase-like 2 [Danio rerio] | |
| contig_0145112 | ↓−21.083 | 1 | Sodium channel protein type 4 subunit alpha B [Danio rerio] | |
| contig_0158020 | ↑20.159 | 1 × 10−7 | Contactin-5 [Danio rerio] | |
| contig_0158413 | ↓−21.083 | 5 | Treslin [Danio rerio] | |
| contig_0292034 | ↑26.614 | 10 | Alpha-protein kinase 2 [Danio rerio] | |
| contig_0402943 | ↑54.564 | 4 | RAB11-binding protein RELCH homolog [Danio rerio] | |
| contig_0430511 | ↓−18.878 | 2 × 10−18 | Creatine kinase, testis isozyme [Oncorhynchus mykiss] | |
| contig_0540911 | ↓−69.586 | 1 | N-acetylneuraminate 9-O-acetyltransferase [Danio rerio] |
| Prototype | Feature ID | Fold Change | Lowest E-Value | Annotation |
|---|---|---|---|---|
| A | contig_0000167 | ↓−606.592 | 8 × 10−23 | Formin-like protein 3 [Danio rerio] |
| contig_0008746 | ↓−527.023 | 0.4 | Serine/threonine-protein kinase N2 [Danio rerio] | |
| contig_0012767 | ↓−606.592 | 5 | Transmembrane protein 198-B [Danio rerio] | |
| contig_0039965 | ↓−575.988 | 1 | Acylglycerol kinase, mitochondrial [Danio rerio] | |
| contig_0046974 | ↓−594.350 | 2 | Xenotropic and polytropic retrovirus receptor 1 homolog [Danio rerio] | |
| contig_0058658 | ↓−563.747 | 2 × 10−11 | Melanocortin-2 receptor accessory protein 2B [Danio rerio] | |
| contig_0061338 | ↓−575.988 | 2 | Hemoglobin subunit beta [Thunnus thynnus] | |
| contig_0083287 | ↓−588.230 | 2 | Neurexin-3b [Danio rerio] | |
| contig_0096893 | ↑40,425.799 | 7 × 10−131 | Elongation factor 1-alpha [Danio rerio] | |
| contig_0097840 | ↓−545.385 | 0 | V(D) J recombination-activating protein 1 [Oncorhynchus mykis] | |
| contig_0143302 | ↓−533.143 | 0.6 | Polycystin-2 [Oryzias latipes] | |
| contig_0185421 | ↑45,501.610 | 6 | Estrogen receptor [Ictalurus punctatus] | |
| contig_0321861 | ↑227,716.450 | 0 | Cytochromec oxidase subunit 1 [Danio rerio] | |
| contig_0552888 | ↑49,171.662 | 5 × 10−3 | Vitellogenin-1 [Fundulus heteroclitus] | |
| contig_0571513 | ↑33,289.567 | 9 | Lysine-specific demethylase phf2 [Dicentrarchus labrax] | |
| contig_0571697 | ↑36,111.859 | 1 | Thrombospondin type-1 domain-containing protein 7A [Danio rerio] | |
| contig_0571705 | ↑28,449.821 | 2 × 10−24 | NADH-ubiquinone oxidoreductase chain 5 [Carassius auratus] | |
| contig_0571764 | ↑39,320.471 | 2 × 10−3 | Dynein heavy chain (Fragment) [Oncorhynchus mykiss] | |
| contig_0571817 | ↑58,078.512 | 3 | Unconventional myosin-IXAa [Danio rerio] | |
| contig_0571876 | ↑30,252.653 | 9 | Sodium channel protein type 4 subunit alpha B [Danio rerio] | |
| B | contig_0016642 | ↓−38.578 | 6 | Collagen alpha-1(XXVII) chain B [Danio rerio] |
| contig_0020177 | ↓−34.554 | 8 | Xin actin-binding repeat-containing protein 1 [Danio rerio] | |
| contig_0030309 | ↓−31.679 | 2 × 10−13 | Palmitoyltransferase [Danio rerio] | |
| contig_0035782 | ↓−36.278 | 0.3 | CCR4-NOT transcription complex subunit 10 [Danio rerio] | |
| contig_0035986 | ↓−34.554 | 2 × 10−74 | Complement component C9 (Fragment) [Oncorhynchus mykiss] | |
| contig_0055961 | ↓−31.104 | −0.8 | 3-hydroxybutyrate dehydrogenase type 2 [Danio rerio] | |
| contig_0062345 | ↑2392.329 | 0.2 | Neuroblastoma-amplified sequence [Danio rerio] | |
| contig_0066929 | ↓−38.578 | 0.5 | Disks large-associated protein 1 [Danio rerio] | |
| contig_0075266 | ↓−35.128 | 0.1 | Eukaryotic translation initiation factor [Danio rerio] | |
| contig_0102002 | ↓−29.954 | 0.6 | Presequence protease, mitochondrial [Danio rerio] | |
| contig_0164965 | ↑2915.165 | 0.03 | Methionine-tRNA ligase, mitochondrial [Takifugu rubripes] | |
| contig_0236931 | ↑2900.227 | 9 | CCR4-NOT transcription complex subunit 1 [Danio rerio] | |
| contig_0287222 | ↓−34.554 | 0.9 | Striatin interacting protein 1 homolog [Danio rerio] | |
| contig_0359850 | ↑2213.070 | 0.3 | Gonadotropin subunit beta-2 [Anguila anguilla] | |
| contig_0454328 | ↑2168.256 | 0.9 | Paired box protein Pax-6 [Oryzias latipes] | |
| contig_0492154 | ↑2332.576 | 7 × 10−10 | WAP, Kazal, immunoglobulin, Kunitz and NTR domain-containing protein [Danio rerio] | |
| contig_0557190 | ↑2511.834 | 1 | Neuron navigator 3 [Danio rerio] | |
| contig_0572018 | ↑2646.278 | 1 | 2-oxoglutarate and iron-dependent oxygenase domain-containing protein 2 [Danio rerio] | |
| contig_0581749 | ↑2362.452 | 4 × 10−15 | Creatine kinase, testisisozyme [Oncorhynchus mykiss] | |
| contig_0635620 | ↑2930.103 | 6 | Lactosylceramide 1,3-Nacetyl-beta-D-glucosaminyl transterase B [Danio rerio] | |
| C | contig_0002190 | ↓−27.536 | 2 | Somatolactin [Cyclopterus lumpus] |
| contig_0010441 | ↓−28.054 | 1 | Ubiquinol-cytochrome-c reductase complex assembly factor 3 [Danio rerio] | |
| contig_0013985 | ↓−28.572 | 4 | Insulin-like growth factor 1, adult form [Cyprinus carpio] | |
| contig_0038889 | ↓−33.756 | 8 | Plexin-A4 [Danio rerio] | |
| contig_0042915 | ↓−28.572 | 2 | SUMO-specific isopeptidase USPL1 [Danio rerio] | |
| contig_0052507 | ↓−35.830 | 1 × 10−4 | Pyridine nucleotide-disulfide oxidoreductase domain-containing protein [Danio reric] | |
| contig_0068071 | ↓−29.091 | 1 | Probable serpin E3 [Danio rerio] | |
| contig_0085140 | ↓−25.980 | 0.5 | CCR4-NOT transcription complex subunit 9 [Danio rerio] | |
| contig_0130362 | ↑1779.731 | 1 | NADH-ubiquinone oxidoreductase chain 2 [Salmo solar] | |
| contig_0141833 | ↓−30.128 | 2 | Neuropilin-1a [Danio rerio] | |
| contig_0153864 | ↓−29.609 | 5 | Polyribonucleotide 5′-hydroxyl-kinase Clp1 [Danio rerio] | |
| contig_0264422 | ↑1765.159 | 2 | Semaphorin-3D [Danio rerio] | |
| contig_0415197 | ↑1677.727 | 0.3 | Vascular endothelial growth factor receptor 2 [Danio rerio] | |
| contig_0434444 | ↑1619.438 | 0.2 | DNA excision repair protein ERCC-6-like [Danio rerio] | |
| contig_0491119 | ↑1692.299 | 1 | Glucagon family neuropeptides [Clarios macrocephalus] | |
| contig_0531987 | ↑2260.609 | 2 × 10−46 | Sarcoplasmic/endoplasmic reticulum calcium ATPase 1 [Makaira nigricans] | |
| contig_0561562 | ↑1590.294 | 0.5 | Membrane progestin receptor alpha [Cynoscion nebulosus] | |
| contig_0572520 | ↑1983.740 | 3 | Protein jagunal homolog 1A [Danio rerio] | |
| contig_0577243 | ↑1910.879 | 2 | Galectin-3-binding protein A [Donio rerio] | |
| contig_0596536 | ↑1867.163 | 0.4 | POU domain, class 6, transcription factor 1 [Danio rerio] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casuso, A.; Valenzuela-Muñoz, V.; Benavente, B.P.; Valenzuela-Miranda, D.; Gallardo-Escárate, C. Exploring Sea Lice Vaccines against Early Stages of Infestation in Atlantic Salmon (Salmo salar). Vaccines 2022, 10, 1063. https://doi.org/10.3390/vaccines10071063
Casuso A, Valenzuela-Muñoz V, Benavente BP, Valenzuela-Miranda D, Gallardo-Escárate C. Exploring Sea Lice Vaccines against Early Stages of Infestation in Atlantic Salmon (Salmo salar). Vaccines. 2022; 10(7):1063. https://doi.org/10.3390/vaccines10071063
Chicago/Turabian StyleCasuso, Antonio, Valentina Valenzuela-Muñoz, Bárbara P. Benavente, Diego Valenzuela-Miranda, and Cristian Gallardo-Escárate. 2022. "Exploring Sea Lice Vaccines against Early Stages of Infestation in Atlantic Salmon (Salmo salar)" Vaccines 10, no. 7: 1063. https://doi.org/10.3390/vaccines10071063
APA StyleCasuso, A., Valenzuela-Muñoz, V., Benavente, B. P., Valenzuela-Miranda, D., & Gallardo-Escárate, C. (2022). Exploring Sea Lice Vaccines against Early Stages of Infestation in Atlantic Salmon (Salmo salar). Vaccines, 10(7), 1063. https://doi.org/10.3390/vaccines10071063








