Assessment of Humoral and Long-Term Cell-Mediated Immune Responses to Recombinant Canarypox-Vectored Equine Influenza Virus Vaccination in Horses Using Conventional and Accelerated Regimens Respectively
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Populations
2.2. Animal Ethics
2.3. Statistical Analysis
2.4. Vaccination
2.5. Vaccination Schedules
2.6. Equine Influenza Viruses
2.7. Blood Collection
2.8. Peripheral Blood Mononuclear Cell Separation
2.9. Haemagglutination Inhibition Antibody Assay
2.10. Competitive Enzyme-Linked Immunosorbent Assay
2.11. Measurement of Interferon-Gamma Production
3. Results
3.1. Study Population 1
3.2. Serologic Testing
3.2.1. Competitive ELISA Assay
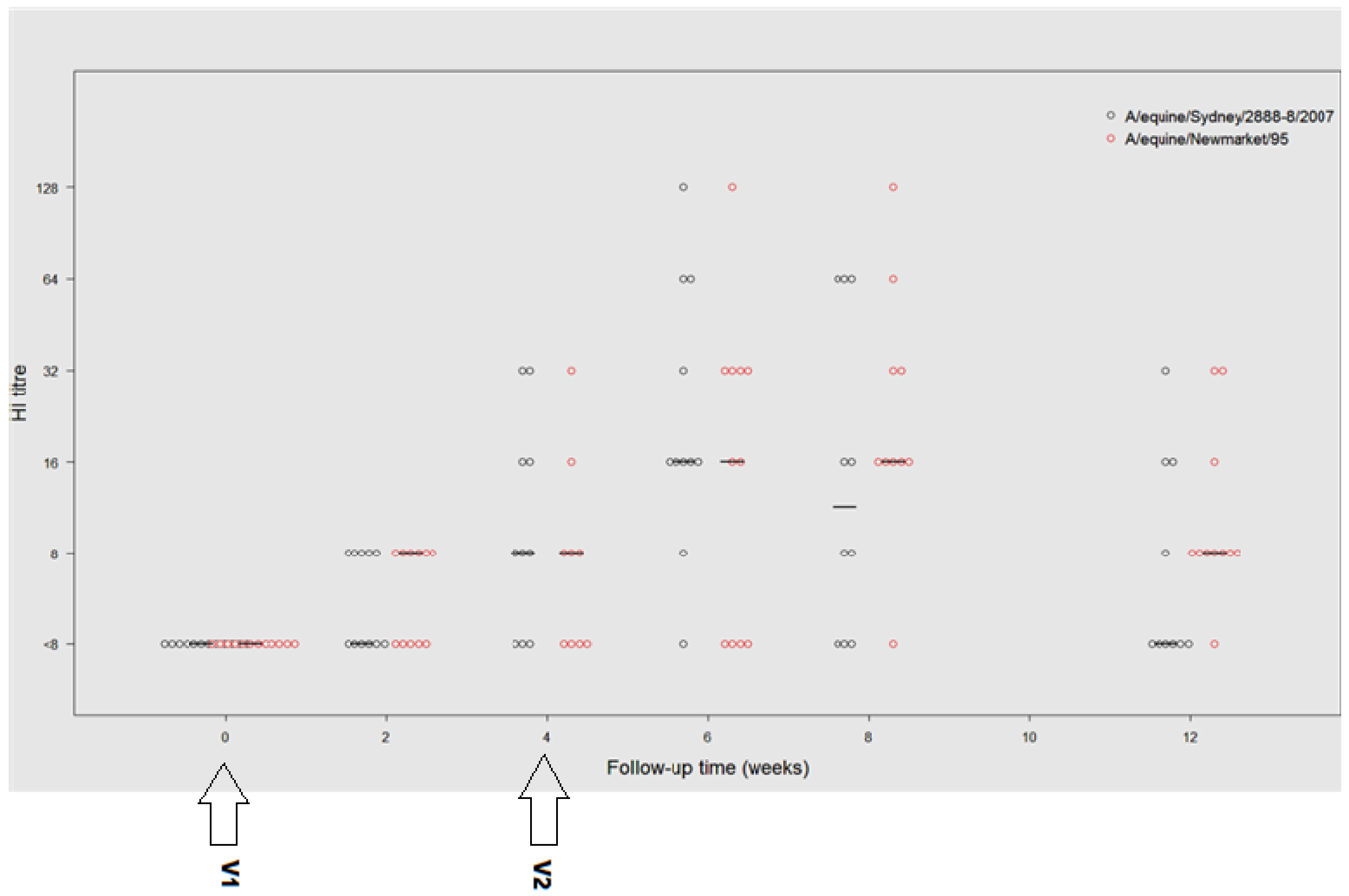
3.2.2. HI Antibody Response to Vaccination
3.2.3. A/Equine/Sydney/07 (H3N8)
3.2.4. A/Equine/Newmarket/95(H3N8)
3.2.5. A/Equine/Prague/1956(H7N7)
3.3. Group 2; Study Population
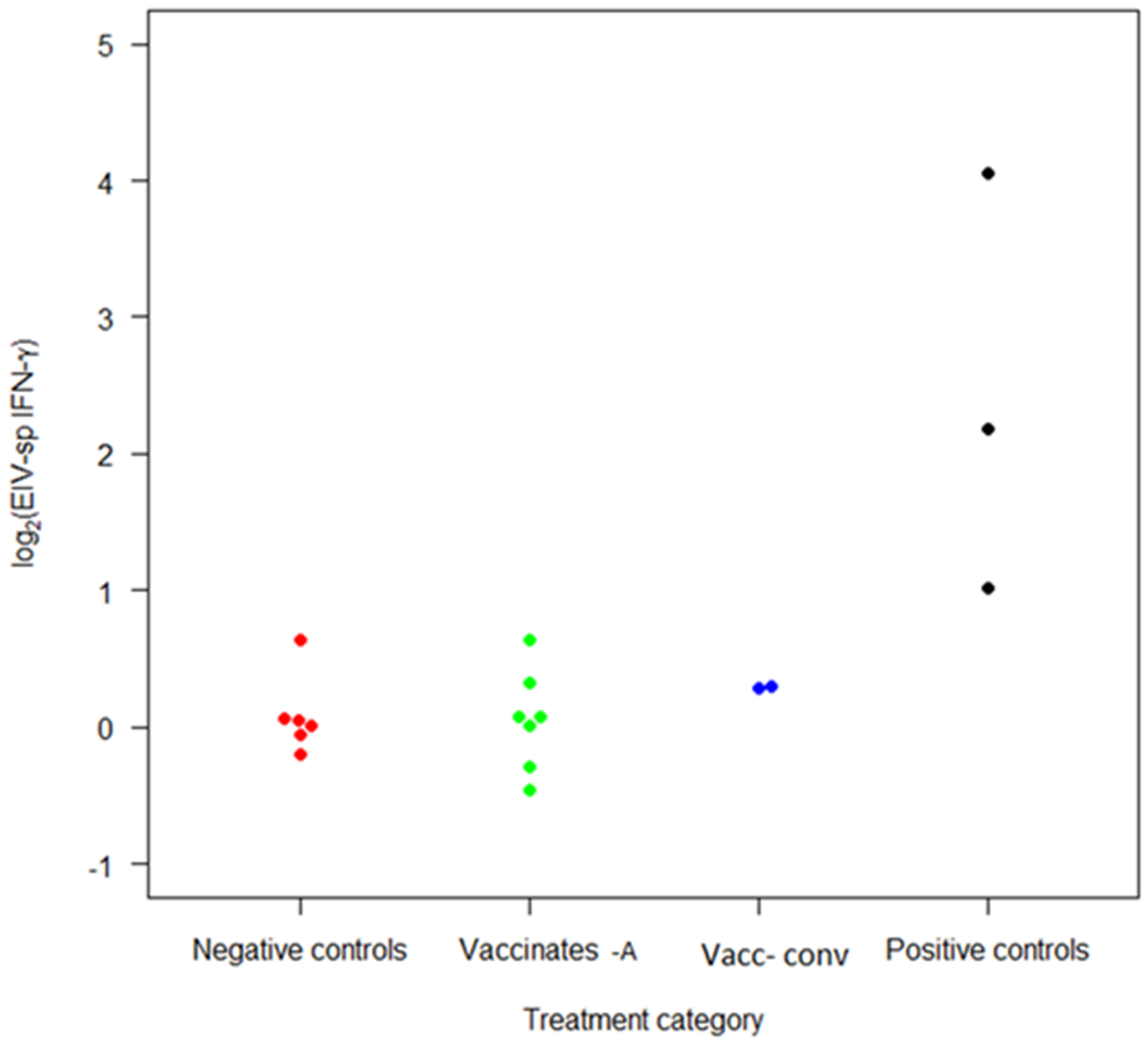
3.4. Interferon-Gamma Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Traub-Dargatz, J.L.; Salman, M.D.; Voss, J.L. Medical problems of adult horses, as ranked by equine practitioners. J. Am. Vet. Med. Assoc. 1991, 198, 1745–1747. [Google Scholar] [PubMed]
- van Maanen, C.; Cullinane, A. Equine influenza virus infections: An update. Vet. Q. 2002, 24, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Cullinane, A.; Newton, J.R. Equine influenza-A global perspective. Vet. Microbiol. 2013, 167, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Elton, D.; Bryant, N. Facing the threat of equine influenza. Equine Vet. J. 2011, 43, 250–258. [Google Scholar] [CrossRef]
- Landolt, G.; Townsend, H.G.; Lunn, D.P. Equine Influenza. In Equine Infectious Diseases; Sellon, D., Long, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 124–134. [Google Scholar]
- Department of Agriculture, Fisheries and Forestry. Recovery of EI Country Free Status-OIE Report; Department of Agriculture, Fisheries and Forestry: Brisbane, Australia, 2008; pp. 27–29. [Google Scholar]
- Callinan, I. Equine Influenza: The August 2007 Outbreak in Australia; Department of Agriculture, Commonwealth of Australia: Canberra, Australia, 2008. [Google Scholar]
- Epidemiology Support Group. Equine Influenza 2007; Australian Experience Report from the EI Epidemiology Support Group to the Consultative Committee on Emergency Animal Disease December; Epidemiology Support Group: Parkville, Australia, 2008; pp. 62–74. [Google Scholar]
- Kannegieter, N.; Frogley, A.; Crispe, E.; Kirkland, P. Clinical outcomes and virology of equine influenza in a naïve population and in horses infected soon after receiving one dose of vaccine. Aust. Vet. J. 2011, 89, 139–142. [Google Scholar] [CrossRef]
- Arthur, R.; Suann, C. Biosecurity and vaccination strategies to minimise the effect of an equine influenza outbreak on racing and breeding. Aust. Vet. J. 2011, 89 (Suppl. 1), 109–113. [Google Scholar] [CrossRef]
- Daly, J.M.; Newton, J.R.; Mumford, J.A. Current perspectives on control of iequine influenza. Vet. Res. 2004, 35, 411–423. [Google Scholar] [CrossRef]
- Paillot, R.; Hannant, D.; Kydd, J.H.; Daly, J.M. Vaccination against equine influenza: Quid novi? Vaccine 2006, 24, 4047–4061. [Google Scholar] [CrossRef]
- Hannant, D.; Mumford, J.; Jessett, D. Duration of circulating antibody and immunity following infection with equine influenza virus. Vet. Rec. 1988, 122, 125–128. [Google Scholar] [CrossRef]
- Mumford, J.; Wood, J.M.; Scott, A.M.; Folkers, C.; Schild, G.C. Studies with inactivated equine influenza vaccine: 2. Protection against experimental infection with influenza virus A/equine/Newmarket/79 (H3N8). J. Hyg. 1983, 90, 385–396. [Google Scholar] [CrossRef]
- European Department for the Quality of Medicines. Equine Influenza Vaccine (Inactivated); European Department for the Quality of Medicines: Strassbourg, France, 2001. [Google Scholar]
- Hannant, D.; Mumford, J.A. Cell mediated immune responses in ponies following infection with equine influenza virus (H3N8): The influence of induction culture conditions on the properties of cytotoxic effector cells. Vet. Immunol. Immunopathol. 1989, 21, 327–337. [Google Scholar] [CrossRef]
- Paillot, R. A Systematic Review of Recent Advances in Equine Influenza Vaccination. Vaccines 2014, 2, 797–831. [Google Scholar] [CrossRef]
- Soboll, G.; Horohov, D.W.; Aldridge, B.M.; Olsen, C.W.; McGregor, M.W.; Drape, R.J.; Macklin, M.D.; Swain, W.F.; Lunn, D.P. Regional antibody and cellular immune responses to equine influenza virus infection, and particle mediated DNA vaccination. Vet. Immunol. Immunopathol. 2003, 94, 47–62. [Google Scholar] [CrossRef]
- Epstein, S.L.; Lo, C.Y.; Misplon, J.A.; Bennink, J.R. Mechanism of protective immunity against influenza virus infection in mice without antibodies. J. Immunol. 1998, 160, 322–327. [Google Scholar] [PubMed]
- Delves, P.J.; Martin, S.J.; Burton, D.R.; Roitt, I.M. Immunological Methods and Application Roitt’s Essential Immunology; Chichester Sussexx; Wiley-Blackwell: Hoboken, NJ, USA, 2012; p. 178. [Google Scholar]
- Salt, J.S. Veterinary Vaccinology; Pastoret, P.P., Blancou, J.P., Vannier, C., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 641–652. [Google Scholar]
- Lunn, D.P. Immunological Basis of Vaccination. In Proceedings of the 46th AAEP Annual Convention, San Antonio, TX, USA, 26–29 November 2000; pp. 1–9. [Google Scholar]
- Nelson, K.M.; Schram, B.R.; McGregor, M.W.; Sheoran, A.S.; Olsen, C.W.; Lunn, D.P. Local and systemic isotype-specific antibody responses to equine influenza virus infection versus conventional vaccination. Vaccine 1998, 16, 1306–1313. [Google Scholar] [CrossRef]
- Paillot, R.; Kydd, J.H.; MacRae, S.; Minke, J.M.; Hannant, D.; Daly, J.M. New assays to measure equine influenza virus-specific Type 1 immunity in horses. Vaccine 2007, 25, 7385–7398. [Google Scholar] [CrossRef]
- Gotch, F.; McMichael, A.; Smith, G.L.; Moss, B. Identification of viral molecules recognized by influenza-specific human cytotoxic T lymphocytes. J. Exp. Med. 1987, 165, 408–416. [Google Scholar] [CrossRef]
- Bryant, N.A.; Paillot, R.; Rash, A.S.; Medcalf, E.; Montesso, F.; Ross, J.; Watson, J.; Jeggo, M.; Lewis, N.S.; Newton, J.R.; et al. Comparison of two modern vaccines and previous influenza infection against challenge with an equine influenza virus from the Australian 2007 outbreak. Vet. Res. 2009, 41, 1–15. [Google Scholar] [CrossRef]
- Paillot, R.; Prowse, L.; Donald, C.; Medcalf, E.; Montesso, F.; Bryant, N.; Watson, J.; Jeggo, M.; Elton, D.; Newton, R.; et al. Efficacy of a whole inactivated EI vaccine against a recent EIV outbreak isolate and comparative detection of virus shedding. Veter-Immunol. Immunopathol. 2010, 136, 272–283. [Google Scholar] [CrossRef]
- Breathnach, C.; Clark, H.; Clark, R.; Olsen, C.; Townsend, H.; Lunn, D. Immunization with recombinant modified vaccinia Ankara (rMVA) constructs encoding the HA or NP gene protects ponies from equine influenza virus challenge. Vaccine 2006, 24, 1180–1190. [Google Scholar] [CrossRef]
- Boehm, U.; Klamp, T.; Groot, M.; Howard, J.C. Cellular responses to interferon-gamma. Annu. Rev. Immunol 1997, 15, 749–795. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Kydd, J.H.; Sindle, T.; Hannant, D.; Toulemonde, C.E.; Audonnet, J.C.; Minke, J.M.; Daly, J.M. Antibody and IFN-gamma responses induced by a recombinant canarypox vaccine and challenge infection with equine influenza virus. Vet. Immunol. Immunopathol. 2006, 112, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.A.; Pavlat, W.A.; Tartaglia, J.; Paoletti, E.; Weinhold, K.J.; Clements, M.L.; Siliciano, R.F. Induction of human immunodeficiency virus type 1 (HIV-1)-specific cytolytic T lymphocyte responses in seronegative adults by a nonreplicating, host-range-restricted canarypox vector (ALVAC) carrying the HIV-1MN env gene. J. Infect. Dis. 1995, 171, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- El Garch, H.; Minke, J.; Rehder, J.; Richard, S.; Toulemonde, C.E.; Dinic, S.; Andreoni, C.; Audonnet, J.; Nordgren, R.; Juillard, V. A West Nile virus (WNV) recombinant canarypox virus vaccine elicits WNV-specific neutralizing antibodies and cell-mediated immune responses in the horse. Vet. Immunol. Immunopathol. 2008, 123, 230–239. [Google Scholar] [CrossRef]
- Poulet, H.; Minke, J.; Pardo, M.C.; Juillard, V.; Nordgren, B.; Audonnet, J.C. Development and registration of recombinant veterinary vaccines. The example of the canarypox vector platform. Vaccine 2007, 25, 5606–5612. [Google Scholar] [CrossRef]
- Adams, A.A.; Sturgill, T.L.; Breathnach, C.C.; Chambers, T.M.; Siger, L.; Minke, J.M.; Horohov, D.W. Humoral and cell-mediated immune responses of old horses following recombinant canarypox virus vaccination and subsequent challenge infection. Vet. Immunol. Immunopathol. 2011, 139, 128–140. [Google Scholar] [CrossRef]
- Heldens, J.; Pouwels, H.; Derks, C.; Van de Zande, S.; Hoeijmakers, M. Duration of immunity induced by an equine influenza and tetanus combination vaccine formulation adjuvanted with ISCOM-Matrix. Vaccine 2010, 28, 6989–6996. [Google Scholar] [CrossRef]
- Thein, P.; Röhm, A.; Voss, J. Experimentelle Untersuchungen zur Tetanusimmunantwort von Fohlen und erwachsenen Pferden unter Einsatz des Fassisi TetaCheck®. Pferdeheilkunde Fundus 2013, 29, 686–699. [Google Scholar] [CrossRef]
- Paillot, R.; Prowse, L.; Montesso, F.; Huang, C.M.; Barnes, H.; Escala, J. Whole inactivated equine influenza vaccine: Efficacy against a representative clade 2 equine influenza virus, IFNgamma synthesis and duration of humoral immunity. Vet. Microbiol. 2013, 162, 396–407. [Google Scholar] [CrossRef]
- Perkins, N.; Webster, W.; Wright, T.; Denney, I.; Links, I. Vaccination program in the response to the 2007 equine influenza outbreak in Australia. Aust. Vet. J. 2011, 89, 126–134. [Google Scholar] [CrossRef]
- Minke, J.M.; El-Hage, C.M.; Tazawa, P.; Homer, D.; Lemaitre, L.; Cozette, V.; Gilkerson, J.R.; Kirkland, P.D. Evaluation of the response to an accelerated immunisation schedule using a canarypox-vectored equine influenza vaccine, shortened interdose intervals and vaccination of young foals. Aust. Vet. J. 2011, 89 (Suppl. 1), 137–139. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, A.J. Equine Influenza in South Africa, 2003 Outbreak. In Proceedings of the 9th International Congress of World Equine Veterinary Association, Marrakech, Morocco, 26 January 2006. [Google Scholar]
- El-Hage, C.; Savage, C.J.; Minke, J.M.; Ficorilli, N.P.; Watson, J.; Gilkerson, J.R. Accelerated vaccination schedule provides protective levels of antibody and complete herd immunity to equine influenza. Equine Vet. J. 2012, 45, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Soboll, G.; Hussey, S.B.; Minke, J.M.; Landolt, G.A.; Hunter, J.S.; Jagannatha, S.; Lunn, D.P. Onset and duration of immunity to equine influenza virus resulting from canarypox-vectored (ALVAC®) vaccination. Vet. Immunol. Immunopathol. 2010, 135, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, P.; Delbridge, G. Use of a blocking ELISA for antibodies to equine influenza virus as a test to distinguish between naturally infected and vaccinated horses: Proof of concept studies. Aust. Vet. J. 2011, 89, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Daly, J.; Juillard, V.; Minke, J.; Hannant, D.; Kydd, J. Equine interferon gamma synthesis in lymphocytes after in vivo infection and in vitro stimulation with EHV-1. Vaccine 2005, 23, 4541–4551. [Google Scholar] [CrossRef]
- Bryant, N.A.; Rash, A.S.; Russell, C.A.; Ross, J.; Cooke, A.; Bowman, S.; MacRae, S.; Lewis, N.S.; Paillot, R.; Zanoni, R.; et al. Antigenic and genetic variations in European and North American equine influenza virus strains (H3N8) isolated from 2006 to 2007. Vet. Microbiol. 2009, 138, 41–52. [Google Scholar] [CrossRef]
- Lai, A.; Chambers, T.M.; Holland, R.E., Jr.; Morley, P.S.; Haines, D.M.; Townsend, H.G.G.; Barrandeguy, M. Diverged evolution of recent equine-2 influenza (H3N8) viruses in the Western Hemisphere. Arch. Virol. 2001, 146, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Minke, J.M.; Audonnet, J.C.; Fischer, L. Equine viral vaccines: The past, present and future. Vet. Res. 2004, 35, 425–443. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.S.; Abdel-Ghany, A.E.; Shany, S.A. Isolation and characterization of highly pathogenic avian influenza virus subtype H5N1 from donkeys. J. Biomed. Sci. 2010, 17, 25. [Google Scholar] [CrossRef]
- Hans, A.; Laabassi, F.; Amelot, G.; Legrand, L.; Gaudaire, D.; Laugier, C.; Pronost, S.; Zientara, S. Serological evidence of circulation of Equine H3N8 Influenza Virus in Algeria and its molecular characterization. J. Equine Vet. Sci. 2012, 32, S11–S12. [Google Scholar] [CrossRef]
- Wilson, A.I.; Cox, N.J. Structural Basis Of Immune Recognition Of Influenza Virus Hemagglutinin. Annu. Rev. Immunol. 1990, 8, 737–771. [Google Scholar] [CrossRef]
- Daly, J.M.; MacRae, S.; Newton, J.R.; Wattrang, E.; Elton, D.M. Equine influenza: A review of an unpredictable virus. Vet. J. 2011, 189, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.M.; Sindle, T.; Tearle, J.; Barquero, N.; Newton, J.R.; Corning, S. Equine influenza vaccine containing older H3N8 strains offers protection against A/eq/South Africa/4/03 (H3N8) strain in a short-term vaccine efficacy study. Equine Vet. J. 2007, 39, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Murcia, P.R.; Baillie, G.J.; Stack, J.C.; Jervis, C.; Elton, D.; Mumford, J.A.; Daly, J.; Kellam, P.; Grenfell, B.T.; Holmes, E.C.; et al. Evolution of equine influenza virus in vaccinated horses. J. Virol. 2013, 87, 4768–4771. [Google Scholar] [CrossRef] [PubMed]
- Madic, J.; Martinovic, S.; Naglic, T.; Hajsig, D.; Cvetnic, S. Serological evidence for the presence of A/equine-1 influenza virus in unvaccinated horses in Croatia. Vet. Rec. 1996, 138, 68. [Google Scholar] [CrossRef]
- Gildea, S.; Arkins, S.; Walsh, C.; Cullinane, A. A comparison of antibody responses to commercial equine influenza vaccines following primary vaccination of Thoroughbred weanlings—A randomised blind study. Vaccine 2011, 29, 9214–9223. [Google Scholar] [CrossRef]
- Elton, D.; Cullinane, A. Equine influenza: Antigenic drift and implications for vaccines. Equine Vet. J. 2013, 45, 768–769. [Google Scholar] [CrossRef]
- Mumford, J.A. Control of Equine Influenza from Man International Perspective. In Proceedings of the Equine Infectious Diseases VIII, Dubai, United Arab Emirates, 23–26 March 1998; R & W Publications: Newmarket, UK, 1998. [Google Scholar]
- Burrows, R.; Goodridge, D.; Denyer, M.; Hutchings, G.; Frank, C.J. Equine influenza infections in Great Britain, 1979. Vet. Rec. 1982, 110, 494–497. [Google Scholar] [CrossRef]
- Wood, J.L.N. A Review of the History and Epidemiology and a Description of Recent Outbreak. Master’s Thesis, University of London, London, UK, 1991. [Google Scholar]
- Hinshaw, V.S.; Naeve, C.W.; Webster, R.G.; Douglas, A.; Skehel, J.J.; Bryans, J. Analysis of antigenic variation in equine 2 influenza A viruses. Bull. World Health Organ. 1983, 61, 153–158. [Google Scholar]
- Murcia, P.R.; Wood, J.L.; Holmes, E.C. Genome-scale evolution and phylodynamics of equine H3N8 influenza A virus. J. Virol. 2011, 85, 5312–5322. [Google Scholar] [CrossRef]
- Newton, J.R.; Lakhan, K.H.; Wood, J.L.N.; Baker, D.J. Risk factors for equine influenza serum antibody titres in young Thoroughbred racehorses given an inactivated vaccine. Prev. Vet. Med. 2000, 46, 129–141. [Google Scholar] [CrossRef]
- Newton, J.R. Equine Influenza Vaccine Performance: Still Learning Lessons from the Field. Vet. J. 2001, 161, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Petermann, H.G.; Stellmann, C.; Graveline, P. A Study of Different Equine Influenza Vaccination Schedules in Seronegative Foals and in Horses. Zent. Veterinärmed. Reihe B 1973, 20, 361–366. [Google Scholar] [CrossRef]
- Cullinane, A.; Gildea, S.; Weldon, E. Comparison of primary vaccination regimes for equine influenza: Working towards an evidence-based regime. Equine Vet. J. 2014, 46, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Paillot, R.; Prowse, L.; Montesso, F.; Stewart, B.; Jordon, L.; Newton, J.R. Duration of equine influenza virus shedding and infectivity in immunised horses after experimental infection with EIV A/eq2/Richmond/1/07. Vet. Microbiol. 2013, 166, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Minke, J.M.; Toulemonde, C.E.; Coupier, H.; Guigal, P.-M.; Dinic, S.; Sindle, T.; Jessett, D.; Black, L.; Bublot, M.; Pardo, M.C.; et al. Efficacy of a canarypox-vectored recombinant vaccine expressing the hemagglutinin gene of equine influenza H3N8 virus in the protection of ponies from viral challenge. Am. J. Vet. Res. 2007, 68, 213–219. [Google Scholar] [CrossRef]
- Mumford, J.; Wood, J. WHO/OIE Meeting: Consultation on Newly Emerging Strains of Equine Influenza. Vaccine 1993, 11, 1172–1174. [Google Scholar] [CrossRef]
- Morley, P.S.; Hanson, L.K.; Bogdan, J.R.; Townsend, H.G.; Appleton, J.A.; Haines, D.M. The relationship between single radial hemolysis, hemagglutination inhibition, and virus neutralization assays used to detect antibodies specific for equine influenza viruses. Vet. Microbiol. 1995, 45, 81–92. [Google Scholar] [CrossRef]
- Chambers, T.M.; Reedy, S.E. Equine Influenza Serological Methods. Anim. Influenza Virus 2014, 1161, 411–422. [Google Scholar]
- OIE. Expert surveillance panel of equine influenza vaccines—Conclusions and recommendations. In Proceedings of the Expert Surveillance Panel of Equine Influenza Vaccines, Amelia Island, FL, USA, 2 February 2010; pp. 44–45. [Google Scholar]
- Paillot, R.; Prowse, L. ISCOM-matrix-based equine influenza (EIV) vaccine stimulates cell-mediated immunity in the horse. Veter-Immunol. Immunopathol. 2012, 145, 516–521. [Google Scholar] [CrossRef]
- Sallusto, F.; Lenig, D.; Forster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Gildea, S.; Quinlivan, M.; Murphy, B.A.; Cullinane, A. Humoral response and antiviral cytokine expression following vaccination of thoroughbred weanlings—A blinded comparison of commercially available vaccines. Vaccine 2013, 31, 5216–5222. [Google Scholar] [CrossRef] [PubMed]
- Bryant, N.A.; Rash, A.; Woodward, A.L.; Medcalf, E.; Helwegen, M.; Wohlfender, F.; Cruz, F.; Herrmann, C.; Borchers, K.; Tiwari, A.; et al. Isolation and characterisation of equine influenza viruses (H3N8) from Europe and North America from 2008 to 2009. Vet. Microbiol. 2010, 147, 19–27. [Google Scholar] [CrossRef] [PubMed]
| EIV | Lineage | Use |
|---|---|---|
| A/equine/Newmarket/95 | Eurasian | HI assay |
| A/equine/Newmarket/2/93 | Eurasian | HA used in vaccine |
| A/equine/Sussex/89 | Eurasian | γ-Interferon assay |
| A/equine/Kentucky/94 | American | HA used in vaccine |
| A/equine/Sydney/07 | American Fc1 | HI assay (local outbreak isolate) |
| A/equine/Richmond/1/07 | American Fc2 | γ-Interferon assay |
| A/equine/Prague/1956 (H7N7) = | N/A | HI assay |
| Horse ID | Age (Years) | Sex |
|---|---|---|
| 1 | 6 | Female |
| 2 | 5 | Female |
| 3 | 4 | Gelding |
| 4 | 5 | Stallion |
| 5 | 8 | Gelding |
| 6 | 3 | Female |
| 7 | 2 | Female |
| 8 | 4 | Gelding |
| 9 | 3 | Female |
| 10 | 3 | Female |
| 11 | 3 | Female |
| 12 | 4 | Gelding |
| Horse ID | Day 0 V1 a | Day 14 | Day28 V2 | Day 42 | Day 56 | Day 84 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sy07b | NM95c | Sy07 | NM95 | Sy07 | NM95 | Sy07 | NM95 | Sy07 | NM95 | Sy07 | NM95 | |
| 0 | <8 | <8 | 8 | 8 | 16 | 16 | NA | NA | 64 | 128 | 16 | 32 |
| 1 | <8 | <8 | <8 | <8 | 8 | 8 | 64 | 128 | 64 | 64 | 8 | 32 |
| 2 | <8 | <8 | 8 | <8 | <8 | <8 | 128 | 16 | 64 | 16 | 16 | 8 |
| F2 | <8 | <8 | 8 | <8 | 16 | <8 | 32 | <8 | NA | NA | NA | NA |
| 20 | <8 | <8 | <8 | <8 | <8 | NA | <8 | <8 | <8 | <8 | <8 | <8 |
| 24 | <8 | <8 | <8 | NA | NA | 8 | <8 | 32 | <8 | 16 | 32 | 8 |
| 26 | <8 | <8 | NA | 8 | 32 | <8 | 16 | 32 | 8 | 32 | 8 | 16 |
| 43 | <8 | <8 | <8 | 8 | 8 | 32 | 8 | 32 | 16 | 32 | <8 | 8 |
| 47 | <8 | <8 | <8 | 8 | 8 | 8 | 64 | 16 | NA | NA | <8 | 8 |
| 126 | <8 | <8 | <8 | <8 | NA | NA | 16 | <8 | 16 | 16 | <8 | 8 |
| 142 | <8 | <8 | 8 | 8 | NA | NA | 16 | 32 | 16 | 16 | <8 | 8 |
| 143 | <8 | <8 | <8 | 8 | <8 | NA | 16 | 16 | 8 | 16 | <8 | 8 |
| EIV Status | Number | Breed |
|---|---|---|
| ProteqFlu-Te V1-V2 14 day interval | 7 | Thoroughbred |
| ProteqFlu-Te V1-V2 42 day interval | 2 | Thoroughbred |
| Previously infected | 3 | Welsh Mountain Pony |
| Uninfected/unvaccinated | 6 | Thoroughbred |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Hage, C.; Hartley, C.; Savage, C.; Watson, J.; Gilkerson, J.; Paillot, R. Assessment of Humoral and Long-Term Cell-Mediated Immune Responses to Recombinant Canarypox-Vectored Equine Influenza Virus Vaccination in Horses Using Conventional and Accelerated Regimens Respectively. Vaccines 2022, 10, 855. https://doi.org/10.3390/vaccines10060855
El-Hage C, Hartley C, Savage C, Watson J, Gilkerson J, Paillot R. Assessment of Humoral and Long-Term Cell-Mediated Immune Responses to Recombinant Canarypox-Vectored Equine Influenza Virus Vaccination in Horses Using Conventional and Accelerated Regimens Respectively. Vaccines. 2022; 10(6):855. https://doi.org/10.3390/vaccines10060855
Chicago/Turabian StyleEl-Hage, Charles, Carol Hartley, Catherine Savage, James Watson, James Gilkerson, and Romain Paillot. 2022. "Assessment of Humoral and Long-Term Cell-Mediated Immune Responses to Recombinant Canarypox-Vectored Equine Influenza Virus Vaccination in Horses Using Conventional and Accelerated Regimens Respectively" Vaccines 10, no. 6: 855. https://doi.org/10.3390/vaccines10060855
APA StyleEl-Hage, C., Hartley, C., Savage, C., Watson, J., Gilkerson, J., & Paillot, R. (2022). Assessment of Humoral and Long-Term Cell-Mediated Immune Responses to Recombinant Canarypox-Vectored Equine Influenza Virus Vaccination in Horses Using Conventional and Accelerated Regimens Respectively. Vaccines, 10(6), 855. https://doi.org/10.3390/vaccines10060855







