Epitope Profiling of Diphtheria Toxoid Provides Enhanced Monitoring for Consistency Testing during Manufacturing Process Changes
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.1.1. Antibodies
2.1.2. Diphtheria Toxin and Toxoid
2.2. mAb Characterization Methods
2.2.1. General BLI Method
2.2.2. Binning Experiment
2.2.3. Inhibition of mAb-DTxn Binding to HB-EGF
2.2.4. Toxin Neutralization Assay
2.2.5. BLI Screening for mAb Sensitivity to Heat-Induced Degradation of DTxd
2.2.6. Western Blot
2.2.7. Single Epitope Antigenicity Testing (SEAT)
SEAT Assay Design
SEAT Qualification
Determination of the Comparability Range
3. Results and Discussion
3.1. mAb Characterization
3.1.1. Determination of Binning Groups and Subunit Binding Sites
3.1.2. Assessment of mAb-DTxn Binding Inhibition by HB-EGF
3.1.3. Determination of Toxin Neutralization Capacity of the mAb Panel
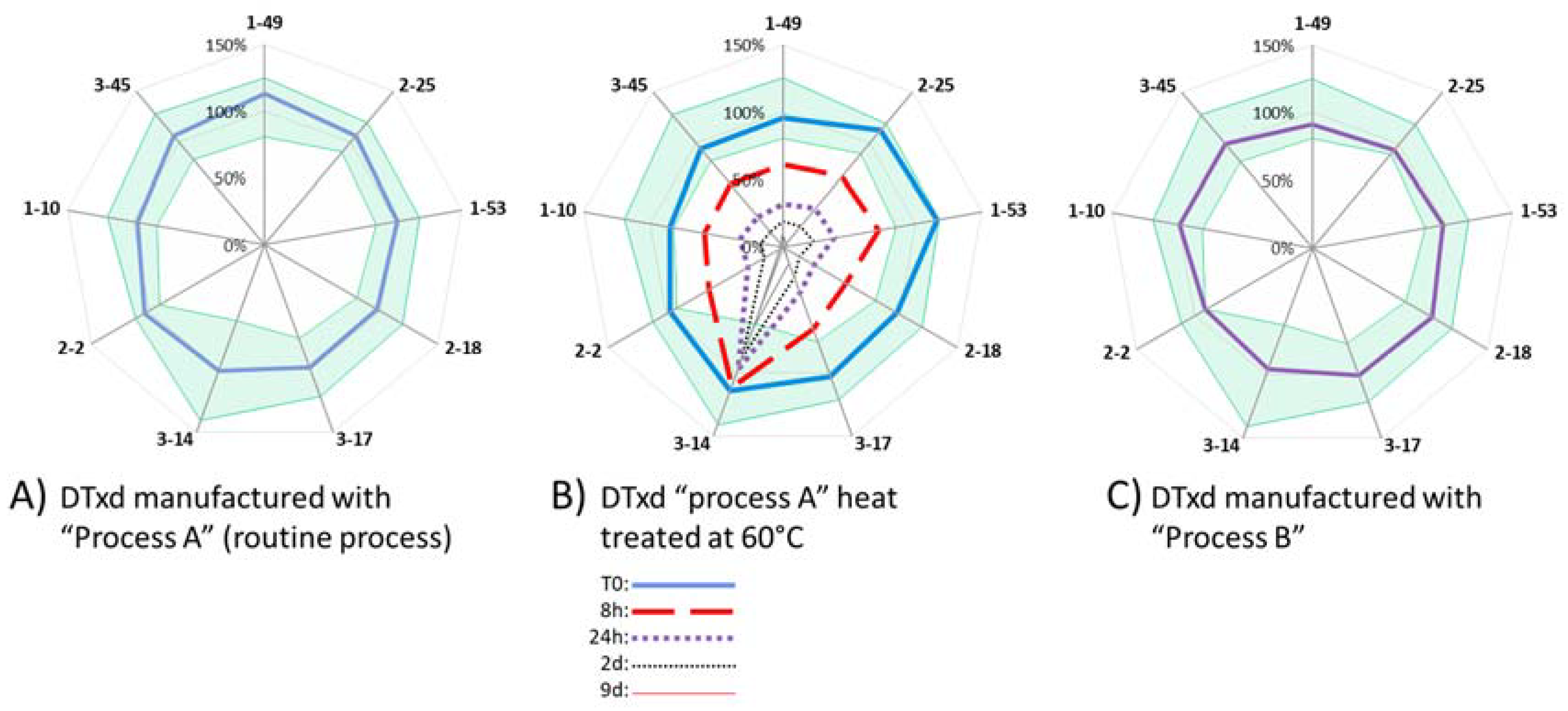
3.1.4. Determination of mAb Ability to Detect Thermally Induced Change in DTxd
3.2. Single Epitope Antigenicity Test (SEAT)
3.2.1. Assay Development
3.2.2. Assay Performance
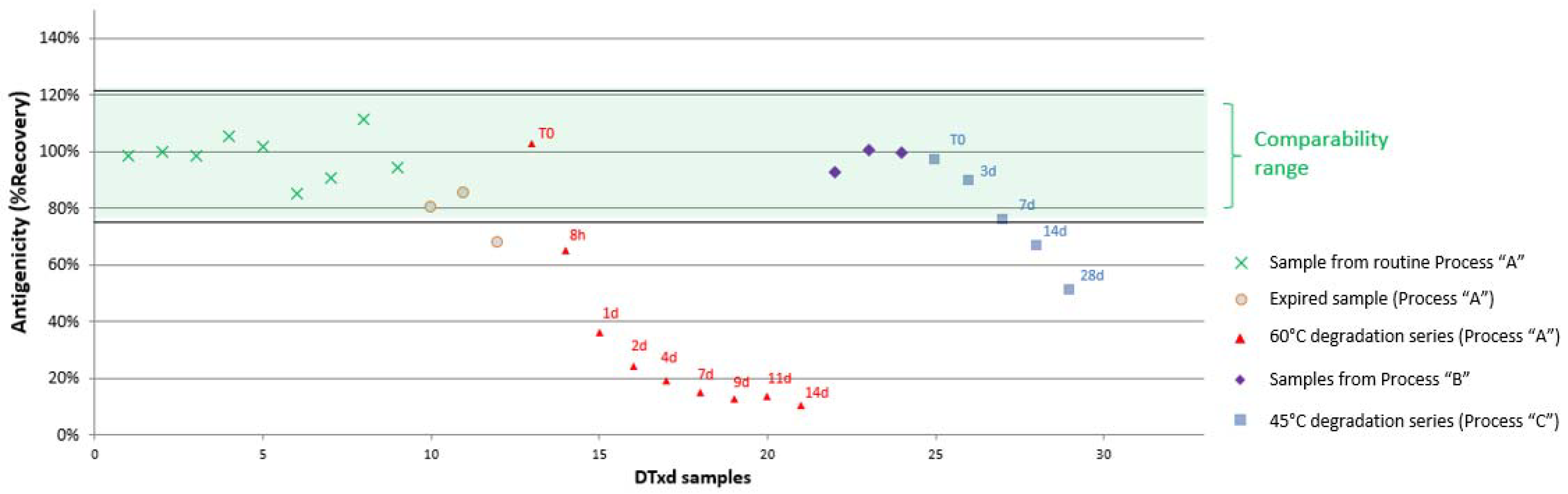
3.2.3. Sample Comparability Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lebron, J.A.; Wolf, J.J.; Kaplanski, C.V.; Ledwith, B.J. Ensuring the Quality, Potency and Safety of Vaccines during Preclinical Development. Expert Rev. Vaccines 2005, 4, 855–866. [Google Scholar] [CrossRef]
- FDA. General Biological Products Standards. In Code of Federal Regulations; Title 21, Part 610 (21 CFR 610); FDA: Silver Spring, MD, USA, 2001. [Google Scholar]
- Q 6 B Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products. 1999. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-6-b-test-procedures-acceptance-criteria-biotechnological/biological-products-step-5_en.pdf (accessed on 29 August 2021).
- CBER; FDA. Content and Format of Chemistry, Manufacturing and Controls Information and Establishment Description Information for a Vaccine or Related Product. In Guidance for Industry; FDA: Silver Spring, MD, USA, 1999. [Google Scholar]
- Hendriksen, C.; Arciniega, J.L.; Bruckner, L.; Chevalier, M.; Coppens, E.; Descamps, J.; Duchêne, M.; Dusek, D.M.; Halder, M.; Kreeftenberg, H.; et al. The Consistency Approach for the Quality Control of Vaccines. Biologicals 2008, 36, 73–77. [Google Scholar] [CrossRef] [PubMed]
- FDA Center for Drug Evaluation and Research and Center for Biologics Evaluation and Research. Q5E Comparability of Biotechnological/Biological Products Subject to Changes in Their Manufacturing Process. In Guidance for Industry; FDA: Silver Spring, MD, USA, 2005. [Google Scholar]
- Metz, B.; Jiskoot, W.; Hennink, W.E.; Crommelin, D.J.A.; Kersten, G.F.A. Physicochemical and Immunochemical Techniques Predict the Quality of Diphtheria Toxoid Vaccines. Vaccine 2003, 22, 156–167. [Google Scholar] [CrossRef]
- Coombes, L.; Stickings, P.; Tierney, R.; Rigsby, P.; Sesardic, D. Development and Use of a Novel in Vitro Assay for Testing of Diphtheria Toxoid in Combination Vaccines. J. Immunol. Methods 2009, 350, 142–149. [Google Scholar] [CrossRef]
- Petersen, R.L. Strategies Using Bio-Layer Interferometry Biosensor Technology for Vaccine Research and Development. Biosensors 2017, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Riches-Duit, R.; Hassall, L.; Kogelman, A.; Westdijk, J.; Dobly, A.; Francotte, A.; Stickings, P. Characterisation of Diphtheria Monoclonal Antibodies as a First Step towards the Development of an in Vitro Vaccine Potency Immunoassay. Biologicals 2021, 69, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Abdiche, Y.N.; Miles, A.; Eckman, J.; Foletti, D.; Blarcom, T.J.V.; Yeung, Y.A.; Pons, J.; Rajpal, A. High-Throughput Epitope Binning Assays on Label-Free Array-Based Biosensors Can Yield Exquisite Epitope Discrimination That Facilitates the Selection of Monoclonal Antibodies with Functional Activity. PLoS ONE 2014, 9, 16. [Google Scholar]
- Xiong, X.; Martin, S.R.; Haire, L.F.; Wharton, S.A.; Daniels, R.S.; Bennett, M.S.; McCauley, J.W.; Collins, P.J.; Walker, P.A.; Skehel, J.J.; et al. Receptor Binding by an H7N9 Influenza Virus from Humans. Nature 2013, 499, 496–499. [Google Scholar] [CrossRef]
- Lad, L.; Clancy, S.; Kovalenko, M.; Liu, C.; Hui, T.; Smith, V.; Pagratis, N. High-Throughput Kinetic Screening of Hybridomas to Identify High-Affinity Antibodies Using Bio-Layer Interferometry. J. Biomol. Screen. 2015, 20, 498–507. [Google Scholar] [CrossRef]
- Sevigny, L.M.; Booth, B.J.; Rowley, K.J.; Leav, B.A.; Cheslock, P.S.; Garrity, K.A.; Sloan, S.E.; Thomas, W.; Babcock, G.J.; Wang, Y. Identification of a Human Monoclonal Antibody To Replace Equine Diphtheria Antitoxin for Treatment of Diphtheria Intoxication. Infect. Immun. 2013, 81, 3992–4000. [Google Scholar] [CrossRef]
- Bayne-Jones, S. The Titration of Diphtheria Toxin and Antitoxin by Ramon’s Flocculation Method. J. Immunol. 1924, 9, 481–504. [Google Scholar]
- Collier, R.J. Understanding the Mode of Action of Diphtheria Toxin: A Perspective on Progress during the 20th Century. Toxicon 2001, 39, 1793–1803. [Google Scholar] [CrossRef]
- Metz, B.; Michiels, T.; Uittenbogaard, J.; Danial, M.; Tilstra, W.; Meiring, H.; Hennink, W.; Crommelin, D.; Kersten, G.; Jiskoot, W. Identification of Formaldehyde-Induced Modifications in Diphtheria Toxin. J. Pharm. Sci. 2019, 109, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J. Diphtheria Toxin: Mode of Action and Structure. Bacteriol. Rev. 1975, 39, 54–85. [Google Scholar] [CrossRef]
- Louie, G.V.; Yang, W.; Bowman, M.E.; Choe, S. Crystal Structure of the Complex of Diphtheria Toxin with an Extracellular Fragment of Its Receptor. Mol. Cell 1997, 1, 67–78. [Google Scholar] [CrossRef]
- Naglich, J.G.; Metherall, J.E.; Russell, D.W.; Eidels, L. Expression Cloning of a Diphtheria Toxin Receptor: Identity with a Heparin-Binding EGF-like Growth Factor Precursor. Cell 1992, 69, 1051–1061. [Google Scholar] [CrossRef]
- Wenzel, E.V.; Bosnak, M.; Tierney, R.; Schubert, M.; Brown, J.; Dübel, S.; Efstratiou, A.; Sesardic, D.; Stickings, P.; Hust, M. Human Antibodies Neutralizing Diphtheria Toxin in Vitro and in Vivo. Sci. Rep. 2020, 10, 571. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.M.; Mawas, F.; Bolgiano, B.; Lemercinier, X.; Crane, D.T.; Huskisson, R.; Corbel, M.J. Physico-Chemical and Immunological Examination of the Thermal Stability of Tetanus Toxoid Conjugate Vaccines. Vaccine 2002, 20, 3509–3522. [Google Scholar] [CrossRef]
- Galazka, A.M.; Milstien, J.B.; Zaffran, M. Thermostability of Vaccines; WHO/GPV/98.07. 1998. Available online: https://apps.who.int/iris/handle/10665/64980 (accessed on 28 April 2022).
- Alsarraf, H.; Dedic, E.; Bjerrum, M.J.; Østergaard, O.; Kristensen, M.P.; Petersen, J.W.; Jørgensen, R. Biophysical Comparison of Diphtheria and Tetanus Toxins with the Formaldehyde-Detoxified Toxoids, the Main Components of Diphtheria and Tetanus Vaccines. Virulence 2017, 8, 1880–1889. [Google Scholar] [CrossRef] [PubMed][Green Version]
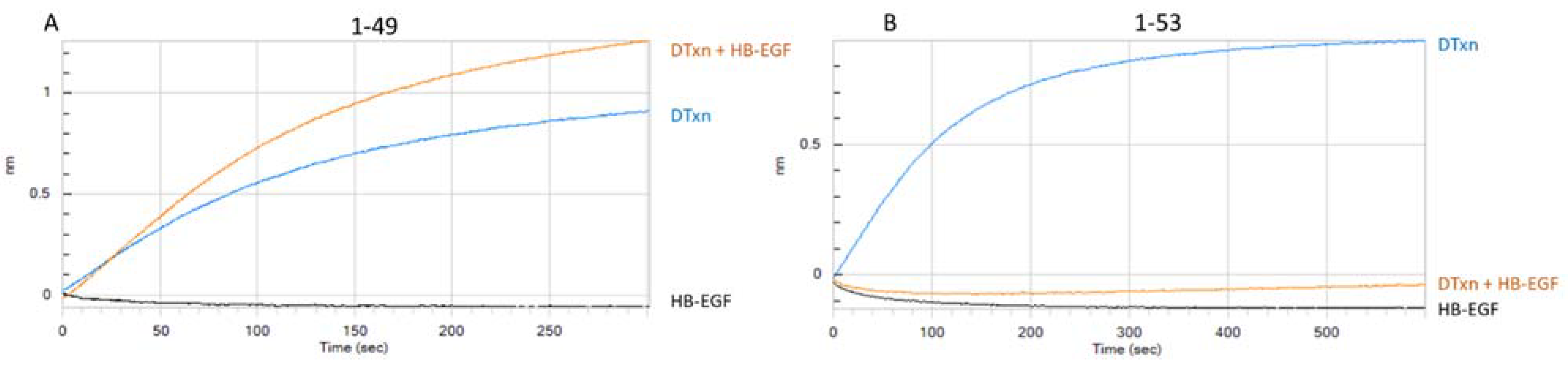

| mAb | Clone ID | Binding Site on DTxn (Subunit A or B) | Bin | Distinguishes Native from Heat Stressed DTxd | Inhibits DTxn Binding to HB-EGF Target | Neutralization Capacity |
|---|---|---|---|---|---|---|
| 1-10 | 1-10.3.1.5.10 | B | 2 | Yes | Yes | weak |
| 1-49 | 1-49.1.2.8.6.3.8 | A | 1 | Yes | No | strong |
| 1-53 | 1-53.3.2.4.6.6 | B | 2 | Yes | Yes | moderate |
| 2-1 | 2-1.3.21 | B | 2 | Yes | inconsistent | weak |
| 2-18 | 2-18.1.8.8.3 | A | 3 | Yes | No | strong |
| 2-2 | 2-2.2.11 | A | 3 | Yes | No | moderate |
| 2-25 | 2-25.1.21.9.7.4.7 | B | 2 | Yes | Yes | moderate |
| 3-11 | 3-11.1.9 | B | 4 | Yes | No | weak |
| 3-14 | 3-14.1.1 | B | 4 | No | inconsistent | weak |
| 3-17 | 3-17.1.2 | B | 4 | Yes | No | strong |
| 3-44 | 3-44.2.7 | A | 3 | Yes | No | weak |
| 3-45 | 3-45.2.3 | A | 3 | Yes | No | moderate |
| mAb | Neutralizing Titre (×10−3 U/mg) | Category | n |
|---|---|---|---|
| 1-10 | <1 | weak | 2 |
| 1-49 | 10–100 | strong | 4 |
| 1-53 | 1–9 | moderate | 2 |
| 2-1 | <1 | weak | 2 |
| 2-18 | 10–100 | strong | 5 |
| 2-2 | 1–9 | moderate | 1 |
| 2-25 | 1–9 | moderate | 4 |
| 3-11 | <1 | weak | 1 |
| 3-14 | <1 | weak | 1 |
| 3-17 | 10–100 | strong | 1 |
| 3-44 | <1 | weak | 1 |
| 3-45 | 1–9 | moderate | 1 |
| Quality Check | Sample/ Parameter | 1-49 | 1-53 | 2-25 | 2-18 | 3-14 | 3-17 | 1-10 | 2-2 | 3-45 |
|---|---|---|---|---|---|---|---|---|---|---|
| Intermediate Precision (%CV, n = 3) | 1X | 7% | 8% | 2% | 4% | 4% | 4% | 8% | 9% | 7% |
| 0.5X | 4% | 3% | 3% | 14% | 6% | 1% | 7% | 5% | 3% | |
| 0.25X | 9% | 2% | 2% | 10% | 10% | 6% | 5% | 2% | 3% | |
| 0.1X | 4% | 4% | 6% | 9% | 16% | 9% | 2% 1 | 9% | 4% | |
| Accuracy (%Recovery range, n = 3) | 1X | 96–111% | 94–108% | 99–104% | 98–105% | 99–108% | 98–107% | 91–105% | 97–113% | 101–115% |
| 0.5X | 100–108% | 95–101% | 97–102% | 80–105% | 92–102% | 96–97% | 93–106% | 98–107% | 98–105% | |
| 0.25X | 93–110% | 93–97% | 93–96% | 84–103% | 86–104% | 90–101% | 93–103% | 96–99% | 97–103% | |
| 0.1X | 95–103% | 87–93% | 86–96% | 80–95% | 72–100% | 81–96% | 90–92% 1 | 87–103% | 92–99% | |
| Linearity (1X, 0.5X, 0.25X, 0.1X) | R2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Slope | 1.02 | 1.06 | 1.05 | 1.05 | 1.07 | 1.06 | 1.02 | 1.03 | 1.04 | |
| Sensitive to Heat Induced Degradation 2 | N/Ap | Yes | Yes | Yes | Yes | poor 3 | Yes | Yes | Yes | Yes |
| Comparability Range (%Recovery) | N/Ap | [80–125%] | [85–118%] | [90–119%] | [80–119%] | [61–141%] | [75–122%] | [82–120%] | [91–112%] | [83–128%] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houy, C.; Ming, M.; Ettorre, L.; Jin, R.; Thangavadivel, N.; Chen, T.; Su, J.; Gajewska, B. Epitope Profiling of Diphtheria Toxoid Provides Enhanced Monitoring for Consistency Testing during Manufacturing Process Changes. Vaccines 2022, 10, 775. https://doi.org/10.3390/vaccines10050775
Houy C, Ming M, Ettorre L, Jin R, Thangavadivel N, Chen T, Su J, Gajewska B. Epitope Profiling of Diphtheria Toxoid Provides Enhanced Monitoring for Consistency Testing during Manufacturing Process Changes. Vaccines. 2022; 10(5):775. https://doi.org/10.3390/vaccines10050775
Chicago/Turabian StyleHouy, Camille, Marin Ming, Luciano Ettorre, Robbie Jin, Nemika Thangavadivel, Tricia Chen, Jin Su, and Beata Gajewska. 2022. "Epitope Profiling of Diphtheria Toxoid Provides Enhanced Monitoring for Consistency Testing during Manufacturing Process Changes" Vaccines 10, no. 5: 775. https://doi.org/10.3390/vaccines10050775
APA StyleHouy, C., Ming, M., Ettorre, L., Jin, R., Thangavadivel, N., Chen, T., Su, J., & Gajewska, B. (2022). Epitope Profiling of Diphtheria Toxoid Provides Enhanced Monitoring for Consistency Testing during Manufacturing Process Changes. Vaccines, 10(5), 775. https://doi.org/10.3390/vaccines10050775






