Immunogenicity of the 13-Valent Pneumococcal Conjugate Vaccine (PCV13) Followed by the 23-Valent Pneumococcal Polysaccharide Vaccine (PPSV23) in Adults with and without Immunosuppressive Therapy
Abstract
:1. Introduction
2. Materials and Methods
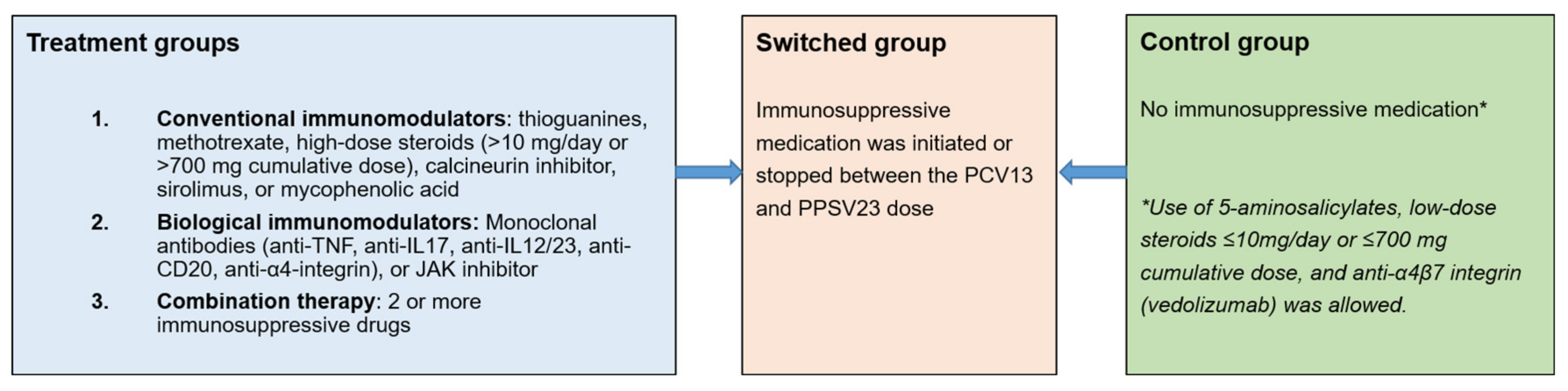
2.1. Study Population
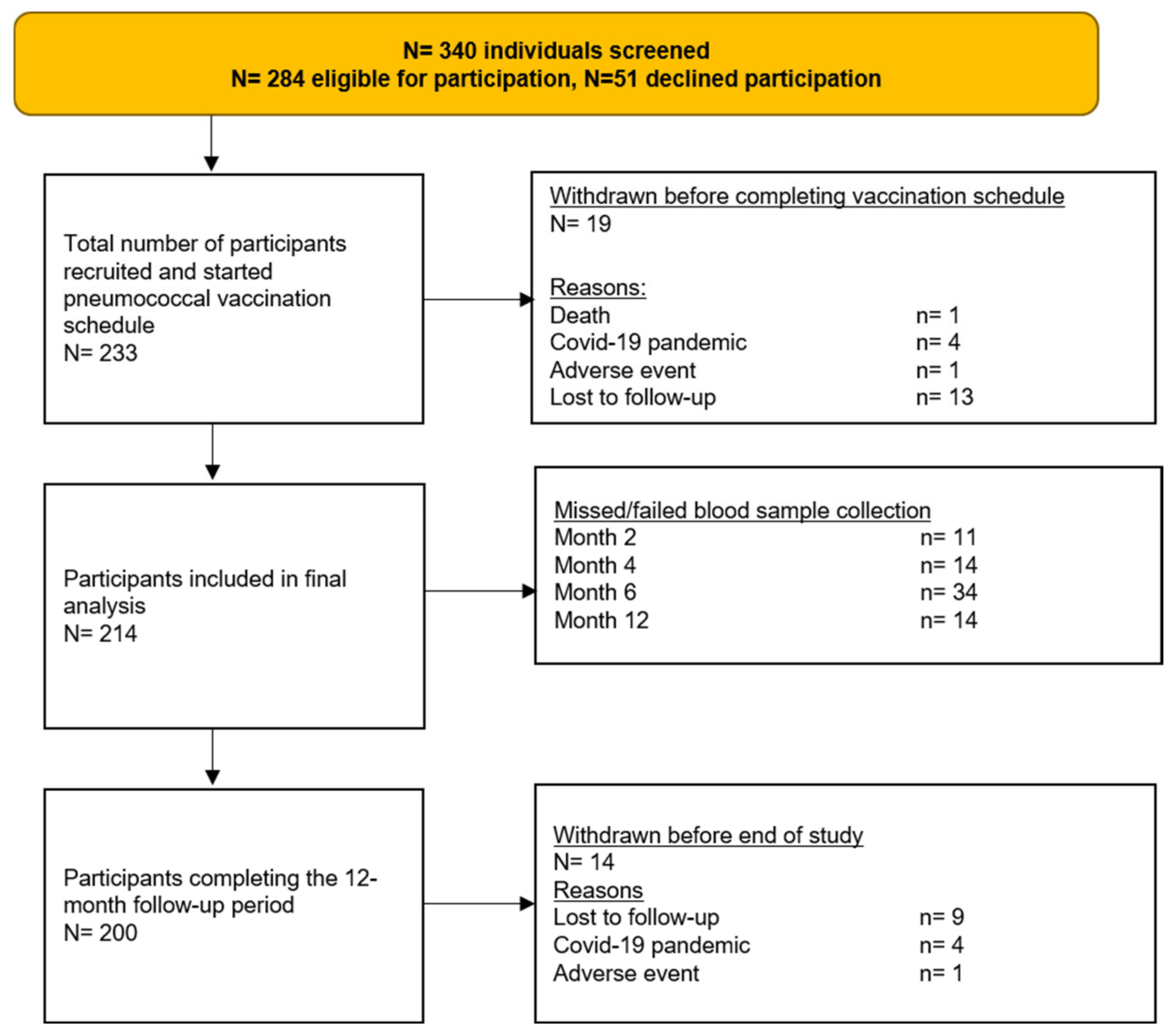
2.2. Sample Size
2.3. Study Procedures
2.4. Outcomes and Analysis
2.5. Ethical Considerations
3. Results
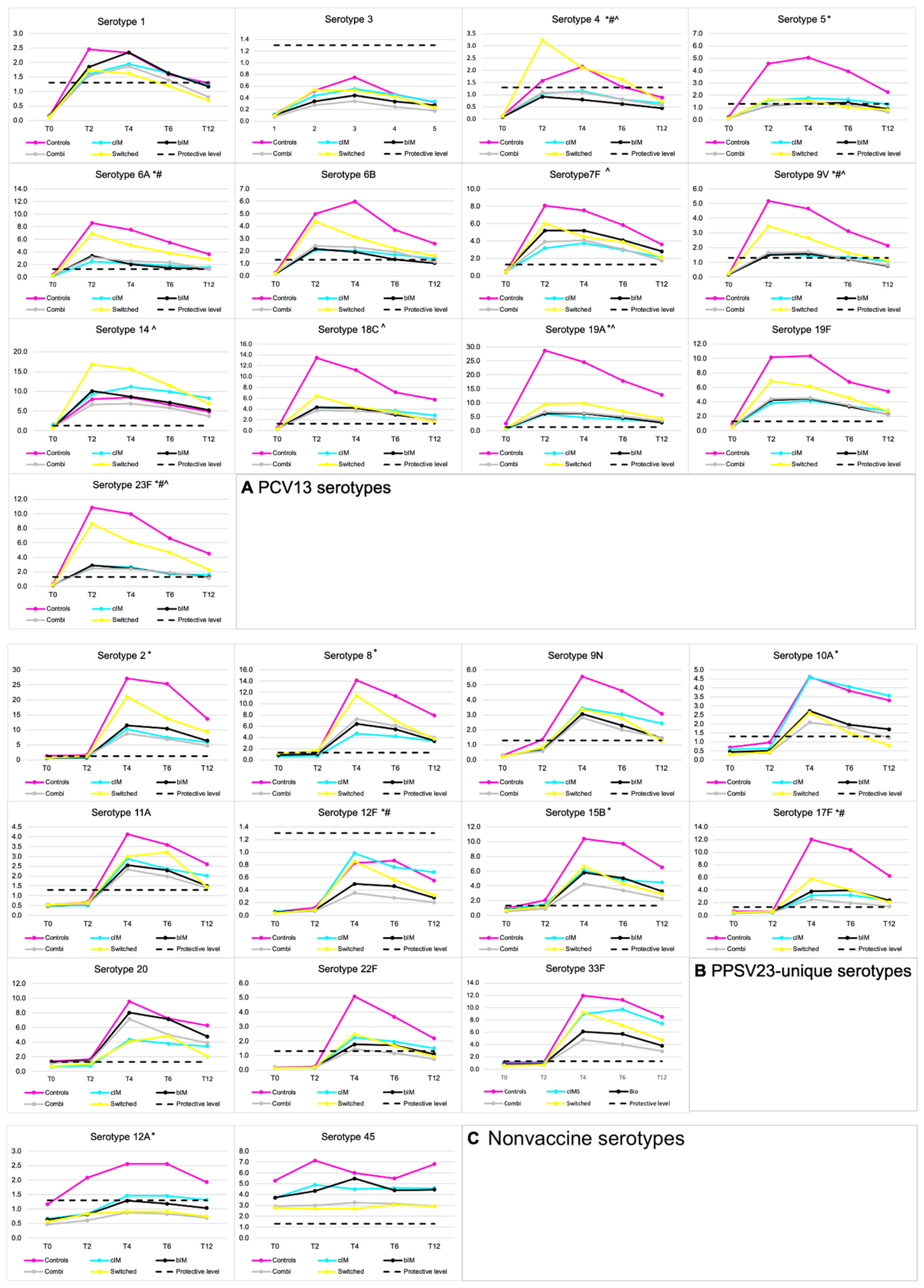
3.1. Seroprotection Rates
3.2. Predictors of Seroconversion
3.3. Waning Immunity
3.4. Safety
4. Discussion
4.1. The Effect of Different Immunosuppressive Agents on Seroprotection
4.2. Different Pneumococcal Vaccination Schedules
4.3. Duration of Protection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Aalst, M.; Lötsch, F.; Spijker, R.; van der Meer, J.T.; Langendam, M.W.; Goorhuis, A.; Grobusch, M.P.; de Bree, G.J. Incidence of invasive pneumococcal disease in immunocompromised patients: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2018, 24, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Shigayeva, A.; Rudnick, W.; Green, K.; Chen, D.K.; Demczuk, W.; Gold, W.L.; Johnstone, J.; Kitai, I.; Krajden, S.; Lovinsky, R.; et al. Invasive Pneumococcal Disease Among Immunocompromised Persons: Implications for Vaccination Programs. Clin. Infect. Dis. 2016, 62, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schurder, J.; Goulenok, T.; Jouenne, R.; Dossier, A.; Van Gysel, D.; Papo, T.; Sacre, K. Pneumococcal infection in patients with systemic lupus erythematosus. Jt. Bone Spine 2018, 85, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, M.F.; Sotgiu, G.; Gramegna, A.; Radovanovic, D.; Terraneo, S.; Reyes, L.F.; Rupp, J.; González del Castillo, J.; Blasi, F.; Aliberti, S.; et al. Prevalence and Etiology of Community-acquired Pneumonia in Immuno-compromised Patients. Clin. Infect. Dis. 2019, 68, 1482–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Aalst, M.; Garcia Garrido, H.M.; Van Der Leun, J.; Meek, B.; Van Leeuwen, E.M.; Löwenberg, M.; D’haens, G.R.; Ponsioen, C.Y.; Grobusch, M.P.; Goorhuis, A.; et al. Immunogenicity of the currently recommended pneumococcal vaccination schedule in patients with inflammatory bowel disease. Clin. Infect. Dis. 2020, 70, 595–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia Garrido, H.M.; Veurink, A.M.; Leeflang, M.; Spijker, R.; Goorhuis, A.; Grobusch, M.P. Hepatitis A vaccine immunogenicity in patients using immunosuppressive drugs: A systematic review and meta-analysis. Travel Med. Infect. Dis. 2019, 32, 101479. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Mariette, X.; Bachelez, H.; Belot, A.; Bonnotte, B.; Hachulla, E.; Lahfa, M.; Lortholary, O.; Loulergue, P.; Paul, S.; et al. Vaccination recommendations for the adult immunosuppressed patient: A systematic review and comprehensive field synopsis. J. Autoimmun. 2017, 80, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.G.; Levin, M.J.; Ljungman, P.; Davies, E.G.; Avery, R.; Tomblyn, M.; Bousvaros, A.; Dhanireddy, S.; Sung, L.; Keyserling, H.; et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin. Infect. Dis. 2014, 58, 309–318. [Google Scholar] [CrossRef]
- Jackson, L.A.; Gurtman, A.; van Cleeff, M.; Frenck, R.W.; Treanor, J.; Jansen, K.U.; Scott, D.A.; Emini, E.A.; Gruber, W.C.; Schmoele-Thoma, B. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine 2013, 31, 3594–3602. [Google Scholar] [CrossRef] [Green Version]
- Van Aalst, M.; Langedijk, A.C.; Spijker, R.; de Bree, G.J.; Grobusch, M.P.; Goorhuis, A. The effect of immunosuppressive agents on immunogenicity of pneumococcal vaccination: A systematic review and meta-analysis. Vaccine 2018, 36, 5832–5845. [Google Scholar] [CrossRef]
- Kumar, D.; Chen, M.H.; Wong, G.; Cobos, I.; Welsh, B.; Siegal, D.; Humar, A. A Randomized, Double-Blind, Placebo-Controlled Trial to Evaluate the Prime-Boost Strategy for Pneumococcal Vaccination in Adult Liver Transplant Recipients. Clin. Infect. Dis. 2008, 47, 885–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, M.T.; Lindegaard, H.; Hendricks, O.; Jørgensen, C.S.; Kantsø, B.; Friis-Møller, N. Initial Serological Response after Prime-boost Pneumococcal Vaccination in Rheumatoid Arthritis Patients: Results of a Randomized Controlled Trial. J. Rheumatol. 2017, 44, 1794–1803. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Invasive Pneumococcal Disease. In ECDC: Annual Epidemiological Report for 2018; ECDC: Stockholm, Sweden, 2020; Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2018_IPD.pdf (accessed on 16 March 2022).
- Ladhani, S.N.; Collins, S.; Djennad, A.; Sheppard, C.L.; Borrow, R.; Fry, N.K.; Andrews, N.J.; Miller, E.; Ramsay, M.E. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000–2017: A prospective national observational cohort study. Lancet Infect. Dis. 2018, 18, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Stuck, A.E.; Minder, C.E.; Frey, F.J. Risk of Infectious Complications in Patients Taking Glucocorticosteroids. Clin. Infect. Dis. 1989, 11, 954–963. [Google Scholar] [CrossRef]
- Garrido, H.M.G.; Haggenburg, S.; Schoordijk, M.C.E.; Meijer, E.; Tanck, M.W.T.; Hazenberg, M.D.; Rutten, C.E.; de Bree, G.J.; Nur, E.; Meek, B.; et al. Immunogenicity of a 5-dose pneumococcal vaccination schedule following allogeneic hematopoietic stem cell transplantation. Am. J. Hematol. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Orange, J.S.; Ballow, M.; Stiehm, E.R.; Ballas, Z.; Chinen, J.; De La Morena, M.; Kumararatne, D.; Harville, T.O.; Hesterberg, P.; Koleilat, M.; et al. Use and interpretation of diagnostic vaccination in primary immunodeficiency: A working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J. Allergy Clin. Immunol. 2012, 130 (Suppl. S3), S1–S24. [Google Scholar] [CrossRef]
- Andrews, N.J.; Waight, P.A.; Burbidge, P.; Pearce, E.; Roalfe, L.; Zancolli, M.; Slack, M.; Ladhani, S.N.; Miller, E.; Goldblatt, D. Serotype-specific effectiveness and correlates of protection for the 13-valent pneumococcal conjugate vaccine: A postlicensure indirect cohort study. Lancet Infect. Dis. 2014, 14, 839–846. [Google Scholar] [CrossRef]
- Bonten, M.J.M.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; van Werkhoven, C.H.; Van Deursen, A.M.M.; Sanders, E.A.M.; Verheij, T.J.M.; et al. Polysaccharide Conjugate Vaccine against Pneumococcal Pneumonia in Adults. N. Engl. J. Med. 2015, 372, 1114–1125. [Google Scholar] [CrossRef] [Green Version]
- Sorensen, R.U.; Leiva, L.E.; Javier, F.C.; Sacerdote, D.M.; Bradford, N.; Butler, B.; Giangrosso, P.A.; Moore, C. Influence of age on the response to Streptococcus pneumoniae vaccine in patients with recurrent infections and normal immunoglobulin concentrations. J. Allergy Clin. Immunol. 1998, 102, 215–221. [Google Scholar] [CrossRef]
- Fiorino, G.; Peyrin-Biroulet, L.; Naccarato, P.; Szabò, H.; Sociale, O.R.; Vetrano, S.; Fries, W.; Montanelli, A.; Repici, A.; Malesci, A.; et al. Effects of immunosuppression on immune response to pneumococcal vaccine in inflammatory bowel disease: A prospective study. Inflamm. Bowel Dis. 2012, 18, 1042–1047. [Google Scholar] [CrossRef]
- Kapetanovic, M.C.; Nagel, J.; Nordstrom, I.; Saxne, T.; Geborek, P.; Rudin, A. Methotrexate reduces vaccine-specific immunoglobulin levels but not numbers of circulating antibody-producing B cells in rheumatoid arthritis after vaccination with a conjugate pneumococcal vaccine. Vaccine 2017, 35, 903–908. [Google Scholar] [CrossRef] [Green Version]
- Kapetanovic, M.C.; Roseman, C.; Jonsson, G.; Truedsson, L.; Saxne, T.; Geborek, P. Antibody response is reduced following vac-cination with 7-valent conjugate pneumococcal vaccine in adult methotrexate-treated patients with established arthritis, but not those treated with tumor necrosis factor inhibitors. Arthritis Rheum. 2011, 63, 3723–3732. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.M.; Pratt, A.; Isaacs, J. Mechanism of action of methotrexate in rheumatoid arthritis, and the search for biomarkers. Nat. Rev. Rheumatol. 2016, 12, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Heusele, M.; Clerson, P.; Guery, B.; Lambert, M.; Launay, D.; Lefevre, G.; Morell-Dubois, S.; Maillard, H.; Le Gouellec, N.; Hatron, P.Y.; et al. Risk factors for severe bacterial infections in patients with systemic autoimmune diseases receiving rituximab. Clin. Rheumatol. 2014, 33, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Kumar, D.; Rotstein, C.; Miyata, G.; Arlen, D.; Humar, A. Randomized, double-blind, controlled trial of pneumococcal vaccination in renal transplant recipients. J. Infect. Dis. 2003, 187, 1639–1645. [Google Scholar] [CrossRef]
- Barton, M.; Wasfy, S.; Dipchand, A.I.; Hébert, D.; Ng, V.; Solomon, M.; Fecteau, A.; Stephen, D.; Allen, U. Seven-valent pneumococcal conjugate vaccine in pediatric solid organ transplant recipients: A prospective study of safety and immunogenicity. Pedr. Infect. Dis. J. 2009, 28, 688–692. [Google Scholar] [CrossRef]
- Cordonnier, C.; Ljungman, P.; Juergens, C.; Maertens, J.; Selleslag, D.; Sundaraiyer, V.; Giardina, P.C.; Clarke, K.; Gruber, W.C.; Scott, D.A.; et al. Immunogenicity, safety, and tolerability of 13-valent pneumococcal conjugate vaccine followed by 23-valent pneumococcal polysaccharide vaccine in recipients of allogeneic hematopoietic stem cell transplant aged >/=2 years: An open-label study. Clin. Infect. Dis. 2015, 61, 313–323. [Google Scholar] [CrossRef] [Green Version]
- Love, B.L.; Finney, C.J.; Gaidos, J.K.J. Effectiveness of Conjugate and Polysaccharide Pneumococcal Vaccines for Prevention of Severe Pneumococcal Disease Among Inflammatory Bowel Disease Patients. J. Crohn’s Colitis 2021, 15, 1279–1283. [Google Scholar] [CrossRef]
- Kumar, D.; Welsh, B.; Siegal, D.; Chen, M.H.; Humar, A. Immunogenicity of pneumococcal vaccine in renal transplant recipients—Three year follow-up of a randomized trial. Am. J. Transpl. 2007, 7, 633–638. [Google Scholar] [CrossRef]
- Bahuaud, M.; Beaudouin-Bazire, C.; Husson, M.; Molto, A.; Launay, O.; Batteux, F.; Dougados, M. Immunogenicity and persistence of a prime-boost re-vaccination strategy for pneumococcal vaccines in patients with rheumatoid arthritis. Hum. Vaccines Immunother. 2018, 14, 1464–1470. [Google Scholar] [CrossRef] [Green Version]

| Total Cohort N = 214 | Groups Based on Use of Immunosuppressive Medication | ||||||
|---|---|---|---|---|---|---|---|
| Conventional Immunomodulators N = 47 | Biological immunomodulators N = 50 | Combination Therapy N = 60 | Switched N = 21 1 | Controls N = 36 | p-Value across Groups | ||
| Males n (%) | 100 (47) | 20 (43) | 26 (52) | 32 (53) | 11 (52) | 11 (31) | 0.20 |
| Age, median (IQR 2) | 41 (26) | 47 (30) | 40 (26) | 41 (20) | 30 (23) | 48 (24) | 0.10 |
| Age group 18–49 n (%) | 139 (65) | 26 (55) | 33 (66) | 45 (75) | 17 (81) | 18 (50) | 0.03 |
| Age group 50–70 n (%) | 75 (35) | 21 (45) | 17 (34) | 15 (25) | 4 (19) | 18 (50) | |
| Body mass index, median (IQR) | 24 (6) | 24 (5) | 25 (6) | 24 (7) | 25 (6) | 23 (7) | 0.42 |
| Current smoker n (%) | 32 (15) | 7 (15) | 7 (14) | 6 (10) | 5 (24) | 7 (19) | 0.55 |
| Previous smoker n (%) | 54 (25) | 10 (21) | 14 (28) | 11 (18) | 7 (33) | 12 (33) | 0.39 |
| Alcohol use >7/week N (%) | 41 (19) | 7 (15) | 6 (12) | 16 (17) | 4 (19) | 8 (22) | 0.33 |
| Drug use (yes/no) n (%) | 21 (9.8) | 5 (11) | 2 (4.0) | 6 (10) | 5 (24) | 3 (8.3) | 0.15 |
| Underlying disease | |||||||
| Crohn’s disease n (%) | 48 (22) | 8 (17) | 9 (18) | 9 (15) | 9 (43) | 13 (36) | 0.02 |
| Ulcerative colitis n (%) | 32 (15) | 6 (13) | 9 (18) | 7 (11) | 3 (14) | 7 (19) | 0.8 |
| Rheumatoid arthritis n (%) | 31 (14) | 14 (30) | 6 (12) | 8 (13) | 2 (9.5) | 1 (2.8) | 0.01 |
| Psoriasis/psoriatic arthritis n (%) | 32 (15) | 6 (13) | 14 (28) | 5 (8.3) | 4 (19) | 3 (8.3) | 0.03 |
| Spondylitis ankylopoietica n (%) | 7 (3.3) | 0 (0) | 4 (8.0) | 0 (0) | 2 (9.5) | 1 (2.8) | 0.04 |
| Neurological autoimmune disease n (%) | 7 (3.3) | 1 (2.1) | 5 (10) | 1 (1.7) | 0 (0) | 0 (0) | 0.05 |
| Other autoinflammatory disease 3 n (%) | 21 (9.8) | 10 (21) | 3 (6) | 3 (5) | 1 (4.8) | 4 (11) | 0.04 |
| Solid organ transplant recipient 4 n (%) | 29 (14) | 2 (4.3) | 0 (0) | 27 (45) | 0 (0) | 0 (0) | <0.01 |
| Comorbidity score (Charlson comorbidity index) median (IQR) | 1 (1) | 1 (2) | 1 (1) | 2 (2) | 1 (2] | 1.5 (1) | 0.03 |
| Pulmonary disease in medical history n (%) | 15 (7.0) | 4 (8.5) | 4 (8.0) | 3 (5.0) | 1 (4.8) | 4 (11) | 0.79 |
| Impaired kidney function (eGFR 5 < 60) n (%) | 24 (11) | 3 (6.4) | 2 (4.0) | 17 (28) | 0 (0) | 2 (5.6) | <0.01 |
| Number of drugs at baseline | |||||||
| One drug n (%) | 98 (46) | 47 (100) | 50 (100) | 0 (0) | 1 (4.8) | 0 (0) | <0.01 |
| Two drugs n (%) | 39 (18) | 0 (0) | 0 (0) | 39 (65) | 0 (0) | 0 (0) | |
| Three drugs n (%) | 21 (9.8) | 0 (0) | 0 (0) | 21 (35) | 0 (0) | 0 (0) | |
| Conventional immunomodulator n (%) | 92 (43) | 47 (100) | 0 (0) | 45 (75) | 0 (0) | 0 (0) | <0.01 |
| Prednisolone (>10 mg/day or 700 mg cumulative) (%) | 38 (18) | 2 (4.3) | 0 (0) | 36 (60) | 0 (0) | 0 (0) | <0.01 |
| Thiopurine n (%) | 28 (13) | 15 (32) | 0(0) | 13 (22) | 0 (0) | 0 (0) | <0.01 |
| Methotrexate (7.5–30 mg/week) n (%) | 37 (17) | 21 (45) | 0 (0) | 16 (27) | 0 (0) | 0 (0) | <0.01 |
| Calcineurin inhibitor n (%) | 25 (12) | 2 (4.3) | 0 (0) | 23 (38) | 0 (0) | 0 (0) | <0.01 |
| Mycophenolate mofetil n (%) | 27 (13) | 5 (11) | 0 (0) | 22 (37) | 0 (0) | 0 (0) | <0.01 |
| Other n (%) 6 | 4 (1.9) | 2 (4.3) | 0 (0) | 2 (3.3) | 0 (0) | 0 (0) | 0.38 |
| Biological immunomodulator n (%) | 79 (37) | 0 (0) | 50 (100) | 28 (47) | 1 (4.8) | 0 (0) | <0.01 |
| TNF-alpha inhibitor at baseline n (%) | 60 (28) | 0 (0) | 32 (64) | 24 (40) | 4 (19) | 0 (0) | <0.01 |
| Etanercept n (%) | 7 (3.3) | 0 (0) | 5 (10) | 2 (3.3) | 0 (0) | 0 (0) | 0.03 |
| Infliximab n (%) | 22 (10) | 0 (0) | 10 (20) | 12 (20) | 0 (0) | 0 (0) | <0.01 |
| Adalimumab n (%) | 22 (10) | 0 (0) | 15 (30) | 7 (12) | 0 (0) | 0 (0) | <0.01 |
| Certolizumab pegol n (%) | 3 (1.4) | 0 (0) | 1 (2.0) | 2 (3.3) | 0 (0) | 0 (0) | 0.52 |
| Golimumab n (%) | 2 (0.9) | 0 (0) | 1 (2.0) | 1 (1.7) | 0 (0) | 2 (0.9) | 0.75 |
| Other biological immunomodulators (non-TNF-alpha inhibitor) at baseline n (%) | 24 (11) | 0 (0) | 18 (36) | 5 (8.3) | 1 (4.8) | 0 (0) | <0.01 |
| Ustekinumab (anti-IL-12/23) n (%) | 7 (3.3) | 0 (0) | 4 (8.0) | 3 (5.0) | 0 (0) | 0 (0) | 0.11 |
| Rituximab/ocrelizumab (anti-CD20) n (%)/mean time since last dose in weeks (SD) | 8 (3.7)/11 (6) | 0 (0) | 6 (12)/13 (4.8) | 2 (3.3)/4 (2.8) | 0 (0) | 0 (0) | <0.01 |
| Tofacitinib (JAK 1/3 inhibitor) n (%) | 3 (1.4) | 0 (0) | 3 (6.0) | 0 (0) | 0 (0) | 0 (0) | 0.04 |
| Secukinumab (anti IL-17A) n (%) | 2 (0.9) | 0 (0) | 1 (2.0) | 0 (0) | 1 (4.8) | 0 (0) | 0.26 |
| Other 7 n (%) | 3 (1.4) | 0 (0) | 3 (6.0) | 0 (0) | 0(0) | 0 (0) | 0.04 |
| Luminal agents | |||||||
| Vedolizumab (α4β7-integrin) n (%) | 10 (4.7) | 0 (0) | 1 (2.0) | 1 (1.7) | 1 (4.8) | 7 (19.4) | <0.01 |
| 5-Aminosalicylates n (%) | 15 (7.0) | 6 (13) | 4 (8.0) | 2 (3.3) | 1 (4.8) | 2 (5.6) | 0.41 |
| Low-dose prednisolone n (%) | 15 (7.0) | 5 (11) | 3 (6.0) | 1 (1.7) | 2 (9.5) | 4 (11) | 0.31 |
| All 24 Serotypes | T0 1 | T2 | T4 | T6 | T12 |
|---|---|---|---|---|---|
| Controls | 1/36 (2.8) | 10/32 (31) | 28/34 (82) a | 24/32 (75) a | 22/35 (63) a |
| cIM 2 | 0/47 (0) | 5/44 (11) | 26/45 (58) | 21/39 (54) | 21/44 (48) |
| bIM 3 | 1/50 (2.0) | 6/49 (12) | 26/46 (57) | 19/39 (49) | 18/46 (39) |
| Combination | 1/60 (1.7) | 5/57 (8.8) | 24/54 (44) b | 17/51 (33) b | 15/58 (24) b |
| Switched | 0/21 (0) | 5/21 (24) | 12/20 (60) | 10/19 (53) | 7/17 (41) |
| p-Value across groups | 0.81 | 0.04 | 0.01 | 0.01 | 0.01 |
| PCV13 4 serotypes | T0 | T2 | T4 | T6 | T12 |
| Controls | 0/36 (0) | 24/32 (75) a | 29/34 (85) a | 24/32 (75) a | 20/35 (57) a |
| cIM | 2/47 (4.3) | 25/44 (57) | 24/45 (53) b | 21/39 (54) | 18/44 (41) |
| bIM | 0/50 (0) | 26/49 (53) | 24/46 (52) b | 19/39 (49) | 16/46 (35) |
| Combination | 3/60 (5.0) | 24/57 (42) b | 25/54 (46) b | 18/51 (35) b | 14/58 (24) b |
| Switched | 0/21 (0) | 15/21 (71) | 12/20 (60) | 11/19 (58) | 7/17 (41) |
| p-v=Value across groups | 0.27 | 0.02 | 0.01 | 0.01 | 0.03 |
| PPSV23 5-exclusive | T0 | T2 | T4 | T6 | T12 |
| Controls | 1/36 (2.8) | 3/32 (9.4) | 28/34 (82) a | 27/32 (84) a | 26/35 (74) a |
| cIM | 0/47 (0) | 0/44 (0) | 29/45 (64) | 21/39 (54) | 23/44 (52) |
| bIM | 2/50 (4.0) | 1/49 (2.0) | 31/46 (67) | 24/39 (62) | 22/46 (48) |
| Combination | 2/60 (3.3) | 2/57 (3.5) | 28/54 (52) b | 26/51 (51) b | 23/58 (40) b |
| Switched | 0/21 (0) | 1/21 (4.8) | 10/20 (50) | 7/19 (37) b | 6/17 (35) |
| p-Value across groups | 0.64 | 0.25 | 0.04 | 0.01 | 0.02 |
| Overall Seroprotection Rate (%) | Raw Odds Ratio (95% CI 1) | Adjusted Odds Ratio (95% CI) | |
|---|---|---|---|
| Males | 55 | ref | ref |
| Females | 61 | 1.3 (0.74–2.3) | 1.5 (0.80–2.7) |
| Age | NA 2 | 0.98 (0.96–1.0) | 0.99 (0.97–1.0) |
| Age group 18–49 | 61 | ref | NIM 3 |
| Age group 50–70 | 54 | 0.75 (0.41–1.3) | |
| BMI 4 | NA | 1.0 (0.95–1.1) | NS 5 |
| Smoking | |||
| Never smoker (ref: ever smoker) | 56 | 0.85 (0.48–1.5) | NIM |
| Current smoker (ref: no current smoker) | 57 | 0.95 (0.42–2.1) | NS |
| Alcohol use >7/week | NS | ||
| No | 60 | ref | NS |
| Yes | 51 | 0.70 (0.35–1.4) | |
| Drug use | |||
| Yes | 63 | ref | NS |
| No | 58 | 0.80 (0.3–2.1) | |
| Comorbidities | |||
| Charlson comorbidity index | NA | 0.87 (0.68–1.1) | NS |
| Normal kidney function | 61 | ref | NS |
| Impaired kidney function (eGFR 6 < 60) | 39 | 0.42 (0.17–1.0) | |
| Crohn’s disease | 63 | 1.3 (0.66–2.6) | NS |
| Ulcerative colitis | 69 | 1.7 (0.73–4.0) | NS |
| Rheumatoid arthritis | 57 | 0.95 (0.42–2.1) | NS |
| Psoriasis/psoriatic arthritis | 48 | 0.99 (0.46–2.2) | NS |
| Solid organ transplant recipient | 42 | 0.48 (0.21–1.1) | 0.40 (0.17–0.97) |
| Time since organ transplantation | NA | 0.96 (0.98–1.00) | NIM |
| ≤12 months | 60 | Ref | |
| >12 months | 38 | 0.41 (0.01–3.01) | |
| Number of drugs at baseline | |||
| No drugs | 74 | ref | NS |
| One drug | 58 | 0.49 (0.23–1.0) | |
| Two drugs | 42 | 0.26 (0.10–0.63) | |
| Three drugs | 50 | 0.36 (0.12–1.1) | |
| cIM 7 | 52 | 0.66 (0.37–1.1) | NS |
| cIM monotherapy | 58 | 0.97 (0.50–1.9) | NIM |
| Prednisolone (>10 mg/day or 700 mg cumulative) | 49 | 0.62 (0.29–1.3) | NS |
| Low-dose prednisolone | 60 | 1.1 (0.40–3.2) | NS |
| Thiopurine | 63 | 1.3 (0.54–2.9) | NS |
| Methotrexate | 42 | 0.37 (0.21–0.91) | 0.37 (0.17–0.81) |
| Calcineurin inhibitor | 50 | 0.69 (0.28–1.7) | NS |
| Mycophenolate mofetil | 42 | 0.57 (0.20–1.1) | NS |
| bIM 8 | 54 | 0.77 (0.43–1.4) | NS |
| bIM monotherapy | 57 | 0.91 (0.47–1.8) | NIM |
| TNF-alpha inhibitor | 59 | 1.04 (0.55–1.9) | NS |
| Other biological immunomodulators (non-TNF-alpha inhibitor) | 45 | 0.55 (0.22–1.4) | NS |
| Ustekinumab (anti IL-12/23) | 43 | 0.52 (0.11–2.4) | NS |
| Rituximab/ocrelizumab (anti-CD20) | 6.0 | 0.14 (0.2–1.2) | 0.01 (0.01–0.81) |
| Nonsystemic agents (combined) | 70 | 1.8 (0.65–4.8) | NS |
| Vedolizumab (α4β7-integrin) | 70 | 1.7 (0.43–6.8) | NIM |
| 5-aminosalicylates | 68 | 1.5 (0.43–5.0) | NIM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia Garrido, H.M.; Vollaard, A.; D’Haens, G.R.; Spuls, P.I.; Bemelman, F.J.; Tanck, M.W.; de Bree, G.J.; Meek, B.; Grobusch, M.P.; Goorhuis, A. Immunogenicity of the 13-Valent Pneumococcal Conjugate Vaccine (PCV13) Followed by the 23-Valent Pneumococcal Polysaccharide Vaccine (PPSV23) in Adults with and without Immunosuppressive Therapy. Vaccines 2022, 10, 795. https://doi.org/10.3390/vaccines10050795
Garcia Garrido HM, Vollaard A, D’Haens GR, Spuls PI, Bemelman FJ, Tanck MW, de Bree GJ, Meek B, Grobusch MP, Goorhuis A. Immunogenicity of the 13-Valent Pneumococcal Conjugate Vaccine (PCV13) Followed by the 23-Valent Pneumococcal Polysaccharide Vaccine (PPSV23) in Adults with and without Immunosuppressive Therapy. Vaccines. 2022; 10(5):795. https://doi.org/10.3390/vaccines10050795
Chicago/Turabian StyleGarcia Garrido, Hannah M., Albert Vollaard, Geert R. D’Haens, Phyllis I. Spuls, Frederike J. Bemelman, Michael W. Tanck, Godelieve J. de Bree, Bob Meek, Martin P. Grobusch, and Abraham Goorhuis. 2022. "Immunogenicity of the 13-Valent Pneumococcal Conjugate Vaccine (PCV13) Followed by the 23-Valent Pneumococcal Polysaccharide Vaccine (PPSV23) in Adults with and without Immunosuppressive Therapy" Vaccines 10, no. 5: 795. https://doi.org/10.3390/vaccines10050795
APA StyleGarcia Garrido, H. M., Vollaard, A., D’Haens, G. R., Spuls, P. I., Bemelman, F. J., Tanck, M. W., de Bree, G. J., Meek, B., Grobusch, M. P., & Goorhuis, A. (2022). Immunogenicity of the 13-Valent Pneumococcal Conjugate Vaccine (PCV13) Followed by the 23-Valent Pneumococcal Polysaccharide Vaccine (PPSV23) in Adults with and without Immunosuppressive Therapy. Vaccines, 10(5), 795. https://doi.org/10.3390/vaccines10050795






