The Frequency and Patterns of Post-COVID-19 Vaccination Syndrome Reveal Initially Mild and Potentially Immunocytopenic Signs in Primarily Young Saudi Women
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population
2.3. Data Collection Tool
2.4. The Questionnaire: Development and Validation of the Questionnaire
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saudagar, V.; Patil, S.; Goh, S.; Pothiawala, S. Vigilance Regarding Immune Thrombocytopenic Purpura after COVID-19 Vaccine. Ir. J. Med. Sci. 2022, 191, 919. [Google Scholar] [CrossRef]
- Favaloro, E.J. Laboratory Testing for Suspected COVID-19 Vaccine–Induced (Immune) Thrombotic Thrombocytopenia. Int. J. Lab. Hematol. 2021, 43, 559–570. [Google Scholar] [CrossRef]
- Jawed, M.; Khalid, A.; Rubin, M.; Shafiq, R.; Cemalovic, N. Acute Immune Thrombocytopenia (ITP) Following COVID-19 Vaccination in a Patient with Previously Stable ITP. Open Forum. Infect. Dis. 2021, 8, ofab343. [Google Scholar] [CrossRef]
- Sharifian-Dorche, M.; Bahmanyar, M.; Sharifian-Dorche, A.; Mohammadi, P.; Nomovi, M.; Mowla, A. Vaccine-Induced Immune Thrombotic Thrombocytopenia and Cerebral Venous Sinus Thrombosis Post COVID-19 Vaccination; A Systematic Review. J. Neurol. Sci. 2021, 428, 117607. [Google Scholar] [CrossRef]
- Akiyama, H.; Kakiuchi, S.; Rikitake, J.; Matsuba, H.; Sekinada, D.; Kozuki, Y.; Iwata, N. Immune Thrombocytopenia Associated with Pfizer-BioNTech’s BNT162b2 MRNA COVID-19 Vaccine. IDCases 2021, 25, e01245. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Banerjee, M. Immune Thrombocytopenia Secondary to COVID-19: A Systematic Review. SN Compr. Clin. Med. 2020, 2, 2048. [Google Scholar] [CrossRef]
- Shah, S.R.A.; Dolkar, S.; Mathew, J.; Vishnu, P. COVID-19 Vaccination Associated Severe Immune Thrombocytopenia. Exp. Hematol. Oncol. 2021, 10, 42. [Google Scholar] [CrossRef]
- Fueyo-Rodriguez, O.; Valente-Acosta, B.; Jimenez-Soto, R.; Neme-Yunes, Y.; Inclán-Alarcón, S.I.; Trejo-Gonzalez, R.; García-Salcido, M.Á. Secondary Immune Thrombocytopenia Supposedly Attributable to COVID-19 Vaccination. BMJ Case Rep. 2021, 14, e242220. [Google Scholar] [CrossRef]
- Candelli, M.; Rossi, E.; Valletta, F.; de Stefano, V.; Franceschi, F. Immune Thrombocytopenic Purpura after SARS-CoV-2 Vaccine. Br. J. Haematol. 2021, 194, 547–549. [Google Scholar] [CrossRef]
- Perry, R.J.; Tamborska, A.; Singh, B.; Craven, B.; Marigold, R.; Arthur-Farraj, P.; Yeo, J.M.; Zhang, L.; Hassan-Smith, G.; Jones, M.; et al. Cerebral Venous Thrombosis after Vaccination against COVID-19 in the UK: A Multicentre Cohort Study. Lancet 2021, 398, 1147–1156. [Google Scholar] [CrossRef]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 NCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef]
- McCrae, K.R. Thrombotic Thrombocytopenia Due to SARS-CoV-2 Vaccination. Clevel. Clin. J. Med. 2021. [Google Scholar] [CrossRef]
- Welsh, K.J.; Baumblatt, J.; Chege, W.; Goud, R.; Nair, N. Thrombocytopenia Including Immune Thrombocytopenia after Receipt of MRNA COVID-19 Vaccines Reported to the Vaccine Adverse Event Reporting System (VAERS). Vaccine 2021, 39, 3329–3332. [Google Scholar] [CrossRef]
- Lee, E.J.; Cines, D.B.; Gernsheimer, T.; Kessler, C.; Michel, M.; Tarantino, M.D.; Semple, J.W.; Arnold, D.M.; Godeau, B.; Lambert, M.P.; et al. Thrombocytopenia Following Pfizer and Moderna SARS-CoV-2 Vaccination. Am. J. Hematol. 2021, 96, 534–537. [Google Scholar] [CrossRef]
- Tarawneh, O.; Tarawneh, H. Immune Thrombocytopenia in a 22-Year-Old Post Covid-19 Vaccine. Am. J. Hematol. 2021, 96, E133–E134. [Google Scholar] [CrossRef]
- Toom, S.; Wolf, B.; Avula, A.; Peeke, S.; Becker, K. Familial Thrombocytopenia Flare-up Following the First Dose of MRNA-1273 Covid-19 Vaccine. Am. J. Hematol. 2021, 96, E134–E135. [Google Scholar] [CrossRef]
- Shazley, O.; Alshazley, M. A COVID-Positive 52-Year-Old Man Presented with Venous Thromboembolism and Disseminated Intravascular Coagulation Following Johnson & Johnson Vaccination: A Case-Study. Cureus 2021, 13, e16383. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 NCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Gambichler, T.; Boms, S.; Susok, L.; Dickel, H.; Finis, C.; Abu Rached, N.; Barras, M.; Stücker, M.; Kasakovski, D. Cutaneous Findings Following COVID-19 Vaccination: Review of World Literature and Own Experience. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 172–180. [Google Scholar] [CrossRef]
- Català, A.; Muñoz-Santos, C.; Galván-Casas, C.; Roncero Riesco, M.; Revilla Nebreda, D.; Solá-Truyols, A.; Giavedoni, P.; Llamas-Velasco, M.; González-Cruz, C.; Cubiró, X.; et al. Cutaneous Reactions after SARS-CoV-2 Vaccination: A Cross-Sectional Spanish Nationwide Study of 405 Cases. Br. J. Dermatol. 2022, 186, 142–152. [Google Scholar] [CrossRef]
- Yamashita, S.; Katsuki, N.E.; Tago, M.; Yamashita, S.I. Dyspnea and Wheezing as the Earliest Manifestations of Severe Fever with Thrombocytopenia Syndrome: The First Case Report. Intern. Med. 2019, 58, 2731. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.J.; Liang, M.F.; Zhang, S.Y.; Liu, Y.; Li, J.D.; Sun, Y.L.; Zhang, L.; Zhang, Q.F.; Popov, V.L.; Li, C.; et al. Fever with Thrombocytopenia Associated with a Novel Bunyavirus in China. N. Engl. J. Med. 2011, 364, 81–84. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; He, B.; Huang, S.Y.; Wei, F.; Zhu, X.Q. Severe Fever with Thrombocytopenia Syndrome, an Emerging Tick-Borne Zoonosis. Lancet Infect. Dis. 2014, 14, 763–772. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, H.; Gao, J.; Zhou, X.; Zhu, R.; Zhang, C.; Bai, H.; Abdullah, A.S.; Pan, H. Two Confirmed Cases of Severe Fever with Thrombocytopenia Syndrome with Pneumonia: Implication for a Family Cluster in East China. BMC Infect. Dis. 2017, 17, 537. [Google Scholar] [CrossRef] [Green Version]
- Frith, J.; Watson, S.; Maggs, P.H.B.B.; Newton, J.L. Cognitive Symptoms Are Common in Immune Thrombocytopenia and Associate with Autonomic Symptom Burden. Eur. J. Haematol. 2012, 88, 224–228. [Google Scholar] [CrossRef]
- Awan, H.A.; Najmuddin Diwan, M.; Aamir, A.; Ali, M.; di Giannantonio, M.; Ullah, I.; Shoib, S.; de Berardis, D. SARS-CoV-2 and the Brain: What Do We Know about the Causality of ’Cognitive COVID? J. Clin. Med. 2021, 10, 3441. [Google Scholar] [CrossRef]
- European Medicines Agency. AstraZeneca’s COVID-19 Vaccine: EMA Finds Possible Link to Very Rare Cases of Unusual Blood Clots with Low Blood Platelets. Available online: https://www.ema.europa.eu/en/news/astrazenecas-covid-19-vaccine-ema-finds-possible-link-very-rare-cases-unusual-blood-clots-low-blood (accessed on 13 May 2022).
- Douxfils, J.; Favresse, J.; Dogné, J.M.; Lecompte, T.; Susen, S.; Cordonnier, C.; Lebreton, A.; Gosselin, R.; Sié, P.; Pernod, G.; et al. Hypotheses behind the Very Rare Cases of Thrombosis with Thrombocytopenia Syndrome after SARS-CoV-2 Vaccination. Thromb. Res. 2021, 203, 163. [Google Scholar] [CrossRef]
- Pottegård, A.; Lund, L.C.; Karlstad, Ø.; Dahl, J.; Andersen, M.; Hallas, J.; Lidegaard, Ø.; Tapia, G.; Gulseth, H.L.; Ruiz, P.L.D.; et al. Arterial Events, Venous Thromboembolism, Thrombocytopenia, and Bleeding after Vaccination with Oxford-AstraZeneca ChAdOx1-S in Denmark and Norway: Population Based Cohort Study. BMJ 2021, 373, n1114. [Google Scholar] [CrossRef]
- Nguyen, T.N.; Siegler, J.E.; Yaghi, S.; Vigilante, N.; Abdalkader, M.; Coutinho, J.M.; Abdul Khalek, F. Cerebral Vein Thrombosis with Vaccine-Induced Immune Thrombotic Thrombocytopenia. Stroke 2021, 52, 3045–3053. [Google Scholar] [CrossRef]
- Fernandez-Nieto, D.; Haemmerle, J.; Fernández Escribano, M. Skin Manifestations of the BNT162b2 MRNA COVID-19 Vaccine in Healthcare Workers. “COVID-Arm”: A Clinical and Histological Characterization. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e425–e427. [Google Scholar] [CrossRef]
- Rinaldi, M.; Perricone, C.; Ortega-Hernandez, O.D.; Perricone, R.; Shoenfeld, Y. Immune Thrombocytopaenic Purpura: An Autoimmune Cross-Link between Infections and Vaccines. Lupus 2014, 23, 554–567. [Google Scholar] [CrossRef]
- Hamiel, U.; Kventsel, I.; Youngster, I. Recurrent Immune Thrombocytopenia After Influenza Vaccination: A Case Report. Pediatrics 2016, 138, e20160124. [Google Scholar] [CrossRef] [Green Version]
- Mantadakis, E.; Farmaki, E.; Thomaidis, S.; Tsalkidis, A.; Chatzimichael, A. A Case of Immune Thrombocytopenic Purpura after Influenza Vaccination: Consequence or Coincidence? J. Pediatric Hematol. Oncol. 2010, 32, e227–e229. [Google Scholar] [CrossRef]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; Baker, S.; Dougan, G.; et al. Age-Related Immune Response Heterogeneity to SARS-CoV-2 Vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef]
- Jasaraj, R.B.; Shrestha, D.B.; Gaire, S.; Kassem, M. Immune Thrombocytopenic Purpura Following Pfizer-BioNTech COVID-19 Vaccine in an Elderly Female. Cureus 2021, 13, e16871. [Google Scholar] [CrossRef]
- Nachtigall, I.; Bonsignore, M.; Hohenstein, S.; Bollmann, A.; Günther, R.; Kodde, C.; Englisch, M.; Ahmad-Nejad, P.; Schröder, A.; Glenz, C.; et al. Effect of Gender, Age and Vaccine on Reactogenicity and Incapacity to Work after COVID-19 Vaccination: A Survey among Health Care Workers. BMC Infect. Dis. 2022, 22, 291. [Google Scholar] [CrossRef]
- Anjorin, A.A.; Odetokun, I.A.; Nyandwi, J.B.; Elnadi, H.; Awiagah, K.S.; Eyedo, J.; Abioye, A.I.; Gachara, G.; Maisara, A.M.; Razouqi, Y.; et al. Public Health Surveillance for Adverse Events Following COVID-19 Vaccination in Africa. Vaccines 2022, 10, 546. [Google Scholar] [CrossRef]
- Green, M.S.; Peer, V.; Magid, A.; Hagani, N.; Anis, E.; Nitzan, D. Gender Differences in Adverse Events Following the Pfizer-BioNTech COVID-19 Vaccine. Vaccines 2022, 10, 233. [Google Scholar] [CrossRef]
- Hu, Z.; van der Ploeg, K.; Chakraborty, S.; Arunachalam, P.; Mori, D.; Jacobson, K.; Bonilla, H.; Parsonnet, J.; Andrews, J.; Hedlin, H.; et al. Early Immune Responses Have Long-Term Associations with Clinical, Virologic, and Immunologic Outcomes in Patients with COVID-19. Res. Sq. 2022, preprint. [CrossRef]
- Malayala, S.V.; Mohan, G.; Vasireddy, D.; Atluri, P. Purpuric Rash and Thrombocytopenia After the MRNA-1273 (Moderna) COVID-19 Vaccine. Cureus 2021, 13, e14099. [Google Scholar] [CrossRef]
- Kong, J.; Cuevas-Castillo, F.; Nassar, M.; Lei, C.M.; Idrees, Z.; Fix, W.C.; Halverstam, C.; Mir, A.; Elbendary, A.; Mathew, A. Bullous Drug Eruption after Second Dose of MRNA-1273 (Moderna) COVID-19 Vaccine: Case Report. J. Infect. Public Health 2021, 14, 1392–1394. [Google Scholar] [CrossRef]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 Vaccines: Comparison of Biological, Pharmacological Characteristics and Adverse Effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1679. [Google Scholar] [CrossRef]
- Neylon, A.J.; Saunders, P.W.G.; Howard, M.R.; Proctor, S.J.; Taylor, P.R.A. Clinically Significant Newly Presenting Autoimmune Thrombocytopenic Purpura in Adults: A Prospective Study of a Population-Based Cohort of 245 Patients. Br. J. Haematol. 2003, 122, 966–974. [Google Scholar] [CrossRef] [Green Version]
- Abrahamson, P.E.; Hall, S.A.; Feudjo-Tepie, M.; Mitrani-Gold, F.S.; Logie, J. The Incidence of Idiopathic Thrombocytopenic Purpura among Adults: A Population-Based Study and Literature Review. Eur. J. Haematol. 2009, 83, 83–89. [Google Scholar] [CrossRef]
- Sarpatwari, A.; Bennett, D.; Logie, J.W.; Shukla, A.; Beach, K.J.; Newland, A.C.; Sanderson, S.; Provan, D. Thromboembolic Events among Adult Patients with Primary Immune Thrombocytopenia in the United Kingdom General Practice Research Database. Haematologica 2010, 95, 1167–1175. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Nardi, M.A.; Borkowsky, W.; Li, Z.; Karpatkin, S. Role of Molecular Mimicry of Hepatitis C Virus Protein with Platelet GPIIIa in Hepatitis C-Related Immunologic Thrombocytopenia. Blood 2009, 113, 4086–4093. [Google Scholar] [CrossRef] [Green Version]
- Aster, R.H. Molecular Mimicry and Immune Thrombocytopenia. Blood 2009, 113, 3887–3888. [Google Scholar] [CrossRef]
- Au, L.; Fendler, A.; Shepherd, S.T.C.; Rzeniewicz, K.; Cerrone, M.; Byrne, F.; Carlyle, E.; Edmonds, K.; del Rosario, L.; Shon, J.; et al. Cytokine Release Syndrome in a Patient with Colorectal Cancer after Vaccination with BNT162b2. Nat. Med. 2021, 27, 1362–1366. [Google Scholar] [CrossRef]
- Ihtesham, A.; Maqbool, S.; Nadeem, M.; Janjua, M.B.A.; Sundus, O.; Naqqash, A.B.; Mohamed, W.I.; Haider, S.T.; Ahmad, M.; Mustafa, M.A.T.; et al. Helicobacter pylori induced Immune Thrombocytopenic Purpura and perspective role of Helicobacter pylori eradication therapy for treating Immune Thrombocytopenic Purpura. AIMS Microbiol. 2021, 7, 284–303. [Google Scholar] [CrossRef]
- Fogagnolo, A.; Campo, G.C.; Mari, M.; Pompei, G.; Pavasini, R.; Volta, C.A.; Spadaro, S. The Underestimated Role of Platelets in Severe Infection a Narrative Review. Cells 2022, 11, 424. [Google Scholar] [CrossRef]
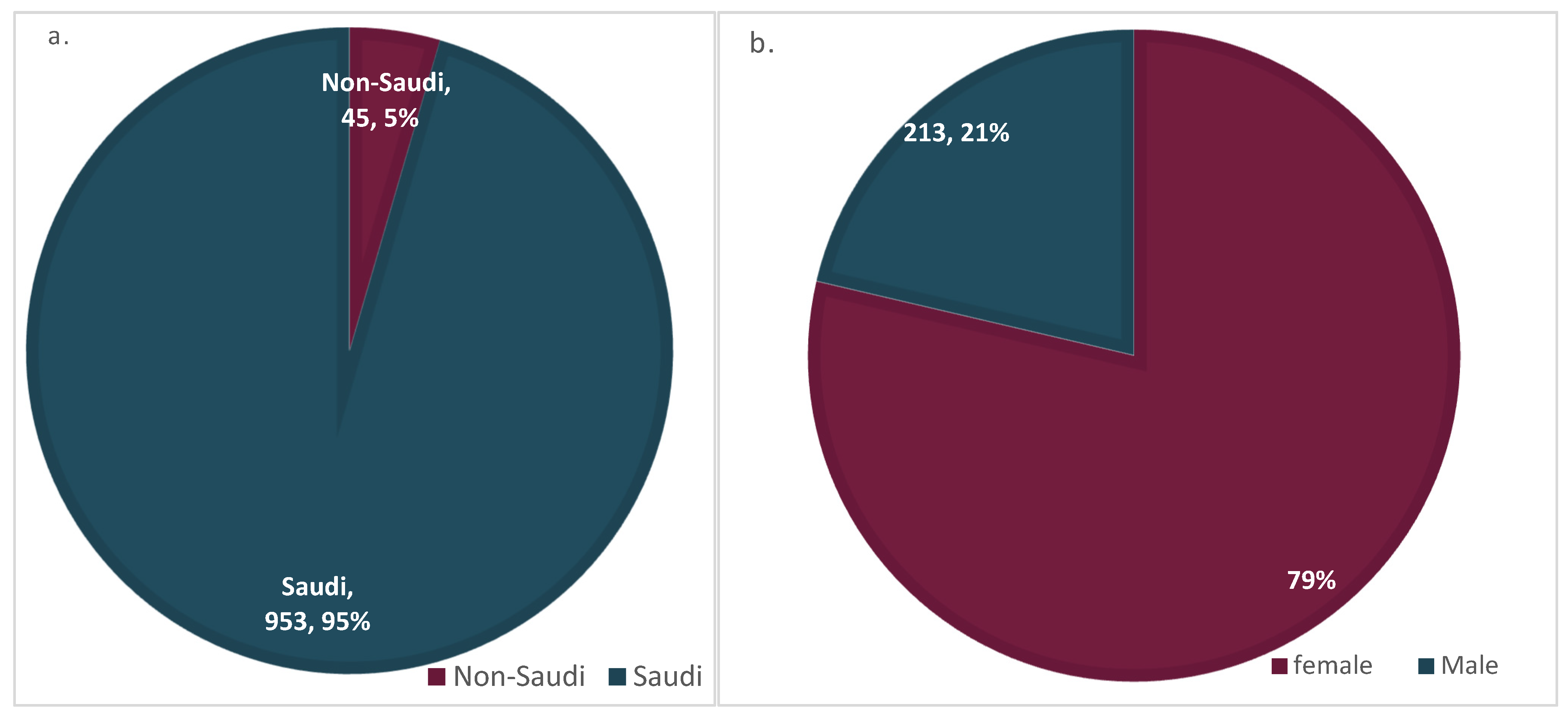

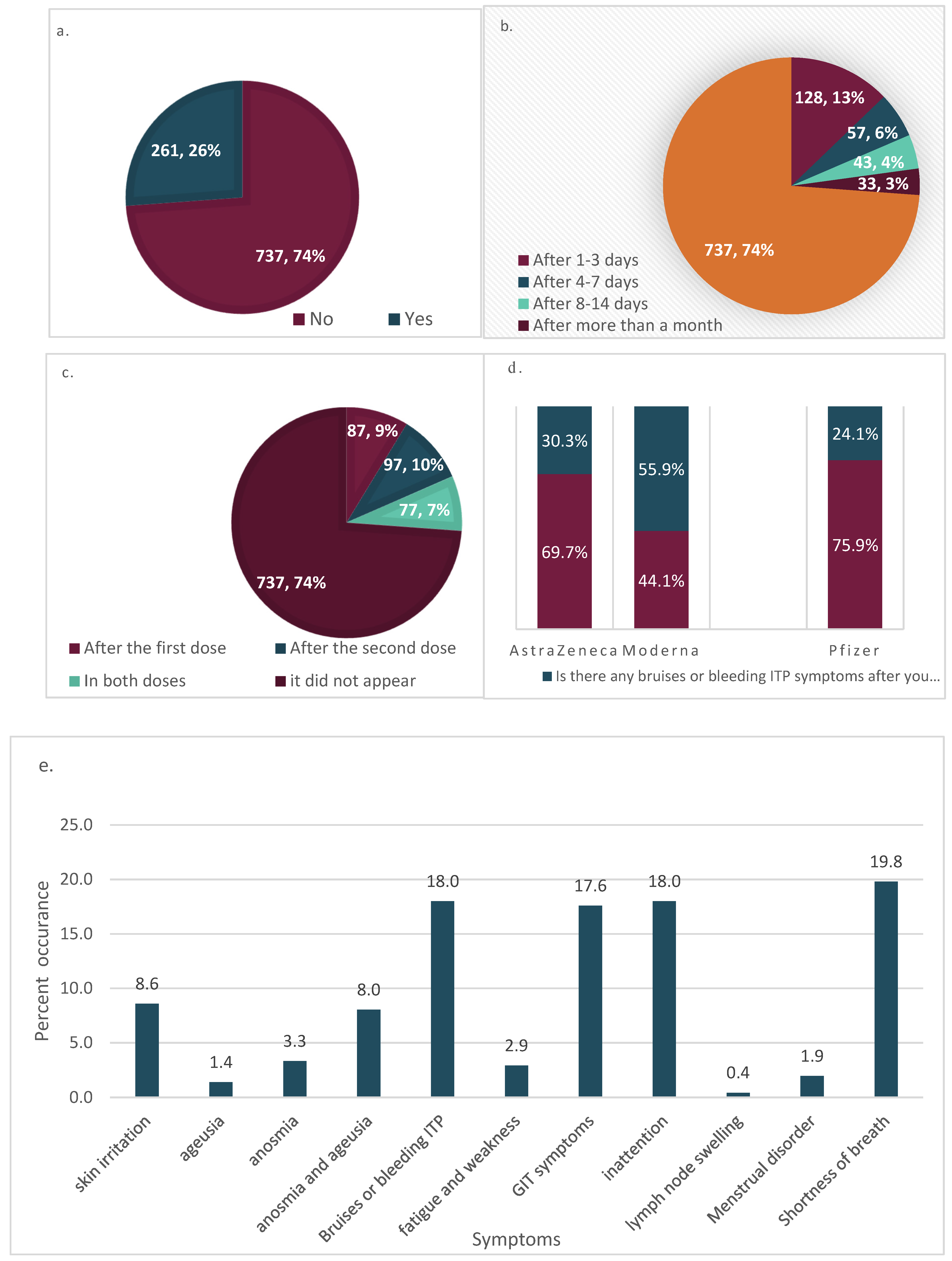
| Frequency | Percent | Valid Percent | Cumulative Percent | ||
|---|---|---|---|---|---|
| Valid | AstraZeneca | 155 | 15.5 | 15.5 | 15.5 |
| Moderna | 34 | 3.4 | 3.4 | 18.9 | |
| Pfizer | 809 | 81.1 | 81.1 | 100.0 | |
| Total | 998 | 100.0 | 100.0 | ||
| Onset Times after Symptoms | Overall Frequency of Bruises or Bleeding ITP Symptoms | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency | Percent | Valid Percent | Cumulative Percent | Frequency | Percent | Valid Percent | Cumulative Percent | ||||
| Valid | After 1–3 days | 128 | 12.8 | 12.8 | 12.8 | Valid | |||||
| After 4–7 days | 57 | 5.7 | 5.7 | 18.5 | |||||||
| After 8–14 days | 43 | 4.3 | 4.3 | 22.8 | No | 737 | 73.8 | 73.8 | 73.8 | ||
| After > month | 33 | 3.3 | 3.3 | 26.2 | Yes | 261 | 26.2 | 26.2 | 100.0 | ||
| it did not appear | 737 | 73.8 | 73.8 | 100.0 | |||||||
| Total | 998 | 100.0 | 100.0 | Total | 998 | 100.0 | 100.0 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Said, K.B.; Al-Otaibi, A.; Aljaloud, L.; Al-Anazi, B.; Alsolami, A.; Alreshidi, F.S.; on behalf of the Ha’il COM Research Unit Group. The Frequency and Patterns of Post-COVID-19 Vaccination Syndrome Reveal Initially Mild and Potentially Immunocytopenic Signs in Primarily Young Saudi Women. Vaccines 2022, 10, 1015. https://doi.org/10.3390/vaccines10071015
Said KB, Al-Otaibi A, Aljaloud L, Al-Anazi B, Alsolami A, Alreshidi FS, on behalf of the Ha’il COM Research Unit Group. The Frequency and Patterns of Post-COVID-19 Vaccination Syndrome Reveal Initially Mild and Potentially Immunocytopenic Signs in Primarily Young Saudi Women. Vaccines. 2022; 10(7):1015. https://doi.org/10.3390/vaccines10071015
Chicago/Turabian StyleSaid, Kamaleldin B., Amal Al-Otaibi, Luluh Aljaloud, Basmah Al-Anazi, Ahmed Alsolami, Fayez Saud Alreshidi, and on behalf of the Ha’il COM Research Unit Group. 2022. "The Frequency and Patterns of Post-COVID-19 Vaccination Syndrome Reveal Initially Mild and Potentially Immunocytopenic Signs in Primarily Young Saudi Women" Vaccines 10, no. 7: 1015. https://doi.org/10.3390/vaccines10071015
APA StyleSaid, K. B., Al-Otaibi, A., Aljaloud, L., Al-Anazi, B., Alsolami, A., Alreshidi, F. S., & on behalf of the Ha’il COM Research Unit Group. (2022). The Frequency and Patterns of Post-COVID-19 Vaccination Syndrome Reveal Initially Mild and Potentially Immunocytopenic Signs in Primarily Young Saudi Women. Vaccines, 10(7), 1015. https://doi.org/10.3390/vaccines10071015






