Breakthrough COVID-19 Infections in the US: Implications for Prolonging the Pandemic
Abstract
:1. Introduction
2. Betacoronaviruses
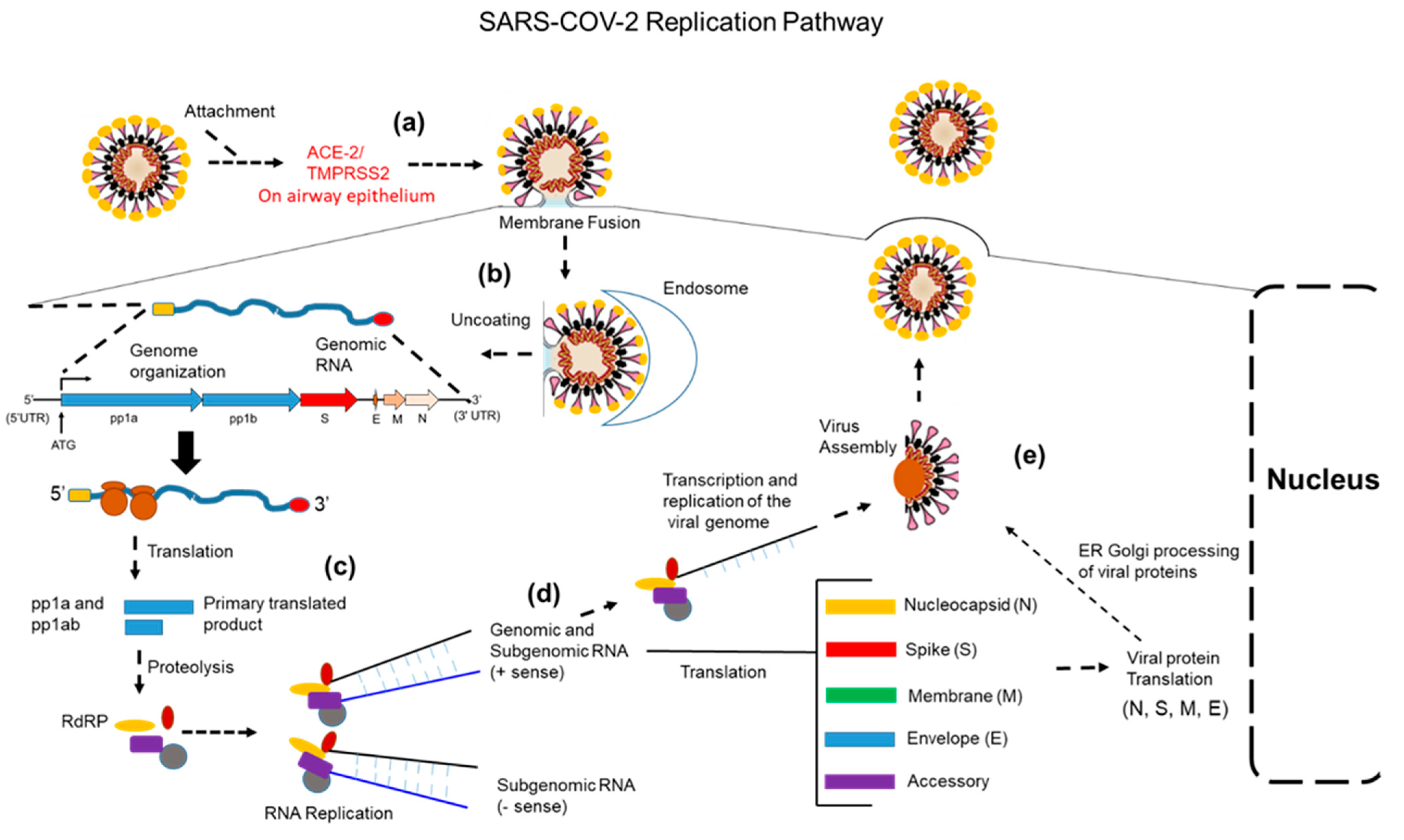
3. Coronavirus Replication
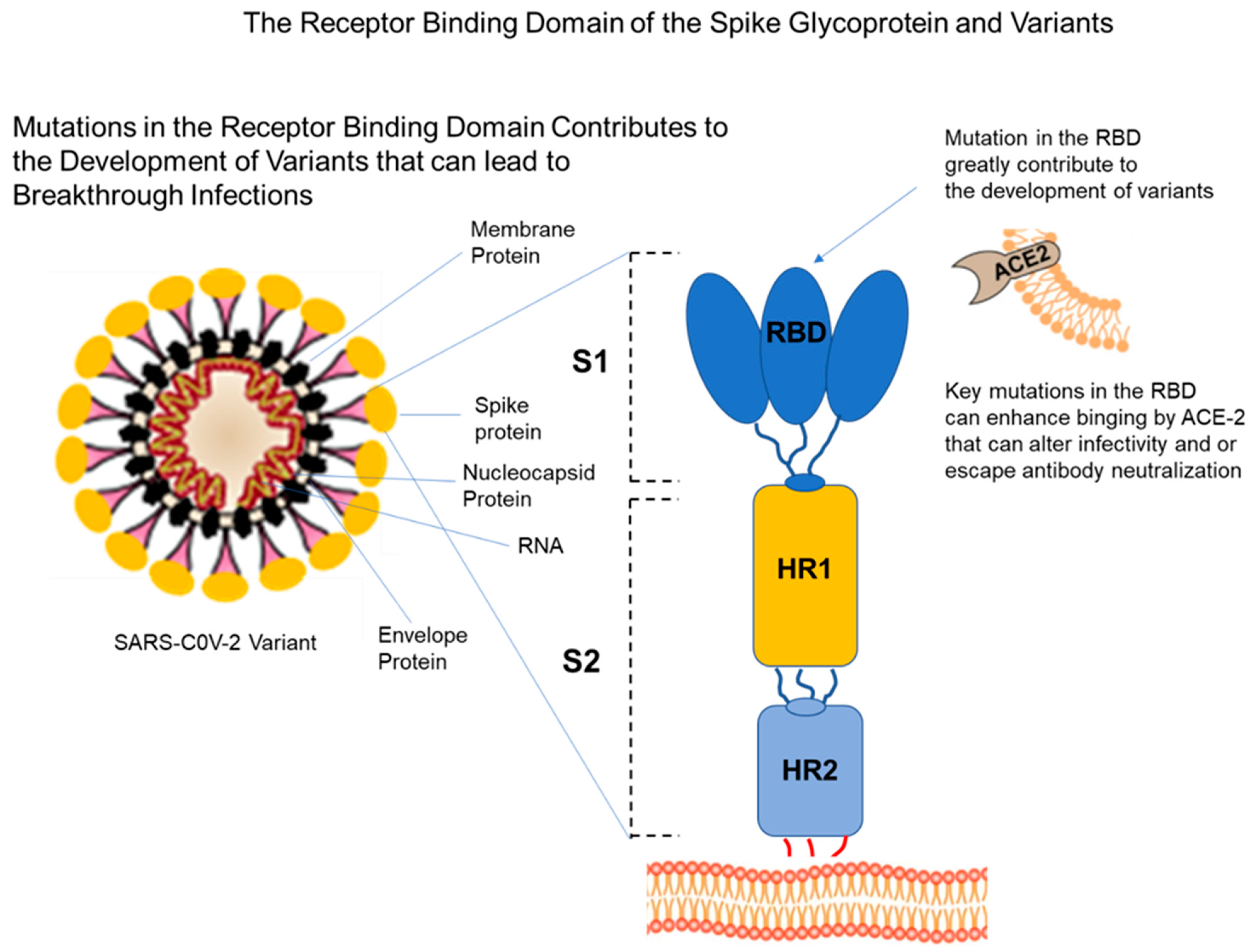
4. Viral Variants
4.1. The Delta Variant
4.2. The Omicron Variant
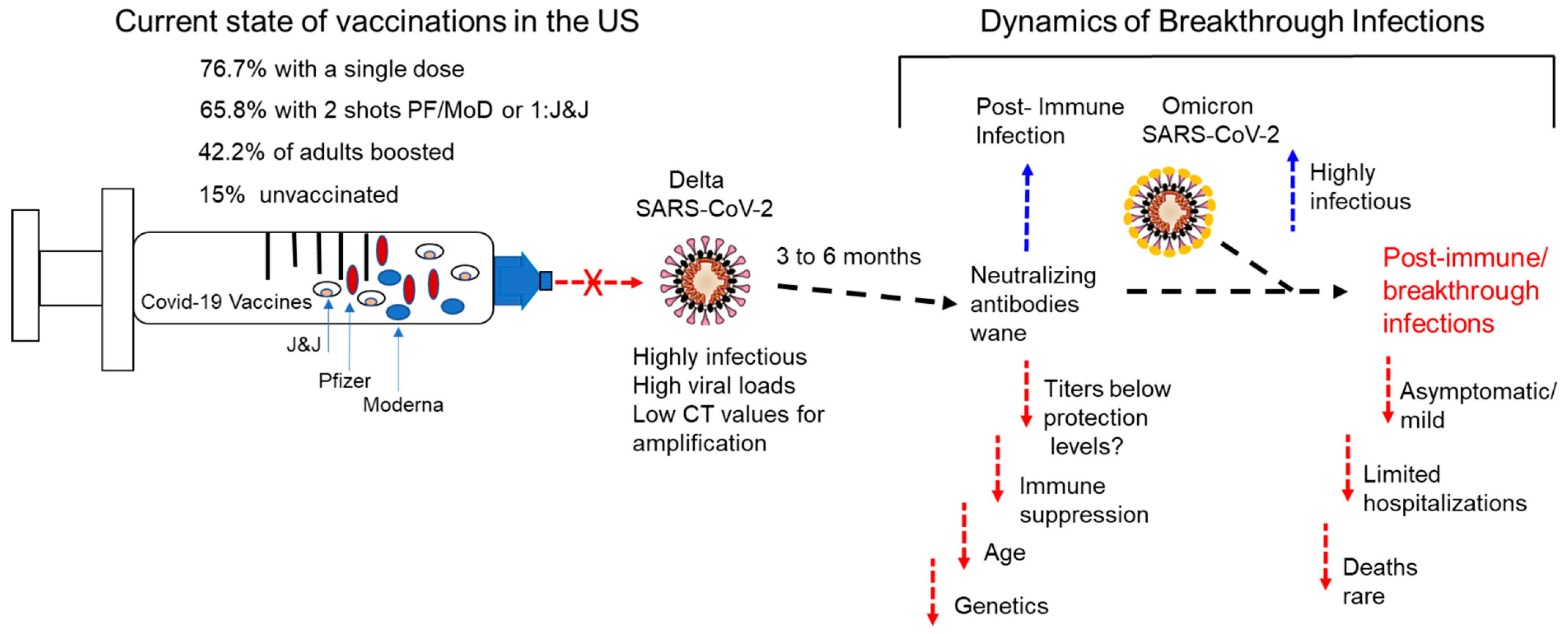
5. Breakthrough Infections (Post-Immune Infections)
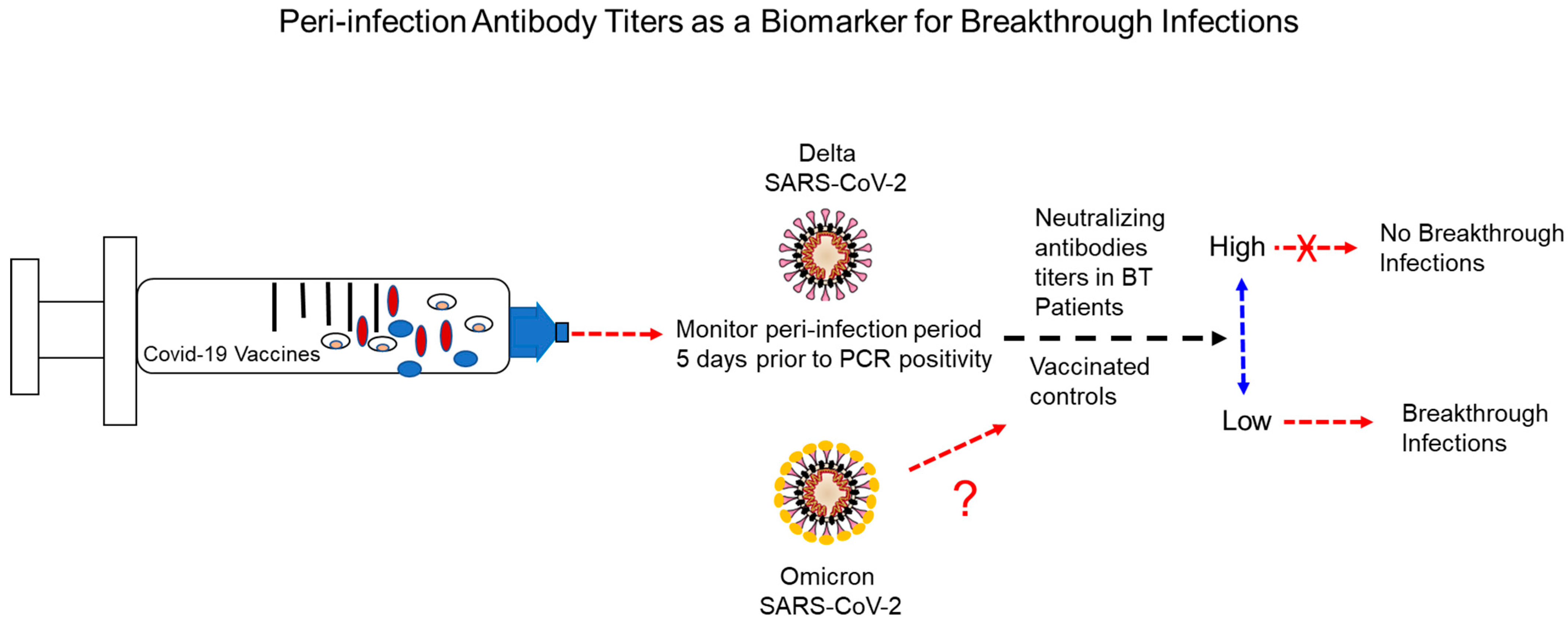
6. Potential Causes and Circumstances Surrounding Breakthrough Infections
6.1. Waning of Immune Protection
6.2. Aging and Immune Responses to Vaccines
6.3. Individuals with Weakened Immune Systems and COVID-19 Vaccines
6.4. Individuals Receiving Biologic Immunosuppressive Therapies
6.5. Genetics and Breakthrough Infections
6.6. Other Factors Implicated in Breakthrough Infections
7. Strategies for Reducing Breakthrough Infections
8. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bassetti, M.; Vena, A.D.; Giacobbe, R. The Novel Chinese Coronavirus (2019nCoV) Infections: Challenges for fighting the storm. Eur. J. Clin. Investig. 2020, 50, e13209. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 2020, 3, 105924. [Google Scholar] [CrossRef]
- Li, Q.; Guan, X.; Wu, P.; Wang, X.; Zhou, L.; Tong, Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020, 13, 1199–1207. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Salacup, G.; Lo, K.B.; Gul, F.; Peterson, E.; De Joy, R.; Bhargav, R.; Pelayo, J.; Albano, J.; Azmaiparashvili, Z.; Patarroyo-Aponte, G.; et al. Characteristics and clinical outcomes of COVID-19 patients in an underserved-inner city population: A single tertiary center cohort. J. Med. Virol. 2021, 93, 416–423. [Google Scholar] [CrossRef]
- Donald, J. Alcendor. Racial Disparities Associated COVID-19 Mortality among Minority Populations in the US. J. Clin. Med. 2020, 8, E2442. [Google Scholar]
- COVID Data Tracker Weekly Review. Available online: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html (accessed on 15 April 2022).
- Alcendor, D.J. Targeting COVID Vaccine Hesitancy in Rural Communities in Tennessee: Implications for Extending the COVID-19 Pandemic in the South. Vaccines 2021, 11, 1279. [Google Scholar] [CrossRef]
- Hildreth, J.E.K.; Alcendor, D.J. Targeting COVID-19 Vaccine Hesitancy in Minority Populations in the US: Implications for Herd Immunity. Vaccines 2021, 5, 489. [Google Scholar] [CrossRef]
- Gupta, R.K.; Topol, E.J. COVID-19 vaccine breakthrough infections. Science 2021, 6575, 1561–1562. [Google Scholar] [CrossRef]
- Hacisuleyman, E.; Hale, C.; Saito, Y.; Blachere, N.E.; Bergh, M.; Conlon, E.G.; Schaefer-Babajew, D.J.; DaSilva, J.; Muecksch, F.; Gaeble, C.; et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021, 23, 2212–2218. [Google Scholar] [CrossRef]
- Khoury, J.; Najjar-Debbiny, R.; Hanna, A.; Jabbour, A.; Abu Ahmad, Y.; Saffuri, A.; Abu-Sinni, M.; Shkeiri, R.; Elemy, A.; Hakim, F. COVID-19 vaccine-Long term immune decline and breakthrough infections. Vaccine 2021, 48, 6984–6989. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 10328, 924–944. [Google Scholar] [CrossRef]
- Kannan, S.; Shaik Syed Ali, P.; Sheeza, A. Omicron (B.1.1.529)—Variant of concern—Molecular profile and epidemiology: A mini review. Eur. Rev. Med. Pharm. Sci. 2021, 25, 8019–8022. [Google Scholar]
- Farinholt, T.; Doddapaneni, H.; Qin, X.; Menon, V.; Meng, Q.; Metcalf, G.; Chao, H.; Gingras, M.C.; Farinholt, P.; Agrawal, C.; et al. Transmission event of SARS-CoV-2 Delta variant reveals multiple vaccine breakthrough infections. medRxiv [Preprint]. Update in: BMC Med. 2021, 1, 255. [CrossRef]
- Levine-Tiefenbrun, M.; Yelin, I.; Alapi, H.; Katz, R.; Herzel, E.; Kuint, J.; Chodick, G.; Gazit, S.; Patalon, T.; Kishony, R. Viral loads of Delta-variant SARS-CoV-2 breakthrough infections after vaccination and booster with BNT162b2. Nat. Med. 2021, 12, 2108–2110. [Google Scholar] [CrossRef]
- Hartenian, E.; Nandakumar, D.; Lari, A.; Ly, M.; Tucker, J.M.; Glaunsinger, B.A. The molecular virology of coronaviruses. J. Biol. Chem. 2020, 37, 12910–12934. [Google Scholar] [CrossRef]
- Satija, N.; Lal, S.K. The molecular biology of SARS coronavirus. Ann. N. Y. Acad. Sci. 2007, 1, 26–38. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.P.; Lam, C.S.F.; Lau, C.C.Y.; Tsang, A.K.L.; Lau, J.H.N.; Bai, R.; Teng, J.L.L.; Tsang, C.C.C.; Wang, M.; et al. Discovery of Seven Novel Mammalian and Avian Coronaviruses in the Genus Deltacoronavirus Supports Bat Coronaviruses as the Gene Source of Alphacoronavirus and Betacoronavirus and Avian Coronaviruses as the Gene Source of Gammacoronavirus and Deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar]
- Chairez, R.; Hollinger, B.; Melnick, J.L.; Dreesman, G.R.; Ghendon, Y.; Porubel, L.; Sasaki, Y.; Sasaki, R.; Cohen, G.H.; Pizer, L.I.; et al. International Committee on Taxonomy of Viruses. Virus Taxonomy: 2017 Release. Available online: https://talk.ictvonline.org/taxonomy/ (accessed on 13 October 2017).
- Du Toit, A. Outbreak of a novel coronavirus. Nat. Rev. Genet. 2020, 18, 123. [Google Scholar] [CrossRef]
- Rothan, H.A.; Byrareddy, S. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020, 109, 102433. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 8, 782–793. [Google Scholar] [CrossRef]
- Hui, D.S.; Azhar, E.E.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; McHugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health. The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.; To, K.K.W.; Tse, H.; Jin, D.-Y.; Yuen, K.-Y. Interspecies transmission, and emergence of novel viruses: Lessons from bats and birds. Trends Microbiol. 2013, 21, 544–555. [Google Scholar] [CrossRef]
- Peiris, J.S.M.; Yuen, K.Y.; Osterhaus, A.D.M.E.; Stöhr, K. The severe acute respiratory syndrome. N. Engl. J. Med. 2003, 349, 2431–2441. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.; Hui, D.; Wu, A.; Chan, P.; Cameron, P.; Joynt, G.M.; Ahuja, A.; Yung, M.Y.; Leung, C.B.; To, K.F.; et al. A major outbreak of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003, 348, 1986–1994. [Google Scholar] [CrossRef]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Al-Abdallat, M.M.; Payne, D.C.; Alqasrawi, S. The Jordan MERS-CoV Investigation Team Hospital-associated outbreak of Middle East respiratory syndrome coronavirus: A serologic, epidemiologic, and clinical description. Clin. Infect Dis. 2014, 59, 1225–1233. [Google Scholar] [CrossRef] [Green Version]
- van Boheemen, S.; de Graaf, M.; Lauber, C. Genomic characterization of a newly discovered coronavirus associated with acute respiratory distress syndrome in humans. MBio 2012, 3, e00473–e00512. [Google Scholar] [CrossRef] [Green Version]
- Kirtipal, N.; Bharadwaj, S.; Kang, S.G. From SARS to SARS-CoV-2, insights on structure, pathogenicity, and immunity aspects of pandemic human coronaviruses. Infect. Genet. Evol. 2020, 85, 104502. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 3, 155–170. [Google Scholar] [CrossRef]
- Souaid, T.; Hindy, J.R.; Kanj, S.S. What is Currently Known About the SARS-CoV2 Variants of Concern. J. Epidemiol. Glob. Health 2021, 3, 257–259. [Google Scholar] [CrossRef] [PubMed]
- Aleem, A.; Akbar Samad, A.B.; Slenker, A.K. Emerging Variants of SARS-CoV-2 And Novel Therapeutics Against Coronavirus (COVID-19). In Treasure Island; StatPearls Publishing: Tresure Island, FL, USA, 2022. [Google Scholar]
- Xia, X. Domains and Functions of Spike Protein in Sars-Cov-2 in the Context of Vaccine Design. Viruses 2021, 1, 109. [Google Scholar] [CrossRef] [PubMed]
- Plante, J.A.; Liu, Y.; Liu, J.; Xia, H.; Johnson, B.A.; Lokugamage, K.G.; Zhang, X.; Muruato, A.E.; Zou, J.; Fontes-Garfias, C.R.; et al. Spike mutation D614G alters SARS-CoV-2 fitness. Nature 2021, 7852, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Lambrou, A.S.; Shirk, P.; Steele, M.K.; Paul, P.; Paden, C.R.; Cadwell, B.; Reese, H.E.; Aoki, Y.; Hassell, N.; Zheng, X.Y.; et al. Strain Surveillance and Emerging Variants Bioinformatic Working Group; Strain Surveillance and Emerging Variants NS3 Working Group. Genomic Surveillance for SARS-CoV-2 Variants: Predominance of the Delta (B.1.617.2) and Omicron (B.1.1.529) Variants—United States, June 2021–January 2022. MMWR Morb. Mortal Wkly. Rep. 2022, 6, 206–211. [Google Scholar]
- Teyssou, E.; Delagrèverie, H.; Visseaux, B.; Lambert-Niclot, S.; Brichler, S.; Ferre, V.; Marot, S.; Jary, A.; Todesco, E.; Schnuriger, A.; et al. The Delta SARS-CoV-2 variant has a higher viral load than the Beta and the historical variants in nasopharyngeal samples from newly diagnosed COVID-19 patients. J. Infect. 2021, 4, e1–e3. [Google Scholar] [CrossRef]
- Harder, T.; Külper-Schiek, W.; Reda, S.; Treskova-Schwarzbach, M.; Koch, J.; Vygen-Bonnet, S.; Wichmann, O. Effectiveness of COVID-19 vaccines against SARS-CoV-2 infection with the Delta (B.1.617.2) variant: Second interim results of a living systematic review and meta-analysis, 1 January to 25 August 2021. Euro Surveill. 2021, 41, 2100920. [Google Scholar] [CrossRef]
- Nanduri, S.; Pilishvili, T.; Derado, G.; Soe, M.M.; Dollard, P.; Wu, H.; Li, Q.; Bagchi, S.; Dubendris, H.; Link-Gelles, R.; et al. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant—National Healthcare Safety Network, March 1-August 1, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 34, 1163–1166. [Google Scholar]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 2021, 7871, 276–280. [Google Scholar] [CrossRef]
- Luo, C.H.; Morris, C.P.; Sachithanandham, J.; Amadi, A.; Gaston, D.; Li, M.; Swanson, N.J.; Schwartz, M.; Klein, E.Y.; Pekosz, A.; et al. Infection with the SARS-CoV-2 Delta Variant is Associated with Higher Infectious Virus Loads Compared to the Alpha Variant in both Unvaccinated and Vaccinated Individuals. Clin. Infect. Dis. 2021, ciab986. [Google Scholar] [CrossRef]
- Migueres, M.; Dimeglio, C.; Trémeaux, P.; Raymond, S.; Lhomme, S.; Da Silva, I.; Oliveira Mendes, K.; Abravanel, F.; Félicé, M.P.; Mansuy, J.M.; et al. Influence of the Delta Variant and Vaccination on the SARS-CoV-2 Viral Load. Viruses 2022, 2, 323. [Google Scholar] [CrossRef]
- Kumar, S.; Thambiraja, T.S.; Karuppanan, K.; Subramaniam, G. Omicron and Delta variant of SARS-CoV-2: A comparative computational study of spike protein. J. Med. Virol. 2022, 4, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.A.T.M.; Datir, R.; Collier, D.A.; Albeck, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 7883, 114–119. [Google Scholar] [CrossRef] [PubMed]
- SARS-CoV-2 Variant Classifications and Definitions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html (accessed on 16 July 2021).
- Cherian, S.; Potdar, V.; Jadhav, S.; Yadav, P.; Gupta, N.; Das, M.; Rakshit, P.; Singh, S.; Abraham, P.; Panda, S.; et al. SARS-CoV-2 Spike Mutations, L452R, T478K, E484Q and P681R, in the Second Wave of COVID-19 in Maharashtra, India. Microorganisms 2021, 7, 1542. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.R.; Spratt, A.N.; Cohen, A.R.; Naqvi, S.H.; Chand, H.S.; Quinn, T.P.; Lorson, C.L.; Byrareddy, S.N.; Singh, K. Evolutionary analysis of the Delta and Delta Plus variants of the SARS-CoV-2 viruses. J. Autoimmun. 2021, 124, 102715. [Google Scholar] [CrossRef] [PubMed]
- CDC COVID-19 Response Team. SARS-CoV-2 B.1.1.529 (Omicron) Variant—United States, 1–8 December 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 50, 1731–1734. [Google Scholar]
- Hoffmann, M.; Krüger, N.; Schulz, S.; Cossmann, A.; Rocha, C.; Kempf, A.; Nehlmeier, I.; Graichen, L.; Moldenhauer, A.S.; Winkler, M.S.; et al. The Omicron variant is highly resistant against antibody-mediated neutralization: Implications for control of the COVID-19 pandemic. Cell 2022, 3, 447–456. [Google Scholar] [CrossRef]
- Shiehzadegan, S.; Alaghemand, N.; Fox, M.; Venketaraman, V. Analysis of the Delta Variant, B.1.617.2 COVID-19. Clin. Pract. 2021, 4, 778–784. [Google Scholar] [CrossRef]
- Mahase, E. Delta variant: What is happening with transmission, hospital admissions, and restrictions? BMJ 2021, 373, n1513. [Google Scholar] [CrossRef]
- Dougherty, K.; Mannell, M.; Naqvi, O.; Matson, D.; Stone, J. SARS-CoV-2 B.1.617.2 (Delta) Variant COVID-19 Outbreak Associated with a Gymnastics. Facility—Oklahoma, April–May 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 28, 1004–1007. [Google Scholar] [CrossRef]
- Singanayagam, A.; Hakki, S.; Dunning, J.; Madon, K.J.; Crone, M.A.; Koycheva, A.; Derqui-Fernandez, N.; Barnett, J.L.; Whitfield, M.G.; Varro, R.; et al. ATACCC Study Investigators. Community transmission and viral load kinetics of the SARS-CoV-2 delta (B.1.617.2) variant in vaccinated and unvaccinated individuals in the UK: A prospective, longitudinal, cohort study. Lancet Infect. Dis. 2022, 2, 183–195. [Google Scholar] [CrossRef]
- Tian, D.; Sun, Y.; Zhou, J.; Ye, Q. The Global Epidemic of the SARS-CoV-2 Delta Variant, Key Spike Mutations, and Immune Escape. Front. Immunol. 2021, 12, 751778. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Abdool-Karim, S.S.; Baden, L.R.; Farrar, J.J.; Hamel, M.B.; Longo, D.L.; Morrissey, S.; Rubin, E.J. Addressing Vaccine Inequity—Covid-19 Vaccines as a Global Public Good. N. Engl. J. Med. 2022, 12, 1176–1179. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Woo, H.G. Omicron: A Heavily Mutated SARS-CoV-2 Variant Exhibits Stronger Binding to ACE2 and Potently Escapes Approved COVID-19 Therapeutic Antibodies. Front. Immunol. 2022, 12, 830527. [Google Scholar] [CrossRef] [PubMed]
- Bushman, M.; Kahn, R.; Taylor, B.P.; Lipsitch, M.; Hanage, W.P. Population impact of SARS-CoV-2 variants with enhanced transmissibility and/or partial immune escape. Cell 2021, 26, 6229–6242.e18. [Google Scholar] [CrossRef]
- Chen, J.; Wang, R.; Gilby, N.B.; Wei, G.W. Omicron (B.1.1.529): Infectivity, vaccine breakthrough, and antibody resistance. arXiv 2021, arXiv:2112.01318v1. Update in: J. Chem. Inf. Model. 2022, 2, 412–422. [Google Scholar] [CrossRef]
- Sonabend, R.; Whittles, L.K.; Imai, N.; Perez-Guzman, P.N.; Knock, E.S.; Rawson, T.; Gaythorpe, K.A.M.; Djaafara, B.A.; Hinsley, W.; FitzJohn, R.G.; et al. Non-pharmaceutical interventions, vaccination, and the SARS-CoV-2 delta variant in England: A mathematical modelling study. Lancet 2021, 10313, 1825–1835. [Google Scholar] [CrossRef]
- VanBlargan, L.A.; Errico, J.M.; Halfmann, P.J.; Zost, S.J.; Crowe, J.E., Jr.; Purcell, L.A.; Kawaoka, Y.; Corti, D.; Fremont, D.H.; Diamond, M.S. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat. Med. 2022, 28, 490–495. [Google Scholar] [CrossRef]
- CDC COVID-19 Vaccine Breakthrough Case Investigations Team. COVID-19 Vaccine Breakthrough Infections Reported to CDC—United States, January 1–April 30, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 21, 792–793. [Google Scholar]
- Public Health Investigations of COVID-19 Vaccine Breakthrough Cases. Case Investigation Protocol. Available online: https://www.cdc.gov/vaccines/covid-19/downloads/covid-vaccine-breakthrough-case-investigations-protocol.pdf (accessed on 28 October 2021).
- Self, W.H.; Tenforde, M.W.; Rhoads, J.P.; Gaglani, M.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; et al. Comparative Effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) Vaccines in Preventing COVID-19 Hospitalizations Among Adults Without Immunocompromising Conditions-United States, March–August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 38, 1337–1343. [Google Scholar]
- Oliver, S.E.; Gargano, J.W.; Marin, M.; Wallace, M.; Curran, K.G.; Chamberland, M.; McClung, N.; Campos-Outcalt, D.; Morgan, R.L.; Mbaeyi, S. The Advisory Committee on Immunization Practices’ Interim Recommendation for Use of Moderna COVID-19. Vaccine-United States, December 2020. MMWR. Morb. Mortal. Wkly. Rep. 2021, 5152, 1653–1656. [Google Scholar] [CrossRef]
- Livingston, E.H.; Malani, P.N.; Creech, C.B. The Johnson & Johnson Vaccine for COVID-19. JAMA 2021, 325, 1575. [Google Scholar] [PubMed]
- So far, 5800 Fully Vaccinated People Have Caught COVID Anyway in US, CDC Says. Available online: https://www.cnn.com/2021/04/14/health/breakthrough-infections-covid-vaccines-cdc/index.html (accessed on 15 April 2021).
- Juthani, P.V.; Gupta, A.; Borges, K.A.; Price, C.C.; Lee, A.I.; Won, C.H.; Chun, H.J. Hospitalisation among vaccine breakthrough COVID-19 infections. Lancet Infect. Dis. 2021, 11, 1485–1486. [Google Scholar] [CrossRef]
- Bergwerk, M.; Gonen, T.; Lustig, Y.; Amit, S.; Lipsitch, M.; Cohen, C.; Mandelboim, M.; Levin, E.G.; Rubin, C.; Indenbaum, V.; et al. Covid-19 Breakthrough Infections in Vaccinated Health Care Workers. N. Engl. J. Med. 2021, 16, 1474–1484. [Google Scholar] [CrossRef]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 3, 1663–1669. [Google Scholar]
- Liu, Y.; Liu, J.; Johnson, B.A.; Xia, H.; Ku, Z.; Schindewolf, C.; Widen, S.G.; An, Z.; Weaver, S.C.; Menachery, V.D.; et al. Delta spike P681R mutation enhances SARS-CoV-2 fitness over Alpha variant. bioRxiv Prepr. Serv. Biol. 2021, 5. [Google Scholar] [CrossRef]
- Takeda, M. Proteolytic activation of SARS-CoV-2 spike protein. Microbiol. Immunol. 2022, 1, 15–23. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, T.; Cai, Y.; Lavine, C.L.; Peng, H.; Zhu, H.; Anand, K.; Tong, P.; Gautam, A.; Mayer, M.L.; et al. Membrane fusion and immune evasion by the spike protein of SARS-CoV-2 Delta variant. Science. 2021, 6573, 1353–1360. [Google Scholar] [CrossRef]
- Lord, J.M. The effect of ageing of the immune system on vaccination responses. Hum. Vaccine Immunother. 2013, 6, 1364–1367. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, A.L.; McIntyre, P.; Subbarao, K.; Booy, R.; Levin, M.J. Vaccines for older adults. BMJ 2021, 372, 188. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, V.; Gadi, N.; Spihlman, A.P.; Wu, S.C.; Choi, C.H.; Moulton, V.R. Aging, Immunity, and COVID-19: How Age Influences the Host Immune Response to Coronavirus Infections? Front. Physiol. 2021, 11, 571416. [Google Scholar] [CrossRef]
- Chen, Y.; Klein, S.L.; Garibaldi, B.T.; Li, H.; Wu, C.; Osevala, N.M.; Li, T.; Margolick, J.B.; Pawelec, G.; Leng, S.X. Aging in COVID-19: Vulnerability, immunity, and intervention. Ageing Res. Rev. 2021, 65, 101205. [Google Scholar] [CrossRef] [PubMed]
- Connors, J.; Haddad, E.K.; Petrovas, C. Aging alters immune responses to vaccines. Aging 2021, 2, 1568–1570. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Landin, A.M.; Lechner, S.C.; Ryan, J.G.; Schwartz, R.; Riley, R.L.; Blomberg, B.B. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J. Immunol. 2008, 8, 5283–5290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merani, S.; Pawelec, G.; Kuchel, G.A.; McElhaney, J.E. Impact of Aging and Cytomegalovirus on Immunological Response to Influenza Vaccination and Infection. Front. Immunol. 2017, 8, 784. [Google Scholar] [CrossRef] [Green Version]
- Metcalf, T.U.; Cubas, R.A.; Ghneim, K.; Cartwright, M.J.; Grevenynghe, J.V.; Richner, J.M.; Olagnier, D.P.; Wilkinson, P.A.; Cameron, M.J.; Park, B.S.; et al. Global analyses revealed age-related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell 2015, 3, 421–432. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C.; et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020, 370, eabd4570. [Google Scholar] [CrossRef]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, 158–168.e14. [Google Scholar] [CrossRef]
- Smieszek, S.P.; Polymeropoulos, V.M.; Xiao, C.; Polymeropoulos, C.M.; Polymeropoulos, M.H. Loss-of-function mutations in IFNAR2 in COVID-19 severe infection susceptibility. J. Glob. Antimicrob. Resist. 2021, 26, 239–240. [Google Scholar] [CrossRef]
- Crotty, S. T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 2019, 50, 1132–1148. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Gaebler, C.; Muecksch, F.; Lorenzi, J.C.C.; Wang, Z.; Cho, A.; Agudelo, M.; Barnes, C.O.; Gazumyan, A.; Finkin, S.; et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 2020, 584, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Mentzer, A.J.; Liu, G.; Yao, X.; Yin, Z.; Dong, D.; Dejnirattisai, W.; Rostron, T.; Supasa, P.; Liu, C.; et al. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat. Immunol. 2020, 21, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Schulien, I.; Kemming, J.; Oberhardt, V.; Wild, K.; Seidel, L.M.; Killmer, S.; Sagar Daul, F.; Salvat Lago, M.; Decker, A.; Luxenburger, H.; et al. Characterization of pre-existing and induced SARS-CoV-2-specific CD8+ T cells. Nat. Med. 2021, 27, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.R.; Johnson, S.R.; Anthony, D.D.; Arasaratnam, R.J.; Baden, L.R.; Bass, A.R.; Calabrese, C.; Gravallese, E.M.; Harpaz, R.; Sadun, R.E.; et al. American College of Rheumatology Guidance for COVID-19 Vaccination in Patients with Rheumatic and Musculoskeletal Diseases: Version 1. Arthritis Rheumatol. 2021, 7, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Adler, E.; Mekeel, K.; Little, S.J. Clinical effectiveness of COVID-19 vaccination in solid organ transplant recipients. Transpl. Infect. Dis. 2021, 5, e13705. [Google Scholar] [CrossRef] [PubMed]
- Guidance for Cardiothoracic Transplant and Mechanical Circulatory Support Centers Regarding SARS CoV-2 Infection and COVID-19: 17 March 2020. Available online: https://community.ishlt.org/HigherLogic/System/DownloadDocumentFile.ashx?DocumentFileKey=afb06f06-5d63-13d4-c107-d152a9f6cd46 (accessed on 21 March 2020).
- Aslam, S.; Liu, J.; Sigler, R.; Syed, R.R.; Tu, X.M.; Little, S.J.; De Gruttola, V. Coronavirus disease 2019 vaccination is protective of clinical disease in solid organ transplant recipients. Transpl. Infect. Dis. 2022, 24, e13788. [Google Scholar] [CrossRef]
- Jones, M.E.; Kohn, A.H.; Pourali, S.P.; Rajkumar, J.R.; Gutierrez, Y.; Yim, R.M.; Armstrong, A.W. The Use of Biologics During the COVID-19 Pandemic. Dermatol. Clin. 2021, 4, 545–553. [Google Scholar] [CrossRef]
- Pahalyants, V.; Murphy, W.S.; Klebanov, N.; Lu, C.; Theodosakis, N.; Klevens, R.M.; Estir, H.; Lilly, E.; Asgari, M.; Semenov, Y.R. Immunosuppressive biologics did not increase the risk of COVID-19 or subsequent mortality: A retrospective matched cohort study from Massachusetts. J. Am. Acad. Dermatol. 2022, 1, 252–255. [Google Scholar] [CrossRef]
- Carapito, R.; Li, R.; Helms, J.; Carapito, C.; Gujja, S.; Rolli, V.; Guimaraes, R.; Malagon-Lopez, J.; Spinnhirny, P.; Lederle, A.; et al. Identification of driver genes for critical forms of COVID-19 in a deeply phenotyped young patient cohort. Sci. Transl. Med. 2022, 14, eabj7521. [Google Scholar] [CrossRef]
- Shin, J.; Toyoda, S.; Nishitani, S.; Fukuhara, A.; Kita, S.; Otsuki, M.; Shimomura, I. Possible Involvement of Adipose Tissue in Patients With Older Age, Obesity, and Diabetes With SARS-CoV-2 Infection (COVID-19) via GRP78 (BIP/HSPA5): Significance of Hyperinsulinemia Management in COVID-19. Diabetes 2021, 12, 2745–2755. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zheng, Q.; Madhira, V.; Olex, A.L.; Anzalone, A.J.; Vinson, A.; Singh, J.A.; French, E.; Abraham, A.G.; Mathew, J.; et al. National COVID Cohort Collaborative (N3C) Consortium. Association Between Immune Dysfunction and COVID-19 Breakthrough Infection After SARS-CoV-2 Vaccination in the US. JAMA Intern. Med. 2022, 182, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Basso, P.; Negro, C.; Cegolon, L.; Larese Filon, F. Risk of Vaccine Breakthrough SARS-CoV-2 Infection and Associated Factors in Healthcare Workers of Trieste Teaching Hospitals (North-Eastern Italy). Viruses 2022, 14, 336. [Google Scholar] [CrossRef] [PubMed]
- Marín-Hernández, D.; Schwartz, R.E.; Nixon, D.F. Nixon Epidemiological evidence for association between higher influenza vaccine uptake in the elderly and lower COVID-19 deaths in Italy. J. Med. Virol. 2021, 93, 64–65. [Google Scholar] [CrossRef]
- Salem, M.L.; El-Hennawy, D. The possible beneficial adjuvant effect of influenza vaccine to minimize the severity of COVID-19. Med. Hypotheses 2020, 140, 109752. [Google Scholar] [CrossRef]
- Conlon, A.; Ashur, C.; Washer, L.; Eagle, K.A.; Hofmann Bowman, M.A. Impact of the influenza vaccine on COVID-19 infection rates and severity. Am. J. Infect. Control 2021, 49, 694–700. [Google Scholar] [CrossRef]
- Fink, G.; Orlova-Fink, N.; Schindler, T.; Grisi, S.; Ferrer, A.P.S.; Daubenberger, C.; Brentani, A. Inactivated trivalent influenza vaccination is associated with lower mortality among patients with COVID-19 in Brazil. BMJ Evid. Based Med. 2020, 26, 192–193. [Google Scholar] [CrossRef]
- Hale, V.L.; Dennis, P.M.; McBride, D.S.; Nolting, J.M.; Madden, C.; Huey, D.; Ehrlich, M.; Grieser, J.; Winston, J.; Lombardi, D.; et al. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature 2022, 7897, 481–486. [Google Scholar] [CrossRef]
- Sharun, K.; Tiwari, R.; Natesan, S.; Dhama, K. SARS-CoV-2 infection in farmed minks, associated zoonotic concerns, and importance of the One Health approach during the ongoing COVID-19 pandemic. Vet. Q. 2021, 1, 50–60. [Google Scholar] [CrossRef]
- Sharun, K.; Dhama, K.; Pawde, A.M.; Gortázar, C.; Tiwari, R.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J.; de la Fuente, J.; Michalak, I.; Attia, Y.A. SARS-CoV-2 in animals: Potential for unknown reservoir hosts and public health implications. Vet. Q. 2021, 1, 181–201. [Google Scholar] [CrossRef]
- Hoffmann, M.; Hofmann-Winkler, H.; Krüger, N.; Kempf, A.; Nehlmeier, I.; Graichen, L.; Arora, P.; Sidarovich, A.; Moldenhauer, A.-S.; Winkler, M.S.; et al. SARS-CoV-2 variant B.1.617 is resistant to Bamlanivimab and evades antibodies induced by infection and vaccination. Cell Rep. 2021, 3, 109415. [Google Scholar] [CrossRef] [PubMed]
- Dejnirattisai, W.; Huo, J.; Zhou, D.; Zahradník, J.; Supasa, P.; Liu, C.; Duyvesteyn, H.M.E.; Ginn, H.M.; Mentzer, A.J.; Tuekprakhon, A.; et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell 2022, 185, 467–484.e15. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; St Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Hause, A.M.; Baggs, J.; Marquez, P.; Myers, T.R.; Gee, J.; Su, J.R.; Zhang, B.; Thompson, D.; Shimabukuro, T.T.; Shay, D.K. COVID-19 Vaccine Safety in Children Aged 5-11 Years—United States, November 3–December 19, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 5152, 1755–1760. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcendor, D.J.; Matthews-Juarez, P.; Smoot, D.; Hildreth, J.E.K.; Lamar, K.; Tabatabai, M.; Wilus, D.; Juarez, P.D. Breakthrough COVID-19 Infections in the US: Implications for Prolonging the Pandemic. Vaccines 2022, 10, 755. https://doi.org/10.3390/vaccines10050755
Alcendor DJ, Matthews-Juarez P, Smoot D, Hildreth JEK, Lamar K, Tabatabai M, Wilus D, Juarez PD. Breakthrough COVID-19 Infections in the US: Implications for Prolonging the Pandemic. Vaccines. 2022; 10(5):755. https://doi.org/10.3390/vaccines10050755
Chicago/Turabian StyleAlcendor, Donald J., Patricia Matthews-Juarez, Duane Smoot, James E. K. Hildreth, Kimberly Lamar, Mohammad Tabatabai, Derek Wilus, and Paul D. Juarez. 2022. "Breakthrough COVID-19 Infections in the US: Implications for Prolonging the Pandemic" Vaccines 10, no. 5: 755. https://doi.org/10.3390/vaccines10050755
APA StyleAlcendor, D. J., Matthews-Juarez, P., Smoot, D., Hildreth, J. E. K., Lamar, K., Tabatabai, M., Wilus, D., & Juarez, P. D. (2022). Breakthrough COVID-19 Infections in the US: Implications for Prolonging the Pandemic. Vaccines, 10(5), 755. https://doi.org/10.3390/vaccines10050755






