The Global Impact of Hepatitis B Vaccination on Hepatocellular Carcinoma
Abstract
1. Introduction
2. The Impact of Hepatitis B Vaccination on HCC Incidence: Real-World Data
2.1. Taiwan
2.2. China
2.3. The Gambia
2.4. Alaska
3. The Impact of Hepatitis B Vaccination on HCC Incidence-Modelling Data
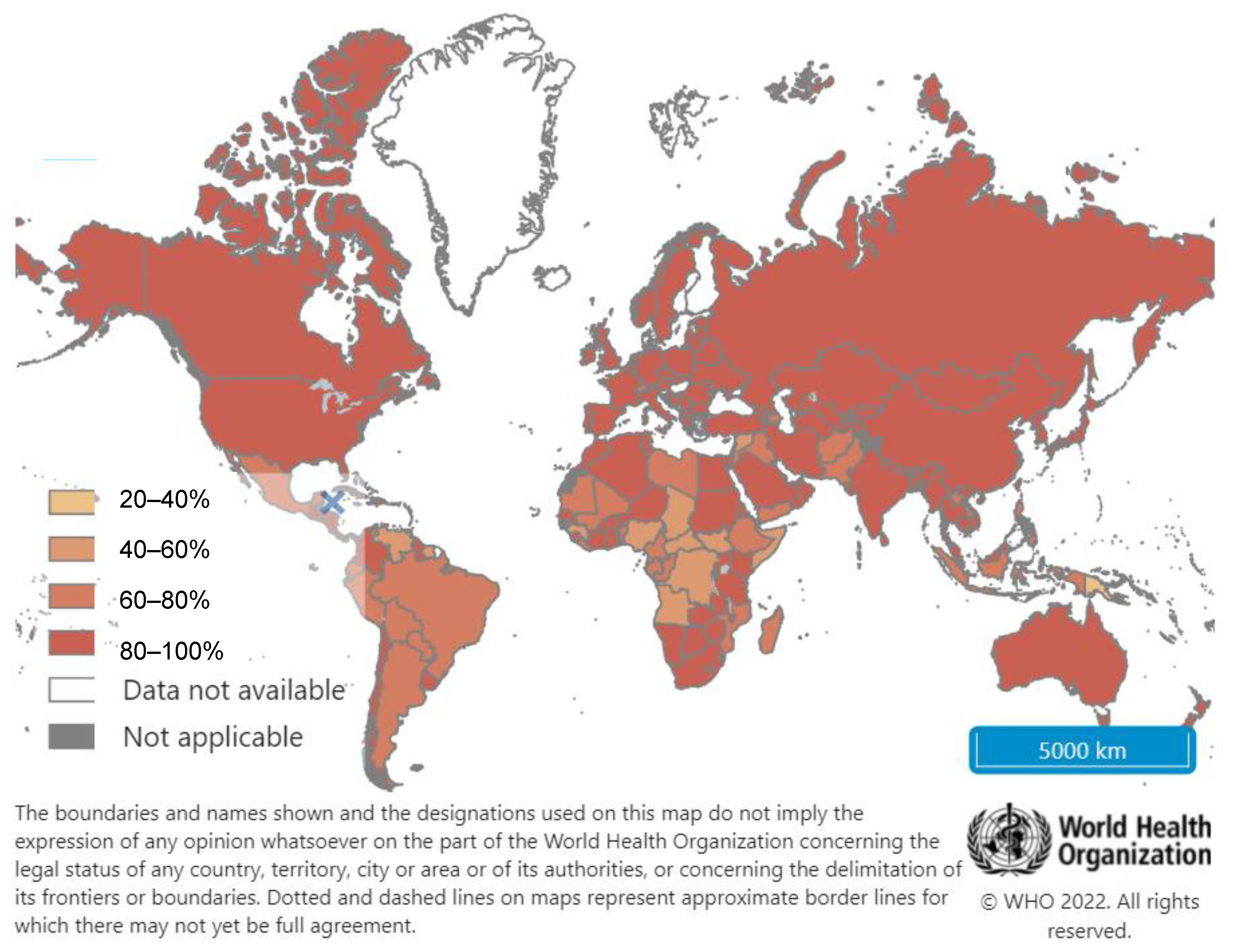
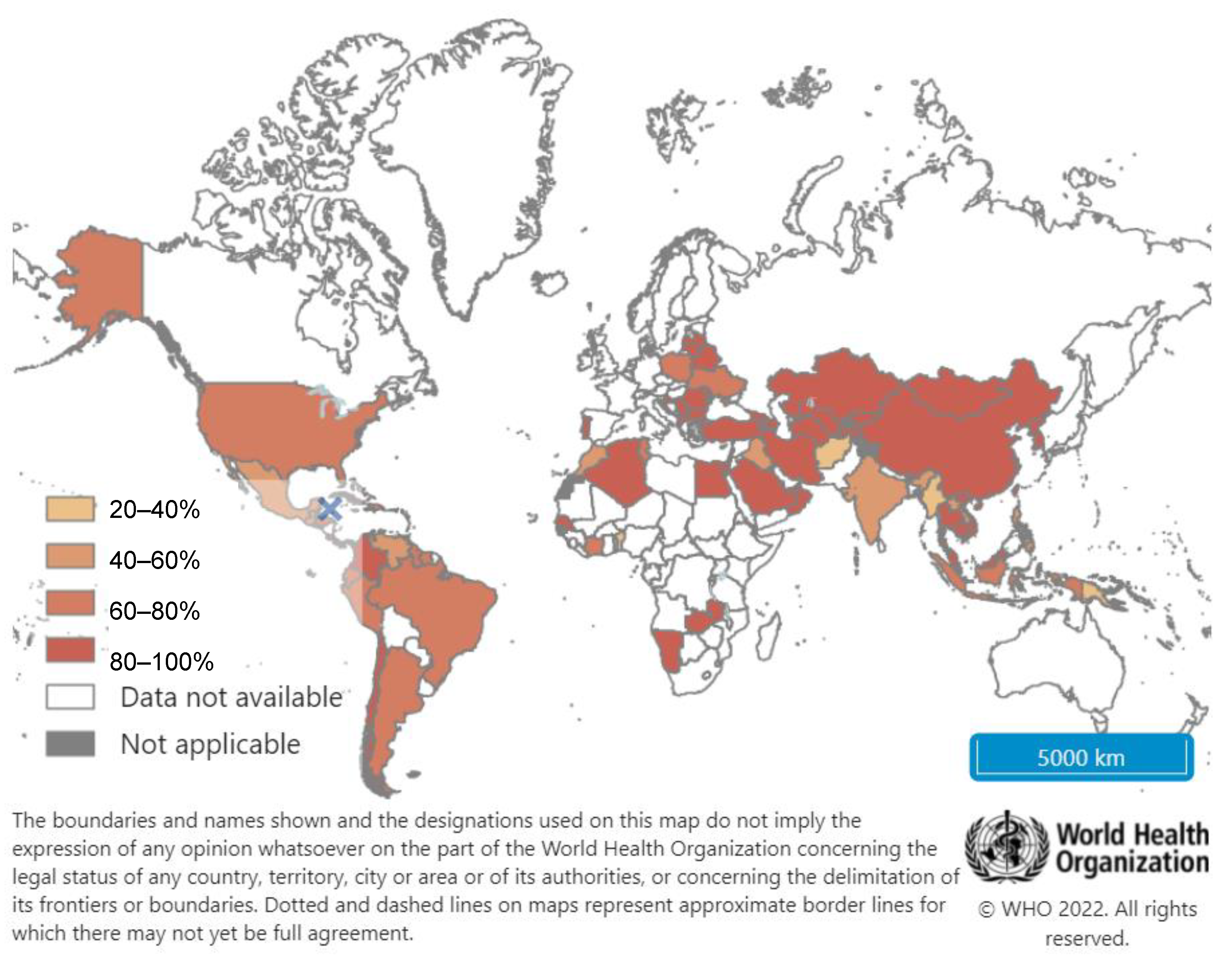
4. Hepatitis B Vaccination: Current Recommendations and Global Coverage
4.1. Hepatitis B Vaccination Preparations and Usage
4.2. Global Hepatitis B Vaccination Coverage
5. Cost-Effectiveness of Hepatitis B Vaccination for Prevention of HCC
6. Challenges Facing Hepatitis B Vaccination Programs
7. Future Directions
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. Hepatitis B Fact Sheet 2021 [Updated 27 July 2021]. Available online: https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-b (accessed on 16 September 2021).
- Global Burden of Disease Liver Cancer Collaboration; Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies From 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [PubMed]
- Chang, M.H. Prevention of hepatitis B virus infection and liver cancer. In Viruses and Human Cancer; Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 2014; Volume 193, pp. 75–95. [Google Scholar]
- MacLachlan, J.H.; Cowie, B.C. Liver cancer is the fastest increasing cause of cancer death in Australians. Med. J. Aust. 2012, 197, 492–493. [Google Scholar] [CrossRef] [PubMed]
- Maucort-Boulch, D.; de Martel, C.; Franceschi, S.; Plummer, M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int. J. Cancer 2018, 142, 2471–2477. [Google Scholar] [CrossRef]
- Lin, W.C.; Lin, Y.S.; Chang, C.W.; Chang, C.W.; Wang, T.E.; Wang, H.Y.; Chen, M.J. Impact of direct-acting antiviral therapy for hepatitis C-related hepatocellular carcinoma. PLoS ONE 2020, 15, e0233212. [Google Scholar] [CrossRef]
- Levrero, M.; Zucman-Rossi, J. Mechanisms of HBV-induced hepatocellular carcinoma. J. Hepatol. 2016, 64 (Suppl. S1), S84–S101. [Google Scholar] [CrossRef]
- McGlynn, K.A.; Petrick, J.L.; London, W.T. Global epidemiology of hepatocellular carcinoma: An emphasis on demographic and regional variability. Clin. Liver Dis. 2015, 19, 223–238. [Google Scholar] [CrossRef]
- Kim, M.N.; Han, K.H.; Ahn, S.H. Prevention of hepatocellular carcinoma: Beyond hepatitis B vaccination. Semin. Oncol. 2015, 42, 316–328. [Google Scholar] [CrossRef]
- World Health Organisation. Hepatitis B vaccines: WHO position paper–July 2017. Wkly. Epidemiol. Rec. 2017, 92, 369–392. [Google Scholar]
- Hyams, K.C. Risks of chronicity following acute hepatitis B virus infection: A review. Clin. Infect. Dis. 1995, 20, 992–1000. [Google Scholar] [CrossRef]
- Leroy, V.; Asselah, T. Universal hepatitis B vaccination: The only way to eliminate hepatocellular carcinoma? J. Hepatol. 2015, 63, 1303–1305. [Google Scholar] [CrossRef]
- Shimakawa, Y.; Lemoine, M.; Bottomley, C.; Njai, H.F.; Ndow, G.; Jatta, A.; Tamba, S.; Bojang, L.; Taal, M.; Nyan, O.; et al. Birth order and risk of hepatocellular carcinoma in chronic carriers of hepatitis B virus: A case-control study in The Gambia. Liver Int. 2015, 35, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Anugwom, C.M.; Allaire, M.; Akbar, S.M.F.; Sultan, A.; Bollipo, S.; Mattos, A.Z.; Debes, J.D. Hepatitis B-related hepatocellular carcinoma: Surveillance strategy directed by immune-epidemiology. Hepatoma Res. 2021, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.; Henshaw, C.; Youlden, D.R.; Clark, P.J.; Aitken, J.F.; Baade, P.D. Global Trends in Incidence Rates of Primary Adult Liver Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.H.; Chen, C.J.; Lai, M.S.; Hsu, H.M.; Wu, T.C.; Kong, M.S.; Liang, D.C.; Shau, W.Y.; Chen, D.S.; Taiwan Childhood Hepatoma Study Group. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. N. Engl. J. Med. 1997, 336, 1855–1859. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Hsieh, K.S.; Ko, Y.C. Trends in the incidence of hepatocellular carcinoma in boys and girls in Taiwan after large-scale hepatitis B vaccination. Cancer Epidemiol. Biomark. Prev. 2003, 12, 57–59. [Google Scholar]
- Chang, M.H.; You, S.L.; Chen, C.J.; Liu, C.J.; Lee, C.M.; Lin, S.M.; Chu, H.C.; Wu, T.C.; Yang, S.S.; Kuo, H.S.; et al. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: A 20-year follow-up study. J. Natl. Cancer Inst. 2009, 101, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.H.; You, S.L.; Chen, C.J.; Liu, C.J.; Lai, M.W.; Wu, T.C.; Wu, S.F.; Lee, C.M.; Yang, S.S.; Chu, H.C.; et al. Long-term Effects of Hepatitis B Immunization of Infants in Preventing Liver Cancer. Gastroenterology 2016, 151, 472–480.e1. [Google Scholar] [CrossRef]
- Hung, G.Y.; Horng, J.L.; Yen, H.J.; Lee, C.Y.; Lin, L.Y. Changing incidence patterns of hepatocellular carcinoma among age groups in Taiwan. J. Hepatol. 2015, 63, 1390–1396. [Google Scholar] [CrossRef]
- Chien, Y.C.; Jan, C.F.; Chiang, C.J.; Kuo, H.S.; You, S.L.; Chen, C.J. Incomplete hepatitis B immunization, maternal carrier status, and increased risk of liver diseases: A 20-year cohort study of 3.8 million vaccinees. Hepatology 2014, 60, 125–132. [Google Scholar] [CrossRef]
- Liao, S.H.; Chen, C.L.; Hsu, C.Y.; Chien, K.L.; Kao, J.H.; Chen, P.J.; Chen, T.H.H.; Chen, C.H. Long-term effectiveness of population-wide multifaceted interventions for hepatocellular carcinoma in Taiwan. J. Hepatol. 2021, 75, 132–141. [Google Scholar] [CrossRef]
- Wang, X.Y.; Huang, J.M.; Lu, X.M.; Harrison, T.J.; Liu, H.B.; Jia, H.H.; Fang, Z.L. Changing risk factors for hepatocellular carcinoma in hyperendemic regions in the era of universal hepatitis B vaccination. Cancer Epidemiol. 2020, 67, 101775. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Chen, T.; Fan, C.; Zhan, Q.; Wang, Y.; Lu, J.; Lu, L.L.; Ni, Z.; Huang, F.; Yao, H.; et al. Efficacy of neonatal HBV vaccination on liver cancer and other liver diseases over 30-year follow-up of the Qidong hepatitis B intervention study: A cluster randomized controlled trial. PLoS Med. 2014, 11, e1001774. [Google Scholar] [CrossRef] [PubMed]
- The Gambia Hepatitis Study Group. The Gambia Hepatitis Intervention Study. The Gambia Hepatitis Study Group. Cancer Res. 1987, 47, 5782–5787. [Google Scholar]
- Viviani, S.; Carrieri, P.; Bah, E.; Hall, A.J.; Kirk, G.D.; Mendy, M.; Montesano, R.; Plymoth, A.; Sam, O.; Van der Sande, M.; et al. 20 years into the Gambia Hepatitis Intervention Study: Assessment of initial hypotheses and prospects for evaluation of protective effectiveness against liver cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3216–3223. [Google Scholar] [CrossRef] [PubMed]
- McMahon, B.J.; Bulkow, L.R.; Singleton, R.J.; Williams, J.; Snowball, M.; Homan, C.; Parkinson, A.J. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology 2011, 54, 801–807. [Google Scholar] [CrossRef]
- Howell, J.; Lemoine, M.; Thursz, M. Prevention of materno-foetal transmission of hepatitis B in sub-Saharan Africa: The evidence, current practice and future challenges. J. Viral Hepat. 2014, 21, 381–396. [Google Scholar] [CrossRef]
- Montesano, R. Hepatitis B immunization and hepatocellular carcinoma: The Gambia Hepatitis Intervention Study. J. Med. Virol. 2002, 67, 444–446. [Google Scholar] [CrossRef]
- Montesano, R. Preventing primary liver cancer: The HBV vaccination project in the Gambia (West Africa). Environ. Health 2011, 10 (Suppl. S1), S6. [Google Scholar] [CrossRef]
- Bah, E.; Carrieri, M.P.; Hainaut, P.; Bah, Y.; Nyan, O.; Taal, M. 20-years of population-based cancer registration in hepatitis B and liver cancer prevention in the Gambia, West Africa. PLoS ONE 2013, 8, e75775. [Google Scholar] [CrossRef]
- The Gambia Hepatitis Study Group. Hepatitis B vaccine in the expanded programme of immunisation: The Gambian experience. Lancet 1989, 1, 1057–1059. [Google Scholar]
- Shimakawa, Y.; Lemoine, M.; Mendy, M.; Njai, H.F.; D’alessandro, U.; Hall, A.; Thursz, M.; Njie, R. Population-based interventions to reduce the public health burden related with hepatitis B virus infection in the gambia, west Africa. Trop. Med. Health 2014, 42 (Suppl. S2), 59–64. [Google Scholar] [CrossRef]
- Morio, S.; Okamoto, N.; Minowa, M.; Mori, H.; Nishioka, K. Preventive effect of HB vaccination against liver cancer: An estimation by simulation. Jpn. J. Cancer Res. 1987, 78, 899–907. [Google Scholar]
- Goldstein, S.T.; Zhou, F.; Hadler, S.C.; Bell, B.P.; Mast, E.E.; Margolis, H.S. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int. J. Epidemiol. 2005, 34, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Nayagam, S.; Thursz, M.; Sicuri, E.; Conteh, L.; Wiktor, S.; Low-Beer, D.; Hallett, T.B. Requirements for global elimination of hepatitis B: A modelling study. Lancet Infect. Dis. 2016, 16, 1399–1408. [Google Scholar] [CrossRef]
- World Health Organisation. Prevention of Mother-to-Child Transmission of Hepatitis B Virus: Guidelines on Antiviral Prophylaxis in Pregnancy; World Health Organisation: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organisation. Immunization Agenda 2030: A Global Strategy to Leave No One Behind; World Health Organisation: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Expanded Programme on Immunization: Global Advisory Group—Part II. Wkly. Epidemiol. Record. 1991, 66, 9–12. [Google Scholar]
- World Health Organisation. Global Hepatitis Report 2017; World Health Organisation: Geneva, Switzerland, 2017. [Google Scholar]
- Alliance GtV. Pentavalent Vaccine Support 2020 [Updated 19 February 2020; Cited 2021]. Available online: https://www.gavi.org/types-support/vaccine-support/pentavalent (accessed on 24 October 2021).
- Alliance GtV. Annex C: Hepatitis B Birth Dose Investment Case. In Proceedings of the Vaccine Investment Strategy Programme and Policy Committee Meeting, Geneva, Switzerland, 18–19 October 2018. [Google Scholar]
- Van Herck, K.; Van Damme, P. Benefits of early hepatitis B immunization programs for newborns and infants. Pediatr. Infect. Dis. J. 2008, 27, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Kao, J.H. Review article: The prevention of hepatitis B-related hepatocellular carcinoma. Aliment Pharm. Ther. 2018, 48, 5–14. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, S.; Luo, C.; Liu, Q.; Zhou, Y.H. Gaps in the prevention of perinatal transmission of hepatitis B virus between recommendations and routine practices in a highly endemic region: A provincial population-based study in China. BMC Infect. Dis. 2012, 12, 221. [Google Scholar] [CrossRef]
- Vryheid, R.E.; Kane, M.A.; Muller, N.; Schatz, G.C.; Bezabeh, S. Infant and adolescent hepatitis B immunization up to 1999: A global overview. Vaccine 2000, 19, 1026–1037. [Google Scholar] [CrossRef]
- Kao, J.H. Hepatitis B vaccination and prevention of hepatocellular carcinoma. Best Pr. Res. Clin. Gastroenterol. 2015, 29, 907–917. [Google Scholar] [CrossRef]
- Steffen, G.; Sperle, I.; Harder, T.; Sarma, N.; Beermann, S.; Thamm, R.; Bremer, V.; Zimmermann, R.; Dudareva, S. Hepatitis B vaccination coverage in Germany: Systematic review. BMC Infect. Dis. 2021, 21, 817. [Google Scholar] [CrossRef] [PubMed]
- Mipatrini, D.; Stefanelli, P.; Severoni, S.; Rezza, G. Vaccinations in migrants and refugees: A challenge for European health systems. A Syst. Rev. Curr. Sci. Evid. Pathog. Glob. Health 2017, 111, 59–68. [Google Scholar] [CrossRef]
- Marschall, T.; Kretzschmar, M.; Mangen, M.J.; Schalm, S. High impact of migration on the prevalence of chronic hepatitis B in the Netherlands. Eur. J. Gastroenterol. Hepatol. 2008, 20, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Immunization Coverage 2021 [Updated 15 July 2021]. Available online: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage (accessed on 13 October 2021).
- Childs, L.; Roesel, S.; Tohme, R.A. Status and progress of hepatitis B control through vaccination in the South-East Asia Region, 1992–2015. Vaccine 2018, 36, 6–14. [Google Scholar] [CrossRef]
- World Health Organisation. Hepatitis B (HepB3) Immunization Coverage among 1-Year-Olds (%) 2021. 2021. Available online: https://www.who.int/data/maternal-newborn-child-adolescent-ageing/indicator-explorer-new/mca/hepatitis-b-(hepb3)-immunization-coverage-among-1-year-olds-(-) (accessed on 13 October 2021).
- Chemin, I. Evaluation of a hepatitis B vaccination program in Taiwan: Impact on hepatocellular carcinoma development. Future Oncol. 2010, 6, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.; Ruff, T.A.; Rana, B.J.; Leydon, J.; Locarnini, S. The effectiveness of the infant hepatitis B immunisation program in Fiji, Kiribati, Tonga and Vanuatu. Vaccine 2000, 18, 3059–3066. [Google Scholar] [CrossRef]
- Mann, J.; Roberts, M. Modelling the epidemiology of hepatitis B in New Zealand. J. Theor. Biol. 2011, 269, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Qama, A.; Allard, N.; Cowie, B.; Davis, J.S.; Davies, J. Hepatitis B in the Northern Territory: Insights into the changing epidemiology of an ancient condition. Intern. Med. J. 2021, 51, 910–922. [Google Scholar] [CrossRef]
- Harpaz, R.; McMahon, B.J.; Margolis, H.S.; Shapiro, C.N.; Havron, D.; Carpenter, G.; Bulkow, L.R.; Wainwright, R.B. Elimination of new chronic hepatitis B virus infections: Results of the Alaska immunization program. J. Infect. Dis. 2000, 181, 413–418. [Google Scholar] [CrossRef]
- Ropero, A.M.; Danovaro-Holliday, M.C.; Andrus, J.K. Progress in vaccination against hepatitis B in the Americas. J. Clin. Virol. 2005, 34 (Suppl. S2), S14–S19. [Google Scholar] [CrossRef]
- Souto, F.J. Distribution of hepatitis B infection in Brazil: The epidemiological situation at the beginning of the 21st century. Rev. Soc. Bras. Med. Trop. 2016, 49, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Choconta-Piraquive, L.A.; De la Hoz-Restrepo, F.; Sarmiento-Limas, C.A. Compliance with birth dose of Hepatitis B vaccine in high endemic and hard to reach areas in the Colombian amazon: Results from a vaccination survey. BMC Health Serv. Res. 2016, 16, 293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bechini, A.; Boccalini, S.; Ninci, A.; Zanobini, P.; Sartor, G.; Bonaccorsi, G.; Grazzini, M.; Bonanni, P. Childhood vaccination coverage in Europe: Impact of different public health policies. Expert Rev. Vaccines 2019, 18, 693–701. [Google Scholar] [CrossRef]
- Mandal, S. Introduction of universal infant hepatitis B immunisation in the UK- paving the way to elimination. Hum. Vaccines Immunother. 2019, 15, 440–443. [Google Scholar] [CrossRef]
- Public Health England. Guidance on the Hepatitis B Antenatal Screening and Selective Neonatal Immunisation Pathway. 2020. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/967528/PHE_11596_Hep_B_guidance.pdf (accessed on 28 October 2021).
- Allison, R.D.; Teleb, N.; Al Awaidy, S.; Ashmony, H.; Alexander, J.P.; Patel, M.K. Hepatitis B control among children in the Eastern Mediterranean Region of the World Health Organization. Vaccine 2016, 34, 2403–2409. [Google Scholar] [CrossRef] [PubMed]
- Amponsah-Dacosta, E. Hepatitis B virus infection and hepatocellular carcinoma in sub-Saharan Africa: Implications for elimination of viral hepatitis by 2030? World J. Gastroenterol. 2021, 27, 6025–6038. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.S.; Nguyen, M.H. Epidemiology of hepatitis B and the role of vaccination. Best Pr. Res. Clin. Gastroenterol. 2017, 31, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Spearman, C.W.; Afihene, M.; Ally, R.; Apica, B.; Awuku, Y.; Cunha, L.; Dusheiko, G.; Gogela, N.; Kassianides, C.; Kew, M.; et al. Hepatitis B in sub-Saharan Africa: Strategies to achieve the 2030 elimination targets. Lancet Gastroenterol. Hepatol. 2017, 2, 900–909. [Google Scholar] [CrossRef]
- Bassoum, O.; Kimura, M.; Tal Dia, A.; Lemoine, M.; Shimakawa, Y. Coverage and Timeliness of Birth Dose Vaccination in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. Vaccines 2020, 8, 301. [Google Scholar] [CrossRef]
- Magoni, M.; Ekra, K.D.; Aka, L.N.; Sita, K.S.; Kanga, K. Effectiveness of hepatitis-B vaccination in Ivory Coast: The case of the Grand Bassam health district. Ann. Trop. Med. Parasitol. 2009, 103, 519–527. [Google Scholar] [CrossRef]
- Gram, L.; Soremekun, S.; ten Asbroek, A.; Manu, A.; O’Leary, M.; Hill, Z.; Danso, S.; Amenga-Etego, S.; Owusu-Agyei, S.; Kirkwood, B.R. Socio-economic determinants and inequities in coverage and timeliness of early childhood immunisation in rural Ghana. Trop. Med. Int. Health 2014, 19, 802–811. [Google Scholar] [CrossRef]
- Ginsberg, G.M.; Shouval, D. Cost-benefit analysis of a nationwide neonatal inoculation programme against hepatitis B in an area of intermediate endemicity. J. Epidemiol. Community Health 1992, 46, 587–594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guo, Y.; Yang, Y.; Bai, Q.; Huang, Z.; Wang, Z.; Cai, D.; Li, S.; Man, X.; Shi, X. Cost-utility analysis of newborn hepatitis B immunization in Beijing. Hum. Vaccines Immunother. 2021, 17, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.F.; Chen, T.H. Probabilistic cost-effectiveness analysis of the long-term effect of universal hepatitis B vaccination: An experience from Taiwan with high hepatitis B virus infection and Hepatitis B e Antigen positive prevalence. Vaccine 2009, 27, 6770–6776. [Google Scholar] [CrossRef] [PubMed]
- Petit, D.; Tevi-Benissan, C.; Woodring, J.; Hennessey, K.; Kahn, A.L. Countries’ interest in a hepatitis B vaccine licensed for the controlled temperature chain; survey results from African and Western Pacific regions. Vaccine 2017, 35 Pt B, 6866–6871. [Google Scholar] [CrossRef]
- Seaman, C.P.; Morgan, C.; Howell, J.; Xiao, Y.; Spearman, C.W.; Sonderup, M.; Lesi, O.; Andersson, M.I.; Hellard, M.E.; Scott, N. Use of controlled temperature chain and compact prefilled auto-disable devices to reach 2030 hepatitis B birth dose vaccination targets in LMICs: A modelling and cost-optimisation study. Lancet Glob. Health 2020, 8, e931–e941. [Google Scholar] [CrossRef]
- Patel, M.K.; Kahn, A.-L. Game changing: Hepatitis B vaccine in a controlled temperature chain. Lancet Glob. Health 2018, 6, e596–e597. [Google Scholar] [CrossRef]
- Kolwaite, A.R.; Xeuatvongsa, A.; Ramirez-Gonzalez, A.; Wannemuehler, K.; Vongxay, V.; Vilayvone, V.; Hennessey, K.; Patel, M.K. Hepatitis B vaccine stored outside the cold chain setting: A pilot study in rural Lao PDR. Vaccine 2016, 34, 3324–3330. [Google Scholar] [CrossRef]
- Moturi, E.; Tevi-Benissan, C.; Hagan, J.E.; Shendale, S.; Mayenga, D.; Murokora, D.; Patel, M.; Hennessey, K.; Mihigo, R. Implementing a Birth Dose of Hepatitis B Vaccine in Africa: Findings from Assessments in 5 Countries. J. Immunol. Sci. 2018, 5, 31–40. [Google Scholar] [CrossRef]
- Scott, N.; Palmer, A.; Morgan, C.; Lesi, O.; Spearman, C.W.; Sonderup, M.; Hellard, M. Cost-effectiveness of the controlled temperature chain for the hepatitis B virus birth dose vaccine in various global settings: A modelling study. Lancet Glob. Health 2018, 6, e659–e667. [Google Scholar] [CrossRef]
- Sutanto, A.; Suarnawa, I.M.; Nelson, C.M.; Stewart, T.; Soewarso, T.I. Home delivery of heat-stable vaccines in Indonesia: Outreach immunization with a prefilled, single-use injection device. Bull. World Health Organ. 1999, 77, 119–126. [Google Scholar] [PubMed]
- Levin, C.E.; Nelson, C.M.; Widjaya, A.; Moniaga, V.; Anwar, C. The costs of home delivery of a birth dose of hepatitis B vaccine in a prefilled syringe in Indonesia. Bull. World Health Organ. 2005, 83, 456–461. [Google Scholar] [PubMed]
- Howell, J.; Pedrana, A.; Schroeder, S.E.; Scott, N.; Aufegger, L.; Atun, R.; Baptista-Leite, R.; Hirnschall, G.; Hutchinson, S.J.; Lazarus, J.V.; et al. A global investment framework for the elimination of hepatitis B. J. Hepatol. 2021, 74, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Fadnes, L.T.; Jackson, D.; Engebretsen, I.M.; Zembe, W.; Sanders, D.; Sommerfelt, H.; Tylleskär, T. Vaccination coverage and timeliness in three South African areas: A prospective study. BMC Public Health 2011, 11, 404. [Google Scholar] [CrossRef] [PubMed]
- Okwaraji, Y.B.; Mulholland, K.; Schellenberg, J.R.; Andarge, G.; Admassu, M.; Edmond, K.M. The association between travel time to health facilities and childhood vaccine coverage in rural Ethiopia. A community based cross sectional study. BMC Public Health 2012, 12, 476. [Google Scholar] [CrossRef]
- Allan, S.; Adetifa, I.M.O.; Abbas, K. Inequities in childhood immunisation coverage associated with socioeconomic, geographic, maternal, child, and place of birth characteristics in Kenya. BMC Infect. Dis. 2021, 21, 553. [Google Scholar] [CrossRef]
- Raoofi, A.; Hatefnia, E.; Kazemnejad, A. Helping to prevent hepatitis B in family setting, by educating women to vaccinate before marriage. Soc. Work Public Health 2018, 33, 354–365. [Google Scholar] [CrossRef]
- Summan, A.; Nandi, A.; Deo, S.; Laxminarayan, R. Improving vaccination coverage and timeliness through periodic intensification of routine immunization: Evidence from Mission Indradhanush. Ann. N. Y. Acad. Sci. 2021, 1502, 110–120. [Google Scholar] [CrossRef]
- Miyahara, R.; Jasseh, M.; Gomez, P.; Shimakawa, Y.; Greenwood, B.; Keita, K.; Ceesay, S.; D’alessandro, U.; Roca, A. Barriers to timely administration of birth dose vaccines in The Gambia, West Africa. Vaccine 2016, 34, 3335–3341. [Google Scholar] [CrossRef]
- Le, X.T.T.; Ishizumi, A.; Nguyen, H.T.T.; Duong, H.T.; Dang, H.T.T.; Do, C.M.; Pham, Q.T.; Le, H.T.; Iijima, M.; Tohme, R.A.; et al. Social and behavioral determinants of attitudes towards and practices of hepatitis B vaccine birth dose in Vietnam. Vaccine 2020, 38, 8343–8350. [Google Scholar]
- Hadler, S.C.; Fuqiang, C.; Averhoff, F.; Taylor, T.; Fuzhen, W.; Li, L.; Xiaofeng, L.; Weizhong, Y. The impact of hepatitis B vaccine in China and in the China GAVI Project. Vaccine 2013, 31 (Suppl. S9), J66–J72. [Google Scholar] [CrossRef] [PubMed]
- Dionne-Odom, J.; Njei, B.; Tita, A.T.N. Elimination of Vertical Transmission of Hepatitis B in Africa: A Review of Available Tools and New Opportunities. Clin. Ther. 2018, 40, 1255–1267. [Google Scholar] [CrossRef] [PubMed]
- Doi, H.; Kanto, T. Factors influencing the durability of hepatitis B vaccine responses. Vaccine 2021, 39, 5224–5230. [Google Scholar] [CrossRef] [PubMed]
- Shaw, F.E., Jr.; Guess, H.A.; Roets, J.M.; Mohr, F.E.; Coleman, P.J.; Mandel, E.J.; Roehm, R.R., Jr.; Talley, W.S.; Hadler, S.C. Effect of anatomic injection site, age and smoking on the immune response to hepatitis B vaccination. Vaccine 1989, 7, 425–430. [Google Scholar] [CrossRef]
- Chang, M.H. Cancer prevention by vaccination against hepatitis B. Recent Results Cancer Res. 2009, 181, 85–94. [Google Scholar]
- Liu, Y.C.; Yeh, C.T.; Lin, K.H. Cancer Stem Cell Functions in Hepatocellular Carcinoma and Comprehensive Therapeutic Strategies. Cells 2020, 9, 1331. [Google Scholar] [CrossRef]
- Breakwell, L.; Tevi-Benissan, C.; Childs, L.; Mihigo, R.; Tohme, R. The status of hepatitis B control in the African region. Pan Afr. Med. J. 2017, 27 (Suppl. S3), 17. [Google Scholar] [CrossRef]
- Mwamba, G.N.; Yoloyolo, N.; Masembe, Y.; Nsambu, M.N.; Nzuzi, C.; Tshekoya, P.; Dah, B.; Kaya, G. Vaccination coverage and factors influencing routine vaccination status in 12 high risk health zones in the Province of Kinshasa City, Democratic Republic of Congo (DRC), 2015. Pan Afr. Med. J. 2017, 27 (Suppl. S3), 7. [Google Scholar] [CrossRef]
- Xeuatvongsa, A.; Datta, S.S.; Moturi, E.; Wannemuehler, K.; Philakong, P.; Vongxay, V.; Vilayvone, V.; Patel, M.K. Improving hepatitis B birth dose in rural Lao People’s Democratic Republic through the use of mobile phones to facilitate communication. Vaccine 2016, 34, 5777–5784. [Google Scholar] [CrossRef]
- Seaman, C.; Mvundara, M.; Frivold, C.; Morgan, C.; Jarrahian, C.; Howell, J.; Hellard, M.E.; Scott, N. Evaluating the potential cost-effectiveness of microarray patches to expand access to hepatitis B birth dose vaccination in low-and middle-income countries: A modelling study. PLoS Glob. Public Health 2022, accepted. [Google Scholar]

| Author | Year | Country/ Region | HCC-Related Outcomes |
|---|---|---|---|
| Chang et al. [16] | 1997 | Taiwan |
|
| Lee et al. [17] | 2003 | Taiwan |
|
| Chang et al. [18] | 2009 | Taiwan | Comparing HCC incidence from July 1983 to June 2004:
|
| Chang et al. [19] | 2016 | Taiwan | HCC incidence rates between June 1983 and June 2011 in age group 6–26 years (vaccinated vs. unvaccinated):
|
| Hung et al. [20] | 2015 | Taiwan | Annual percentage change in age-standardized incidence rates for age groups from 2003 to 2011 (all p < 0.05):
|
| Chien et al. [21] | 2014 | Taiwan |
|
| Liao et al. [22] | 2021 | Taiwan |
|
| Wang et al. [23] | 2020 | Guangxi, China | HCC-related mortality in 2017–2018:
|
| Qu et al. [24] | 2014 | Qidong, China | HCC incidence in children born in 1985–1990 in 41 rural towns across 6 clusters
|
| The Gambia Hepatitis Study Group [25] | 1987 | The Gambia, Africa | For the evaluation of protective effect of vaccination on HCC and CLD of children born during the period of 1986–1990 with stepped wedge design of sequential randomization of EPI teams every three months over four-year period, until all EPI teams administering HBV vaccine with other vaccinations. Long term follow up through the national cancer registry continues. Outcomes not available at time of publication. |
| Viviani et al. [26] | 2008 | The Gambia, Africa | 65% subjects available for follow up. With expected cumulative incidence based on age-specific HCC incidence rates from 1987–2002, final outcome for detecting significant impact of vaccination on HCC development will be measurable between 2017 and 2020 when subjects are approximately 30 years old. Outcomes not available at time of publication. |
| McMahon, et al. [27] | 2011 | Alaska, United States of America | HCC incidence identified by national cancer institute cancer registry and HCC surveillance program set up by Liver Disease and Hepatitis Program between 1969 and 2008:
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores, J.E.; Thompson, A.J.; Ryan, M.; Howell, J. The Global Impact of Hepatitis B Vaccination on Hepatocellular Carcinoma. Vaccines 2022, 10, 793. https://doi.org/10.3390/vaccines10050793
Flores JE, Thompson AJ, Ryan M, Howell J. The Global Impact of Hepatitis B Vaccination on Hepatocellular Carcinoma. Vaccines. 2022; 10(5):793. https://doi.org/10.3390/vaccines10050793
Chicago/Turabian StyleFlores, Joan Ericka, Alexander J. Thompson, Marno Ryan, and Jessica Howell. 2022. "The Global Impact of Hepatitis B Vaccination on Hepatocellular Carcinoma" Vaccines 10, no. 5: 793. https://doi.org/10.3390/vaccines10050793
APA StyleFlores, J. E., Thompson, A. J., Ryan, M., & Howell, J. (2022). The Global Impact of Hepatitis B Vaccination on Hepatocellular Carcinoma. Vaccines, 10(5), 793. https://doi.org/10.3390/vaccines10050793






