The Role of Healthcare Providers in Promoting Human Papillomavirus Vaccines among Men Who Have Sex with Men: A Scoping Review
Abstract
:1. Introduction
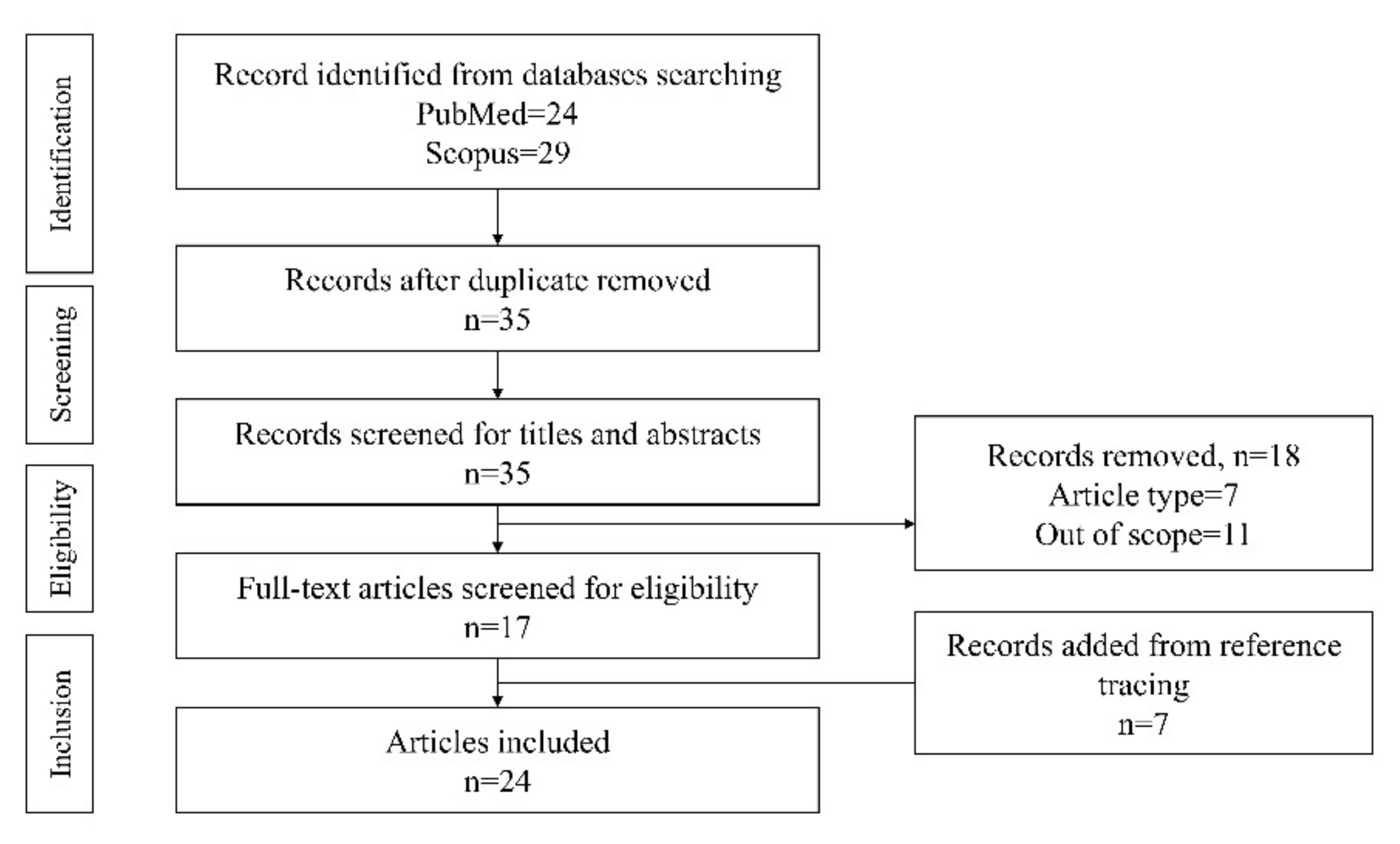
2. Literature Search
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boda, D.; Docea, A.O.; Calina, D.; Ilie, M.A.; Caruntu, C.; Zurac, S.; Neagu, M.; Constantin, C.; Branisteanu, D.E.; Voiculescu, V.; et al. Human papilloma virus: Apprehending the link with carcinogenesis and unveiling new research avenues (Review). Int. J. Oncol. 2018, 52, 637–655. [Google Scholar] [CrossRef] [PubMed]
- Petca, A.; Borislavschi, A.; Zvanca, M.E.; Petca, R.-C.; Sandru, F.; Dumitrascu, M.C. Non-sexual HPV transmission and role of vaccination for a better future (Review). Exp. Ther. Med. 2020, 20, 186. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef]
- Cutts, F.T.; Franceschi, S.; Goldie, S.; Castellsague, X.; de Sanjose, S.; Garnett, G.; Edmunds, W.J.; Claeys, P.; Goldenthal, K.L.; Harper, D.M.; et al. Human papillomavirus and HPV vaccines: A review. Bull. World Health Organ. 2007, 85, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Boakye, E.A.; Tobo, B.B.; Rojek, R.P.; Mohammed, K.; Geneus, C.J.; Osazuwa-Peters, N. Approaching a decade since HPV vaccine licensure: Racial and gender disparities in knowledge and awareness of HPV and HPV vaccine. Hum. Vaccines Immunother. 2017, 13, 2713–2722. [Google Scholar] [CrossRef]
- Wei, F.; Gaisa, M.M.; D’Souza, G.; Xia, N.; Giuliano, A.R.; Hawes, S.E.; Gao, L.; Cheng, S.-H.; Donà, M.G.; Goldstone, S.E.; et al. Epidemiology of anal human papillomavirus infection and high-grade squamous intraepithelial lesions in 29 900 men according to HIV status, sexuality, and age: A collaborative pooled analysis of 64 studies. Lancet HIV 2021, 8, e531–e543. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, Y.; Du, J. Human papillomavirus vaccines: An updated review. Vaccines 2020, 8, 391. [Google Scholar] [CrossRef]
- Centre of Disease Control and Prevention. FDA Licensure of Bivalent Human Papillomavirus Vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR. Morb. Mortal. Wkly. Rep. 2010, 59, 626–629. [Google Scholar]
- Centre of Disease Control and Prevention. Recommendations on the use of Quadrivalent Human Papillomavirus Vaccine in Males—Advisory Committee on Immunization Practices (ACIP). MMWR. Morb. Mortal. Wkly. Rep. 2011, 60, 1705–1708. [Google Scholar]
- Meites, E.; Szilagyi, P.G.; Chesson, H.W.; Unger, E.R.; Romero, J.R.; Markowitz, L.E. Human Papillomavirus Vaccination for Adults: Updated recommendations of the Advisory Committee on Immunization Practices. MMWR. Morb. Mortal. Wkly. Rep. 2019, 68, 698–702. [Google Scholar] [CrossRef]
- UK Health Security Agency. HPV Vaccination Guidance for Healthcare Practitioners (Version 6). Available online: https://www.gov.uk/government/publications/hpv-universal-vaccination-guidance-for-health-professionals/hpv-universal-vaccination-guidance-for-healthcare-practitioners-version-3 (accessed on 7 April 2022).
- Goldstone, S.E.; Giuliano, A.R.; Palefsky, J.M.; Lazcano-Ponce, E.; Penny, M.E.; Cabello, R.E.; Moreira, E.D.; Baraldi, E.; Jessen, H.; Ferenczy, A.; et al. Efficacy, immunogenicity, and safety of a quadrivalent HPV vaccine in men: Results of an open-label, long-term extension of a randomised, placebo-controlled, phase 3 trial. Lancet Infect. Dis. 2022, 22, 413–425. [Google Scholar] [CrossRef]
- Meites, E.; Winer, R.L.; Newcomb, M.E.; Gorbach, P.M.; Querec, T.D.; Rudd, J.; Collins, T.; Lin, J.; Moore, J.; Remble, T.; et al. Vaccine effectiveness against prevalent anal and oral Human Papillomavirus infection among men who have sex with men—United States, 2016–2018. J. Infect. Dis. 2020, 222, 2052–2060. [Google Scholar] [CrossRef]
- Chow, E.P.F.; Tabrizi, S.N.; Fairley, C.K.; Wigan, R.; Machalek, D.A.; Garland, S.M.; Cornall, A.M.; Atchison, S.; Hocking, J.S.; Bradshaw, C.S.; et al. Prevalence of human papillomavirus in young men who have sex with men after the implementation of gender-neutral HPV vaccination: A repeated cross-sectional study. Lancet Infect. Dis. 2021, 21, 1448–1457. [Google Scholar] [CrossRef]
- Winer, R.L.; Lin, J.; Querec, T.D.; Unger, E.R.; Stern, J.E.; Rudd, J.M.; Golden, M.R.; Swanson, F.; Markowitz, L.E.; Meites, E. Effectiveness of Human Papillomavirus (HPV) vaccination against penile HPV infection in men who have sex with men and transgender women. J. Infect. Dis. 2022, 225, 422–430. [Google Scholar] [CrossRef]
- Chambers, C.; Deeks, S.L.; Sutradhar, R.; Cox, J.; de Pokomandy, A.; Grennan, T.; Hart, T.A.; Lambert, G.; Moore, D.M.; Coutlée, F.; et al. Anal human papillomavirus prevalence among vaccinated and unvaccinated gay, bisexual, and other men who have sex with men in Canada. Sex. Transm. Dis. 2022, 49, 123–132. [Google Scholar] [CrossRef]
- Mann, L.; Llata, E.; Flagg, E.W.; Hong, J.; Asbel, L.; Carlos-Henderson, J.; Kerani, R.P.; Kohn, R.; Pathela, P.; Schumacher, C.; et al. Trends in the prevalence of anogenital warts among patients at sexually transmitted disease clinics—Sexually Transmitted Disease Surveillance Network, United States, 2010–2016. J. Infect. Dis. 2019, 219, 1389–1397. [Google Scholar] [CrossRef]
- Loretan, C.; Chamberlain, A.T.; Sanchez, T.; Zlotorzynska, M.; Jones, J. Trends and characteristics associated with human papillomavirus vaccination uptake among men who have sex with men in the United States, 2014–2017. Sex. Transm. Dis. 2019, 46, 465–473. [Google Scholar] [CrossRef]
- Grewal, R.; Grennan, T.; Gillis, J.L.; Ogilvie, G.; Gaspar, M.; Grace, D.; Raboud, J.M.; MacPherson, P.A.; Rosenes, R.; Salit, I.E.; et al. Low human papillomavirus (HPV) vaccine uptake among men living with human immunodeficiency virus (HIV): Cross-sectional findings from a clinical cohort. Prev. Med. 2021, 143, 106329. [Google Scholar] [CrossRef]
- Nadarzynski, T.; Frost, M.; Miller, D.; Wheldon, C.W.; Wiernik, B.M.; Zou, H.; Richardson, D.; Marlow, L.A.; Smith, H.; Jones, C.J.; et al. Vaccine acceptability, uptake and completion amongst men who have sex with men: A systematic review, meta-analysis and theoretical framework. Vaccine 2021, 39, 3565–3581. [Google Scholar] [CrossRef]
- Gerend, M.A.; Madkins, K.; Crosby, S.; Korpak, A.K.; Phillips, G.; Bass, M.; Houlberg, M.; Mustanski, B. A qualitative analysis of young sexual minority men’s perspectives on Human Papillomavirus Vaccination. LGBT Health 2019, 6, 350–356. [Google Scholar] [CrossRef]
- Hao, Z.; Guo, Y.; Bowling, J.; Ledenyi, M. Facilitators and barriers of HPV vaccine acceptance, initiation, and completion among LGBTQ Community in the U.S.: A systematic review. Int. J. Sex. Health 2021, 34, 291–307. [Google Scholar] [CrossRef]
- Beavis, A.L.; Levinson, K.L. Preventing cervical cancer in the United States: Barriers and resolutions for HPV vaccination. Front. Oncol. 2016, 6, 19. [Google Scholar] [CrossRef]
- PRISMA. PRISMA for Scoping Reviews. Available online: http://www.prisma-statement.org/Extensions/ScopingReviews (accessed on 7 April 2022).
- Simatherai, D.; Bradshaw, C.S.; Fairley, C.K.; Bush, M.; Heley, S.; Chen, M.Y. What men who have sex with men think about the human papillomavirus vaccine. Sex. Transm. Infect. 2009, 85, 148–149. [Google Scholar] [CrossRef]
- Reiter, P.L.; Brewer, N.T.; McRee, A.-L.; Gilbert, P.; Smith, J.S. Acceptability of HPV Vaccine Among a National Sample of Gay and Bisexual Men. Sex. Transm. Dis. 2010, 37, 197–203. [Google Scholar] [CrossRef]
- Colón-López, V.; Del Toro-Mejías, L.M.; Ortiz, A.P.; Tortolero-Luna, G.; Palefsky, J.M. HPV awareness and willingness to HPV vaccination among high-risk men attending an STI clinic in Puerto Rico. Puerto Rico Health Sci. J. 2012, 31, 227–231. [Google Scholar]
- Rank, C.; Gilbert, M.; Ogilvie, G.; Jayaraman, G.C.; Marchand, R.; Trussler, T.; Hogg, R.; Gustafson, R.; Wong, T. Acceptability of human papillomavirus vaccination and sexual experience prior to disclosure to health care providers among men who have sex with men in Vancouver, Canada: Implications for targeted vaccination programs. Vaccine 2012, 30, 5755–5760. [Google Scholar] [CrossRef]
- Meites, E.; Markowitz, L.E.; Paz-Bailey, G.; Oster, A.M.; White, J.; Todd, J.; Bautista, G.; Flynn, C.; German, D.; Miminos, M.; et al. HPV vaccine coverage among men who have sex with men—National HIV Behavioral Surveillance System, United States, 2011. Vaccine 2014, 32, 6356–6359. [Google Scholar] [CrossRef]
- Cummings, T.; Kasting, M.L.; Rosenberger, J.G.; Rosenthal, S.L.; Zimet, G.D.; Stupiansky, N.W. Catching up or missing out? Human Papillomavirus Vaccine acceptability among 18- to 26-year-old men who have sex with men in a US national sample. Sex. Transm. Dis. 2015, 42, 601–606. [Google Scholar] [CrossRef]
- Moores, A.; Phillips, J.C.; O’Byrne, P.; MacPherson, P. Anal cancer screening knowledge, attitudes, and experiences among men who have sex with men in Ottawa, Ontario. Can. J. Hum. Sex. 2015, 24, 228–236. [Google Scholar] [CrossRef]
- Reiter, P.L.; McRee, A.-L.; Katz, M.L.; Paskett, E.D. Human Papillomavirus vaccination among young adult gay and bisexual men in the United States. Am. J. Public Health 2015, 105, 96–102. [Google Scholar] [CrossRef]
- Nadarzynski, T.; Smith, H.; Richardson, D.; Pollard, A.; Llewellyn, C. Perceptions of HPV and attitudes towards HPV vaccination amongst men who have sex with men: A qualitative analysis. Br. J. Health Psychol. 2017, 22, 345–361. [Google Scholar] [CrossRef] [PubMed]
- Stupiansky, N.; Liau, A.; Rosenberger, J.; Rosenthal, S.L.; Tu, W.; Xiao, S.; Fontenot, H.; Zimet, G.D. Young men’s disclosure of same sex behaviors to healthcare providers and the impact on health: Results from a US national sample of young men who have sex with men. AIDS Patient Care STDs 2017, 31, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Wheldon, C.W.; Daley, E.M.; Buhi, E.R.; Baldwin, J.A.; Nyitray, A.G.; Giuliano, A.R. HPV vaccine decision-making among young men who have sex with men. Health Educ. J. 2017, 76, 52–65. [Google Scholar] [CrossRef]
- Nadarzynski, T.; Smith, H.; Richardson, D.; Bremner, S.; Llewellyn, C. Men who have sex with men who do not access sexual health clinics nor disclose sexual orientation are unlikely to receive the HPV vaccine in the UK. Vaccine 2018, 36, 5065–5070. [Google Scholar] [CrossRef]
- Kesten, J.M.; Flannagan, C.; Ruane-McAteer, E.; Merriel, S.W.D.; Nadarzynski, T.; Shapiro, G.; Rosberger, Z.; Prue, G. Mixed-methods study in England and Northern Ireland to understand young men who have sex with men’s knowledge and attitudes towards human papillomavirus vaccination. BMJ Open 2019, 9, e025070. [Google Scholar] [CrossRef]
- Petit, B.; Epaulard, O. Men having sex with men and the HPV vaccine in France: A low vaccine coverage that may be due to its infrequent proposal by physicians. Vaccine 2020, 38, 2160–2165. [Google Scholar] [CrossRef]
- Nadarzynski, T.; Smith, H.; Richardson, D.; Ford, E.; Llewellyn, C. Sexual healthcare professionals’ views on HPV vaccination for men in the UK. Br. J. Cancer 2015, 113, 1599–1601. [Google Scholar] [CrossRef]
- Nadarzynski, T.; Llewellyn, C.; Richardson, D.; Pollard, A.; Smith, H. UK healthcare professionals’ uncertainties, barriers and facilitators to the introduction of targeted human papillomavirus vaccination for men who have sex with men. Sex. Health 2017, 14, 372. [Google Scholar] [CrossRef]
- Merriel, S.W.D.; Flannagan, C.; Kesten, J.M.; Shapiro, G.K.; Nadarzynski, T.; Prue, G. Knowledge and Attitudes of General Practitioners and Sexual Health Care Professionals Regarding Human Papillomavirus Vaccination for Young Men Who Have Sex with Men. Int. J. Environ. Res. Public Health 2018, 15, 151. [Google Scholar] [CrossRef]
- Wheldon, C.W.; Sutton, S.K.; Fontenot, H.; Quinn, G.; Giuliano, A.R.; Vadaparampil, S.T. Physician communication practices as a barrier to risk-based HPV vaccine uptake among men who have sex with men. J. Cancer Educ. 2018, 33, 1126–1131. [Google Scholar] [CrossRef]
- Wigfall, L.T.; Bynum, S.A.; Brandt, H.M.; Sebastian, N.; Ory, M.G. HPV-Related cancer prevention and control programs at community-based HIV/AIDS service organizations: Implications for future engagement. Front. Oncol. 2018, 8, 422. [Google Scholar] [CrossRef]
- FitzGerald, S.M.; Savage, E.; Hegarty, J. The Human Papillomavirus: Men’s attitudes and beliefs toward the HPV vaccination and condom use in cancer prevention. J. Men’s Health 2014, 11, 121–129. [Google Scholar] [CrossRef]
- Gerend, M.A.; Madkins, K.; Ii, G.P.; Mustanski, B. Predictors of Human Papillomavirus vaccination among young men who have sex with men. Sex. Transm. Dis. 2016, 43, 185–191. [Google Scholar] [CrossRef]
- Jaiswal, J.; LoSchiavo, C.; Maiolatesi, A.; Kapadia, F.; Halkitis, P.N. Misinformation, gendered perceptions, and low healthcare provider communication around HPV and the HPV vaccine among young sexual minority men in New York City: The P18 cohort study. J. Community Health 2020, 45, 702–711. [Google Scholar] [CrossRef]
- Grace, D.; Gaspar, M.; Rosenes, R.; Grewal, R.; Burchell, A.N.; Grennan, T.; Salit, I.E. Economic barriers, evidentiary gaps, and ethical conundrums: A qualitative study of physicians’ challenges recommending HPV vaccination to older gay, bisexual, and other men who have sex with men. Int. J. Equity Health 2019, 18, 159. [Google Scholar] [CrossRef]
- Sanchooli, A.; Aghayipour, K.; Naghlani, S.K.; Samiee, Z.; Kiasari, B.A.; Makvandi, M. Production of Human Papillomavirus Type-16 L1 VLP in Pichia pastoris. Appl. Biochem. Microbiol. 2020, 56, 51–57. [Google Scholar] [CrossRef]
- Fisher, C.B.; Fried, A.L.; Macapagal, K.; Mustanski, B. Patient–provider communication barriers and facilitators to HIV and STI preventive services for adolescent MSM. AIDS Behav. 2018, 22, 3417–3428. [Google Scholar] [CrossRef]
- Rodríguez-Álvarez, M.I.; Gómez-Urquiza, J.L.; Ahmed, H.H.-E.; Albendín-García, L.; Gómez-Salgado, J.; la Fuente, G.A.C.-D. Prevalence and risk factors of Human Papillomavirus in male patients: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2018, 15, 2210. [Google Scholar] [CrossRef]
- Ejaz, M.; Ekström, A.M.; Ahmed, A.; Haroon, A.; Ali, D.; Ali, T.S.; Salazar, M. Human Papillomavirus associated prevention: Knowledge, attitudes, and perceived risks among men who have sex with men and transgender women in Pakistan: A qualitative study. BMC Public Health 2022, 22, 378. [Google Scholar] [CrossRef]
- Swedish, K.A.; Goldstone, S.E. Prevention of anal condyloma with Quadrivalent Human Papillomavirus Vaccination of Older men who have sex with men. PLoS ONE 2014, 9, e93393. [Google Scholar] [CrossRef]
| Study | Subjects Characteristics | Major Findings | Notes |
|---|---|---|---|
| Simatherai et al. [25] | MSM attending the Melbourne Sexual Health Centre n = 200 Median age = 27 years Age range = 19–71 years | 93% would disclose to healthcare professionals they were MSM if they could obtain HPVV for free. This was valid until a median age of 20 years (2 years post-sexual debut) and a median sexual partner number of 15. | Huge challenge to get MSM vaccinated before HPV exposure. Need to address the low level of awareness first. Subjects could be more health-conscious. Suggest vaccination to all boys. |
| Reiter et al. [26] | A national sample of self-identified gay or bisexual men in the US n = 306 Age range = 18–59 years | Higher HPVV acceptability among those who perceived their doctors would recommend it (vs unbeliever, OR 12.87, 95% CI 4.63–35.79) and those who were doubtful (vs unbeliever, OR 3.15, 95% CI 1.47–6.76), ≥5 lifetime-sexual partners (OR 3.39, 95% CI 1.34–8.55) and perceived higher severity of HPV-related disease (OR 1.92, 95% CI 1.18–3.14), perceived higher HPVV effectiveness (OR 1.97, 95% CI 1.27–3.06), perceived higher regrets if they developed an HPV infection if unvaccinated (OR 2.39, 95% CI 1.57–3.61). | Conducted before HPVV is licensed to be used among men. Inclusion of MSM outside the range of recommended vaccination. Willingness might not translate to behaviours. |
| Colón-López et al. [27] | Men ≥26 years attending an STI Clinic in Puerto Rico n = 46 | Factors increasing vaccination willingness: —A doctor recommended HPVV (95.7%) —Health insurance reimbursed HPVV (91.3%) —Subjects understand the importance of the vaccine (91.3%) Barriers to be vaccinated: —Low perceived susceptibility towards infection —High cost | Subgroup analysis for MSM was not performed. Very small sample size. |
| Rank et al. [28] | MSM aged ≥19 years recruited at community venues in Vancouver n = 1401 | ↑ vaccine acceptability was linked with previous diagnosis of genital warts (OR 1.7, 95% CI 1.1–2.6), sexual behaviour disclosure to healthcare providers (OR 1.6, 95% CI 1.1–2.3), annual income ≥ $20,000 (OR 1.5, 95% CI 1.1–2.1), previous hepatitis A or B vaccines (OR 1.4, 95% CI 1.0–2.0) and absence of recreational drug use (OR 1.4, 95% CI 1.0–2.0) Median time from disclosure to first sexual contact: 6.0 years (IQR 2–14 years) 37% of men ≤26 years who never disclosed to any healthcare provider reported ≥6 lifetime sexual partners. | Recruitment at the public, so MSM must be willing to disclose themselves, thus could be more comfortable discussing with healthcare providers. The cost of HPVV was not factored in. Delay in disclosure will increase the likelihood of exposure to HPV infection before vaccination. |
| Meites et al. [29] | MSM under National HIV Behavioural Surveillance System n = 3221 Age range = 18–26 | Factors predicting HPVV uptake: —Visiting a healthcare provider last year (aPR 2.3, CI 1.2–4.2) —Ever disclosing male-male sexual attraction/behaviour to a healthcare provider (aPR 2.1, CI 1.3–3.3) —A positive test for HIV infection (aPR 2.2, CI 1.5–3.2) —Any hepatitis vaccination (aPR 2.2, CI 1.5–3.2). | Vaccine status was self-reported. Vaccine completion was not evaluated. Venue-based sampling would exclude those who were discrete about their sexual orientation. |
| FitzGerald et al. [44] | Subjects were conveniently sampled in a university n = 12 Age range = 18–28 years | Healthcare professionals were cited as the main referents to approve subjects receiving HPVV. Subjects mainly referred to general practitioners. Cost, low knowledge level on HPV/HPVV and concerns on side effects were barriers to vaccination. | Small sample size. |
| Cummings et al. [30] | Email to registered users of the world largest men who seek social or sexual interactions with other men n = 1457 Mean age = 22.5 (SD = 2.40) years | ↓ HPVV acceptability was linked with HPVV safety concerns (B = −0.262, p < 0.01), greater shame associated with HPV infection/disease (B = −0.103, p < 0.01), and perceived resistance (B = −0.089, p < 0.01). ↑ HPVV acceptability was linked with healthcare provider’s recommendation (B = 0.190, p < 0.01), greater worry about HPV infection (B = 0.139, p < 0.01), and being tested for an STD in the previous year (B = 0.060, p < 0.05). ↑ vaccine uptake was linked with being tested for a sexually transmitted disease in the previous year (OR 3.27, 95% CI 1.87–5.70), disclosure of sexual orientation (OR 2.99, 95% CI, 1.83–4.88), and higher HPV knowledge scores (OR 1.40, 95% CI 1.23–1.59) | Lack of disclosure could lead to no recommendation from doctor to take up HPVV. Low response rate (1457/4801) Self–reported vaccination status. |
| Moores et al. [31] | Men registered for health services at a sexually transmitted infection testing and treatment clinic in Ottawa, Ontario n = 280 Mean age = 37 ± 11.86 years (range 18–69) | 16.2% were vaccinated with HPVV. For unvaccinated individuals, only 27.2% talked to healthcare professionals about vaccinations. 74.9% had family doctors and from those, 75% know their clients were MSM. For MSM who had discussed anal cancer screening and prevention with healthcare providers (n = 30), most were knowledgeable about HPVV. | Over-sampling of MSM open about their sexual orientation. Under-sampling of MSM who went to their GP for screening. |
| Reiter et al. [32] | Harris Interactive LGBT Panel LGBT living in the United States Age range = 18–26 years n = 428 | 13% initiated the vaccination, 54% of them completed all doses. The major reason for vaccination was doctor’s recommendation. 83% of those who received the recommendation for HPVV by a healthcare provider initiated the vaccination. In multivariate analysis, recommendations by healthcare providers remained the strongest correlate of HPVV initiation (OR 110.60; 95% CI 32.67, 374.48). | First study after AICP’s recommendation for routine vaccination of males was released in late 2011. Only includes subjects who self-identified as gay or bisexual. HPV vaccination is self-reported. |
| Gerend et al. [45] | MSM recruited from a geospatial dating app n = 336 Age rage = 18–26 | Provider recommendation was the strongest predictor for HPVV uptake (40 times more likely to be vaccinated). Provider’s recommendation was predicted by sexual identity (↑ others vs gay), ethnicity (↓ Hispanic vs White), condomless anal sex (↑ yes vs no) and HIV status (↑ yes vs no). Lack of recommendation, lack of HPV/HPVV knowledge, not disclosing sexual identity, low susceptibility for HPV and concerns about vaccine safety are barriers to vaccination. | Self-reported vaccination and recommendation. Exclusion of those under 18 years. |
| Nadarzynski et al. [33] * | MSM from community-based LGBTQ venues and organisations n = 33 Median age = 25 years (IQR: 21–27), Age range=16-60 years | All MSM would accept HPVV if offered by a healthcare professional. Barriers: accessing healthcare services or discussing same-sex experience with healthcare professionals, efficacy of vaccines, side effects, fear of needles, fear of interaction between HPVV and HIV treatment. The majority preferred sexual health clinics as a means to reach out to MSM due to openness, some preferred GP because young men had limited access to sexual health clinics. | Self-selection bias. Education level not determined. The area surveyed was more open to the LGBTQ population. The absolute incidence of anal cancer not disclosed to prevent subjects underestimating the risk, which might change the attitudes towards vaccines. |
| Wheldon et al. [35] | n = 9 from student pride groups n = 13 from sexual networking application used by MSM Interview in person = 14 Telephone = 8 Age range = 18–26 years, mean = 22 years | Interpersonal influence of HPVV acceptance: doctor’s opinion was the most important, some stated influence of more senior gay friends. Family support was mixed due to alienation. External control factors: out-of-pocket cost, uncertainties about where to get vaccinated, lack of established relationship with providers, convenience (distance, schedule). Self-efficacy to ask for HPVV was mixed due to uneasiness to disclose sexual orientation to healthcare providers. Relationship with provider: sometimes negative, impacting disclosure and interactions. Some feel ashamed, awkward and judged. Felt the need to understand healthcare providers’ standpoint on LGBT issues for fear that the provider might be biased or incompetent in providing care. | Small sample size. Specific geographical area. All well-educated with healthcare insurance. |
| Stupiansky et al. [34] | US users of an online MSM social and sexual networking website n = 1751 Mean age = 22.7 years | 38% disclosed same-sex relationship to their healthcare provider. Increased ≥1 dose of HPVV was linked with: —Higher disclosure to friends/family —Recent sexually transmitted disease history —Visiting a healthcare provider in the past year —Searching for sexual health information online and disclosure to healthcare providers were important mediators in the relationship between these predictors and vaccine uptake Having visited a healthcare provider in the past year was the most important predictor of disclosure of MSM behaviour. | The high dependence on disclosure reflects HPV vaccination is especially dependent on practice of individual providers. Users of social and sexual networking websites are high-risk groups. The Black population is underrepresented. |
| Nadarzynski et al. [36] | MSM recruited via advertisement via Facebook n = 1508 Median age = 22 years Age range = 14–63 years | 89% would accept HPVV if a healthcare provider offered it. HPVV acceptability was positively associated with: —access to sexual health clinics [OR 1.82, 95% CI 1.29–2.89] —disclosure of sexual orientation to a healthcare provider [OR 2.02, CI 1.39–3.14] —positive HIV status [OR 1.96, CI 1.09–3.53] After receiving HPVV information, the acceptability was positively associated with: —↑ perceived HPV risk (OR 1.31, CI 1.05–1.63) —↑ perceived severity of HPV infection (OR 1.89, CI 1.16–3.01) —↑ perceived HPVV benefits (OR 1.61, CI 1.14–3.01) —↑ perceived HPVV effectiveness (OR 1.54, CI 1.14–2.08) —↓ perceived barriers to HPV vaccination (OR = 4.46, CI 2.95–6.73) | Convenience sampling method. Targeting only men who were already comfortable disclosing their sexuality online. Recall bias and social desirability. |
| Kesten et al. [37] ** | MSM recruited from LGBTQ organisations, university information days, university student union | 65% had never discussed HPVV with a healthcare provider. Mean age of participants willing to disclose sexuality to healthcare providers = 18.3 years (range: 11–23 years). The most comfortable setting to receive HPV vaccine was LGBTQ-specific services than genitourinary medicine clinics. Thematic analysis: A good relationship with general practitioners or sexual healthcare providers is important for HPVV acceptance. The school nurse was suggested as a trusted person to deliver the vaccine. | Small sample size. Self-selection—participants may be more comfortable with their sexuality. |
| Gerend et al. [21] * | Men identified as gay, bisexual or queer recruited via Facebook or a local LGBTQ health and development program n = 29 Mean age = 22.66 (SD = 2.30) Age range = 18–26 years | Some were not sure HPVV is effective for sexually active men. Doubts on the number and timing of doses, age, side effects of HPVV. Provider played a central role in subjects’ decision to be vaccinated. Some providers seemed uncomfortable asking subjects’ sexuality. Some felt stigmatised or judged. Some were hesitant in asking for HPVV if they had to disclose sexuality. The level of comfort relied on their relationship with the providers. | Small sample size. Subjects are from regions with higher socioeconomic status. |
| Petit and Epaulard [38] | MSM under the age of 27 recruited via Facebook, community website or dating application n = 2094 | Among 1728 with a family physician, 9.9% was proposed HPVV (9.1% for those ≤ 27 years), 60.6% disclosed sexual orientation. 17.9% ≤ 27 years had received the vaccine. 37.6% received the proposal accepted HPVV, compared to 1.9% among those who did not. | Self-selection of subjects with greater interest in sexual health. Might have a higher vaccination rate than the general MSM in France. |
| Jaiswal et al. [46] * | Sexual minority men recruited from a larger cohort study of emerging sexual minority adults in New York City. n = 38 Mean age = 25.82 (SD = 0.95) years Age range = 24-27 years | Healthcare providers did not explain the importance of HPVV adequately. Healthcare system did not follow up with clients to complete vaccination. | No in-depth exploration of the topic. Not readily generalisable to sexual minorities of other areas. |
| Study | Subjects Characteristics | Major findings | Notes |
|---|---|---|---|
| Nadarzynski et al. [39] | UK-based sexual health workers (i.e., consultants, nurses, health advisors) n = 325 (70% female, 46% doctors, 75% in sexual health clinics) | 65% recommended targeting MSM for HPVV. 3% believed that HPV poses little cancer risk in MSM to make vaccination necessary. 75% believed that the majority of MSM would want to receive HPVV. 60% believed that HPVV would promote MSM to engage with sexual health services. 3% believed that HPVV increased the likelihood of unsafe sex among MSM. 26% believed in individual assessment of MSM attending sexual health clinic. 74% believed HPVV should be offered by GPs or pharmacies. 51% believed all HPVV offering to MSM should not be based on age. 17% believed it is too late to vaccinate if MSM are sexually active. 49% believed they have the skills to identify MSM that would benefit from HPVV. 44% believed that they are sufficiently informed about HPVV for MSM. Sexual healthcare providers who were vaccinating men had less odds to disagree that MSM are not at risk of HPV-related cancers and that MSM-targeted HPV vaccination is worthwhile (OR 0.34, 95 CI% 0.20–0.70). They also believed they had higher knowledge levels about issues related to HPVV and MSM (OR 8.49, 95% CI 4.50–15.1). Nurses were more likely to agree with individual assessment in MSM-targeted HPV vaccination (OR 3.32, 95% CI 1.69–5.65). | Risk of self-selection. The response rate cannot be determined. Not include GPs and pharmacists. |
| Nadarzynski et al. [40] * | UK-based self-referred healthcare providers (13 doctors, 3 nurses, 3 health advisers) involved in sexual healthcare n = 19 | Issues: healthcare providers were not sure about selection criteria (younger/without a history of genital warts), appropriate healthcare setting (sexual health clinics/GP) and source of vaccination funding (central/local). Barriers: Lack of political and public support (become a sex vaccine), limited access to HPV vaccination by MSM (rural area), delayed disclosure of sexual orientation to healthcare providers, identification of eligibility, poor awareness and motivation to complete vaccination. Facilitating factors to increase coverage: official guidelines, awareness campaigns and integrated clinic procedures (non-judgmental processing in recording sexual behaviours, incentivise recording, encouraging HPVV for MSM not attending sexual health clinics, reminders to complete vaccination). | Gender-neutral vaccination is preferred over MSM-targeted screening. Effects will be compromised if MSM are not willing to attend sexual health clinics or disclose sexual orientation. Suggested effective use of social media such as Facebook, poster advertising and text messages. Small sample size. Self-selection bias from healthcare providers with a particular interest in HPV vaccination. |
| Merriel et al. [41] | General practitioners and sexual healthcare providers (including genitourinary medicine consultants, doctors-in-training and nurses working in sexual health clinics) n = 87 (38 GPs and 49 sexual healthcare providers) Mean age = 40.71 years with a median 14 years of experience (IQR 8. 24). | Sexual healthcare providers were more likely to vaccinate a young MSM, and aware of the recommendation (adjusted OR 0.03, 95% CI 0.01, 0.11) and perceived self-sufficient to engage in informed discussion with HPVV (adjusted OR 0.04, 95% CI 0.01, 0.14). 78.95% general practitioners indicated no to low knowledge of HPV vaccination for young MSM, compared to 12.24% among sexual healthcare providers. GPs were less likely to agree on sex-neutral (adjusted OR 0.30, 95% CI 0.09, 0.98) or HPV vaccination MSM (adjusted OR 0.30, 95% CI 0.09, 0.98), or young MSM would want to be vaccinated (adjusted OR 0.13, 95% CI 0.04, 0.41). GPs were less likely to believe that a young person would disclose their sexual orientation (adjusted OR 0.17, 95% CI 0.06, 0.50), less confident that they could identify young MSM who may benefit from HPVV (adjusted OR 0.03, 95% CI 0.01, 0.15), and in recommending HPVV to young MSM (adjusted OR 0.04, 95% CI 0.01, 0.18). Barriers to deliver HPVV to young MSM: GP—65.79% no time, sexual healthcare providers—staff availability. Solution: GP—73.68% additional training, sexual healthcare providers—51.43% computer prompts. | Convenience sampling approach. Pre-determined survey statements did not allow reason to be given for the opinion. Small sample size. |
| Wheldon et al. [42] | Primary care physicians in Florida n = 770 drawn from American Medical Association Physician Masterfile | 70.5% knew HPVV recommendation for MSM. 13.6% routinely discussed both sexual orientation and HPVV with male patients aged 22-26 years (high potential group). 24.5% did not discuss either. HPVV discussion was positively associated with awareness of the physicians on the recommendation for MSM (OR 3.49; 95%CI 1.80–6.74). Physicians with low HPV knowledge were more likely to discuss sexual orientation and HPVV. Postulation: Those with high knowledge levels failed to act on HPVV recommendations based on assessment data. | Suggest the use of electronic medical systems to prompt providers regarding specific recommendations. Non-probability-based sampling. Modest response rate. Not examining communication in real time 51% response rate. |
| Wigfall et al. [43] | Staff from three community-based HIV/AIDS service organizations n = 30 Mean age = 47.7 (SD = 12.5) years | 100% were aware of HPV and 77% were aware of HPVV. 67% were aware that HPV causes anal cancer. 91–95% were willing to prompt MSM and female clients to talk to a healthcare provider about HPVV. 86–95% were willing to direct clients to adult safety net HPVV providers. 59–67% thought they could exert a positive influence on MSM and female client’s HPVV decision-making. 63% thought HPV stigma was a barrier to HPV cancer prevention tool. | Small sample size—provider level participant. Gay stigma as a potential healthcare access barrier was not evaluated. |
| Grace et al. [47] * | 13 physiciansand 2 clinical researchers in Canada. Most affiliated with HPV-SAVE project. 7 were HIV/sexually transmitted disease specialists 6 general practitioners | The subjects were in favour of HPVV and were not concerned with its safety. They would recommend HPV to MSM < 27 years, those with health insurance, or HIV-positive patients regardless of age and insurance. HPVV recommendation for older men with HIV, the lack of evidence of benefits of vaccinating MSM > 26 years could affect the recommendation. In these situations, the recommendation was based on patients’ contact with HPV and their sexual history. Discussion on HPV and HPVV was the priority. Both healthcare providers and patients had initiated the discussion. Cost was a major factor inhibiting discussion. | The subjects showed a high degree of knowledge on the HPV research and recommendation practice than other physicians. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chin, K.-Y.; Ekeuku, S.O.; Hamzah, M.R. The Role of Healthcare Providers in Promoting Human Papillomavirus Vaccines among Men Who Have Sex with Men: A Scoping Review. Vaccines 2022, 10, 930. https://doi.org/10.3390/vaccines10060930
Chin K-Y, Ekeuku SO, Hamzah MR. The Role of Healthcare Providers in Promoting Human Papillomavirus Vaccines among Men Who Have Sex with Men: A Scoping Review. Vaccines. 2022; 10(6):930. https://doi.org/10.3390/vaccines10060930
Chicago/Turabian StyleChin, Kok-Yong, Sophia Ogechi Ekeuku, and Muhammad Rafie Hamzah. 2022. "The Role of Healthcare Providers in Promoting Human Papillomavirus Vaccines among Men Who Have Sex with Men: A Scoping Review" Vaccines 10, no. 6: 930. https://doi.org/10.3390/vaccines10060930
APA StyleChin, K.-Y., Ekeuku, S. O., & Hamzah, M. R. (2022). The Role of Healthcare Providers in Promoting Human Papillomavirus Vaccines among Men Who Have Sex with Men: A Scoping Review. Vaccines, 10(6), 930. https://doi.org/10.3390/vaccines10060930







