A Prospective Study Investigating the Impact of Obesity on the Immune Response to the Quadrivalent Influenza Vaccine in Children and Adolescents
Abstract
:1. Introduction
2. Materials and Methods
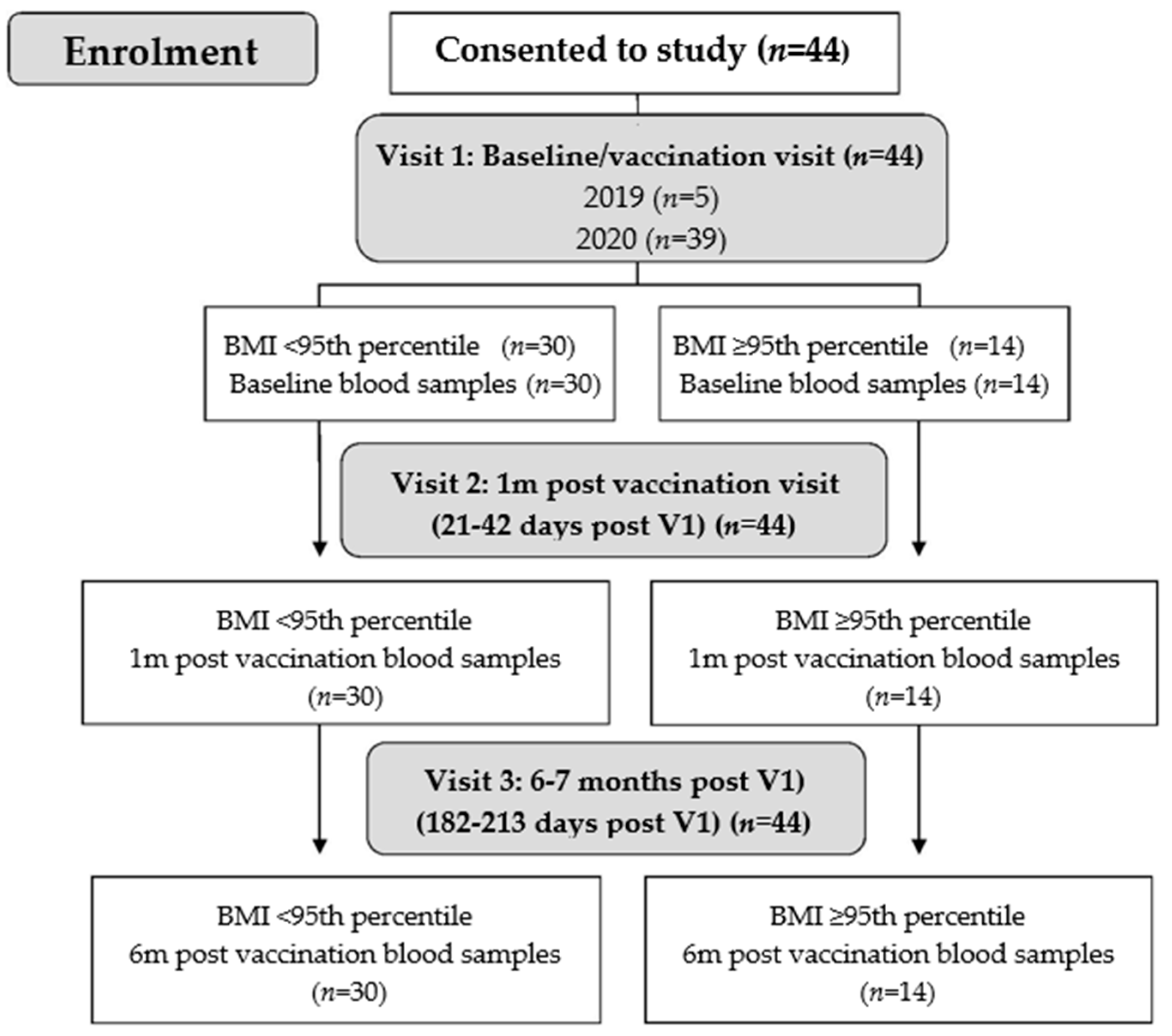
2.1. Study Participants and Procedures
2.2. Influenza Vaccine and Vaccination
2.3. Serology and Assessment of Immunogenicity
2.4. Statistical Analysis
2.5. Ethics Approval and Study Registration
3. Results
3.1. Characteristics of Study Participants
3.2. Vaccine Immunogenicity
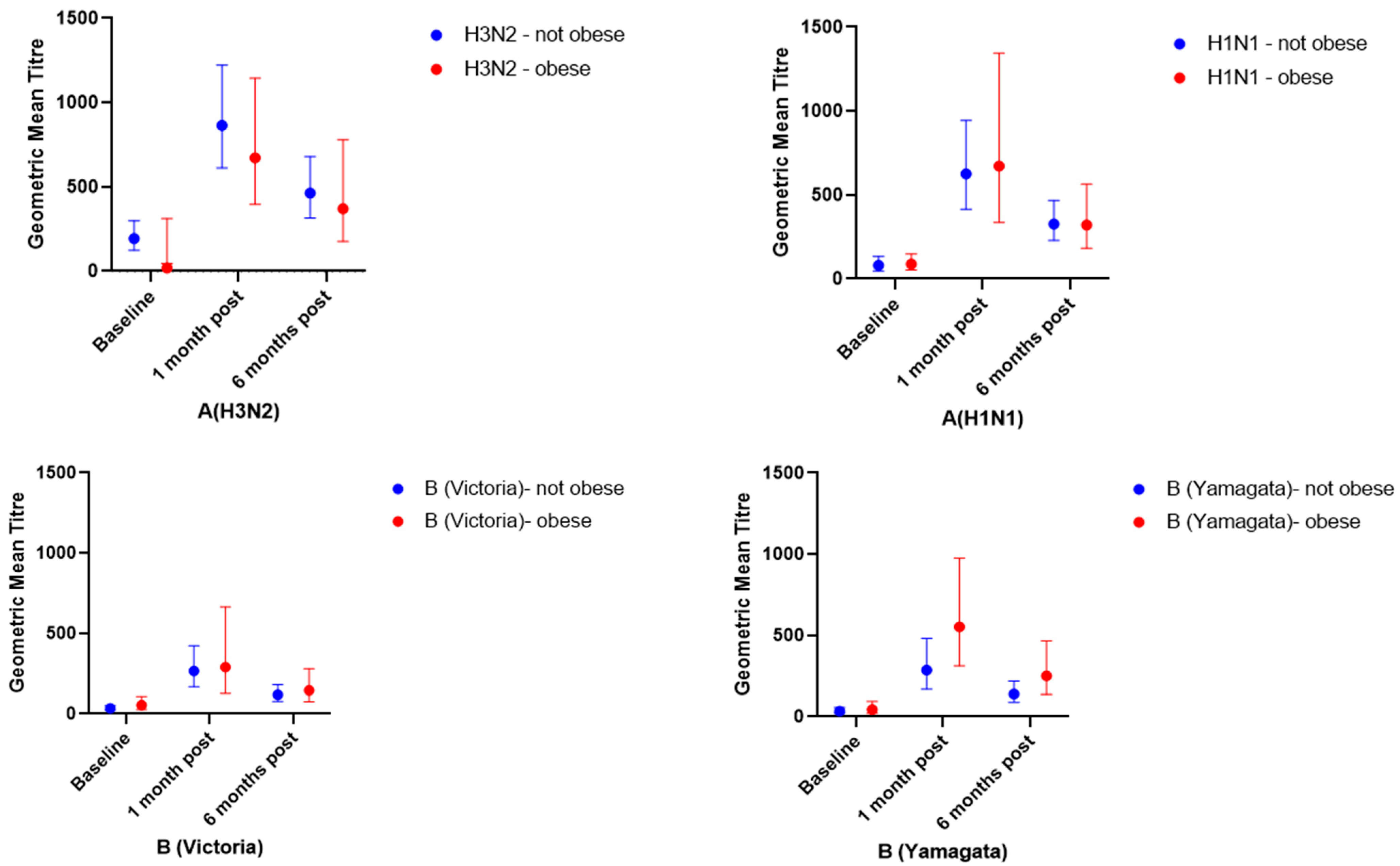
3.2.1. Geometric Mean Titres and Geometric Mean Titre Ratios
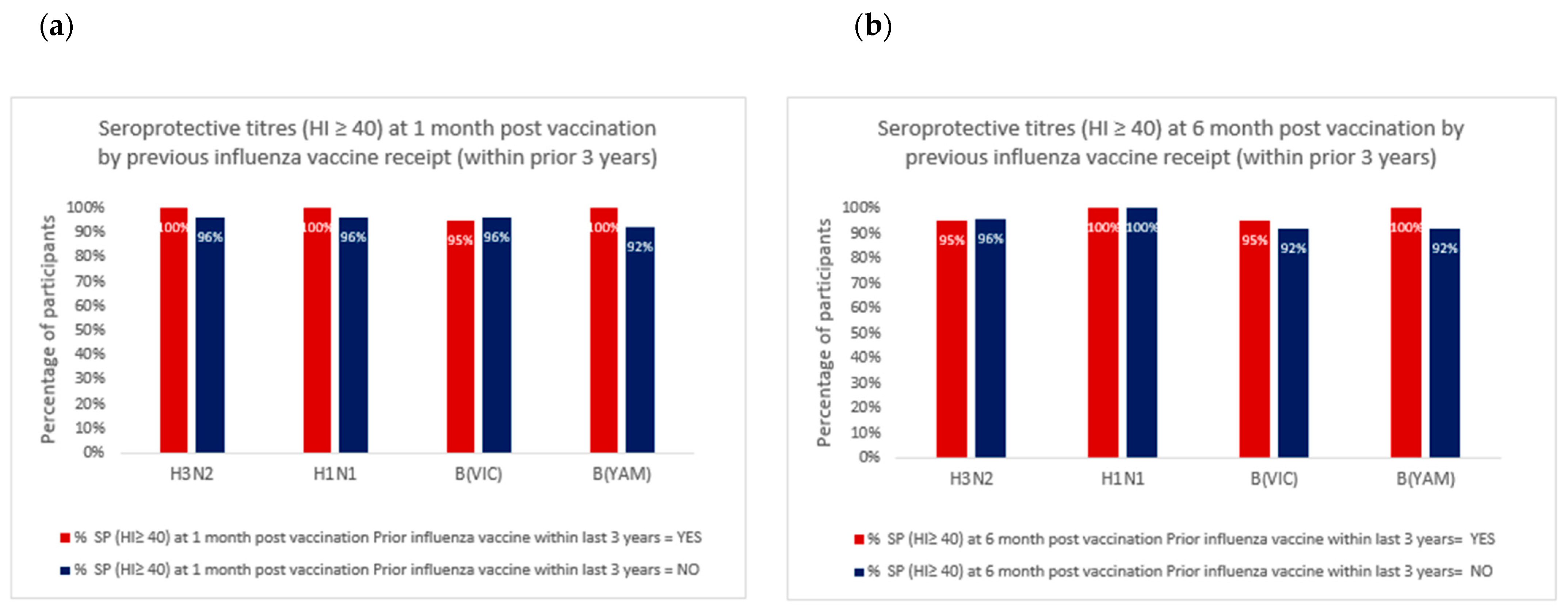
3.2.2. Seroconversion and Seroprotection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. 2021 Overweight and Obesity. Available online: https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 22 April 2022).
- Australian Institute of Health and Welfare. Overweight and Obesity: An Interactive Insight; AIHW: Canberra, Australia, 2020. Available online: https://www.aihw.gov.au/reports/overweight-obesity/overweight-and-obesity-an-interactive-insight/contents/what-is-overweight-and-obesity (accessed on 17 September 2021).
- Fezeu, L.; Julia, C.; Henegar, A.; Bitu, J.; Hu, F.B.; Grobbee, D.E.; Kengne, A.P.; Hercberg, S.; Czernichow, S. Obesity is associated with higher risk of intensive care unit admission and death in influenza A (H1N1) patients: A systematic review and meta-analysis. Obes. Rev. 2011, 12, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gang, X.; He, G.; Li, Z.; Lv, Y.; Han, Q.; Wang, G. Obesity Increases the Severity and Mortality of Influenza and COVID-19: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 595109. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.A.S.; Galindo-Fraga, A.; Ortiz-Hernández, A.A.; Gu, W.; Hunsberger, S.; Galán-Herrera, J.F.; Guerrero, M.L.; Ruiz-Palacios, G.M.; Beigel, J.H.; Magaña-Aquino, M.; et al. Underweight, overweight, and obesity as independent risk factors for hospitalization in adults and children from influenza and other respiratory viruses. Influenza Other Respir. Viruses 2019, 13, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Moser, J.A.S.; Galindo-Fraga, A.; Ortiz-Hernández, A.A.; Gu, W.; Hunsberger, S.; Galán-Herrera, J.F.; Guerrero, M.L.; Ruiz-Palacios, G.M.; Beigel, J.H.; La Red ILI 002 Study Group; et al. The impact of obesity and timely antiviral administration on severe influenza outcomes among hospitalized adults. J. Med. Virol. 2018, 90, 212–218. [Google Scholar]
- Morgan, O.W.; Bramley, A.; Fowlkes, A.; Freedman, D.S.; Taylor, T.H.; Gargiullo, P.; Belay, B.; Jain, S.; Cox, C.; Kamimoto, L.; et al. Morbid obesity as a risk factor for hospitalization and death due to 2009 pandemic influenza A(H1N1) disease. PLoS ONE 2010, 5, e9694. [Google Scholar] [CrossRef]
- Australian Technical Advisory Group on Immunisation (ATAGI). Australian Immunisation Handbook; Australian Government Department of Health: Canberra, Australia, 2021. Available online: https://immunisationhandbook.health.gov.au/ (accessed on 21 April 2022).
- Grohskopf, L.A.; Alyanak, E.; Broder, K.R.; Blanton, L.H.; Fry, A.M.; Jernigan, D.B.; Atmar, R.L. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2020–2021 Influenza Season. MMWR Recomm. Rep. 2020, 69, 1–24. [Google Scholar] [CrossRef]
- Mancuso, P. Obesity and respiratory infections: Does excess adiposity weigh down host defense? Pulm. Pharmacol. Ther. 2013, 26, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Honce, R.; Schultz-Cherry, S. Impact of Obesity on Influenza A Virus Pathogenesis, Immune Response, and Evolution. Front. Immunol. 2019, 10, 1071. [Google Scholar] [CrossRef]
- Neidich, S.D.; Green, W.D.; Rebeles, J.; Karlsson, E.A.; Schultz-Cherry, S.; Noah, T.L.; Chakladar, S.; Hudgens, M.G.; Weir, S.S.; Beck, M.A. Increased risk of influenza among vaccinated adults who are obese. Int. J. Obes. 2017, 41, 1324–1330. [Google Scholar] [CrossRef] [Green Version]
- Tagliabue, C.; Principi, N.; Giavoli, C.; Esposito, S. Obesity: Impact of infections and response to vaccines. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 325–331. [Google Scholar] [CrossRef]
- Okoli, G.N.; Racovitan, F.; Abdulwahid, T.; Righolt, C.H.; Mahmud, S.M. Variable seasonal influenza vaccine effectiveness across geographical regions, age groups and levels of vaccine antigenic similarity with circulating virus strains: A systematic review and meta-analysis of the evidence from test-negative design studies after the 2009/10 influenza pandemic. Vaccine 2021, 39, 1225–1240. [Google Scholar] [PubMed]
- Sheridan, P.A.; Paich, H.A.; Handy, J.; Karlsson, E.A.; Hudgens, M.G.; Sammon, A.B.; Holland, L.A.; Weir, S.; Noah, T.L.; Beck, M.A. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012, 36, 1072–1077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Heredia, F.P.; Gomez-Martinez, S.; Marcos, A. Obesity, inflammation and the immune system. Proc. Nutr. Soc. 2012, 71, 332–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Callahan, S.T.; Wolff, M.; Hill, H.R.; Edwards, K.M.; Vaccine, N.; Treatment Evaluation Unit Pandemic HNVSG. Impact of body mass index on immunogenicity of pandemic H1N1 vaccine in children and adults. J. Infect. Dis. 2014, 210, 1270–1274. [Google Scholar] [CrossRef] [Green Version]
- Esposito, S.; Giavoli, C.; Trombetta, C.; Bianchini, S.; Montinaro, V.; Spada, A.; Montomoli, E.; Principi, N. Immunogenicity, safety and tolerability of inactivated trivalent influenza vaccine in overweight and obese children. Vaccine 2016, 34, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Martin, J.M.; Cole, K.S.; Zimmerman, R.K.; Susick, M.; Moehling, K.K.; Levine, M.Z.; Spencer, S.; Flannery, B.; Nowalk, M.P. Are children’s vitamin D levels and BMI associated with antibody titers produced in response to 2014–2015 influenza vaccine? Hum. Vaccines Immunother. 2017, 13, 1661–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shypailo, R.J. Age-Based Pediatric Growth Reference Charts; Children’s Nutrition Research Center, Body Composition Laboratory Web Site: Houston, TX, USA, 2020; Available online: http://www.bcm.edu/bodycomplab/BMIapp/BMI-calculator-kids.html (accessed on 16 August 2021).
- WHO Global Influenza Surveillance Network. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza 29 July 2011; WHO: Geneva, Switzerland, 2011; Available online: https://apps.who.int/iris/handle/10665/44518 (accessed on 29 July 2021).
- Committee for Proprietary Medicinal Products. Note for Guidance on Harmonisation of Requirement for Influenza Vaccines; (CPMP/BWP/214/96); The European Agency for the Evaluation of Medicinal Products: Amsterdam, The Netherlands; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/note-guidance-harmonisation-requirements-influenza-vaccines_en.pdf (accessed on 17 September 2021).
- Ng, S.; Fang, V.J.; Ip, D.K.; Chan, K.H.; Leung, G.M.; Peiris, J.M.; Cowling, B.J. Estimation of the association between antibody titers and protection against confirmed influenza virus infection in children. J. Infect. Dis. 2013, 208, 1320–1324. [Google Scholar] [CrossRef]
- Black, S.; Nicolay, U.; Vesikari, T.; Knuf, M.; Del Giudice, G.; Della Cioppa, G.; Tsai, T.; Clemens, R.; Rappuoli, R. Hemagglutination inhibition antibody titers as a correlate of protection for inactivated influenza vaccines in children. Pediatric Infect. Dis. J. 2011, 30, 1081–1085. [Google Scholar] [CrossRef]
- Jagielska, A.M.; Brydak, L.B.; Nitsch-Osuch, A.S. Immunogenicity of Split Inactivated Quadrivalent Influenza Vaccine in Adults with Obesity in the 2017/2018 Season. Med. Sci. Monit. 2021, 27, e929572. [Google Scholar] [CrossRef]
- Talbot, H.K.; Coleman, L.A.; Crimin, K.; Zhu, Y.; Rock, M.T.; Meece, J.; Shay, D.K.; Belongia, E.A.; Griffin, M.R. Association between obesity and vulnerability and serologic response to influenza vaccination in older adults. Vaccine 2012, 30, 3937–3943. [Google Scholar] [CrossRef] [Green Version]
- Clarke, M.; Goodchild, L.M.; Evans, S.; Giles, L.C.; Sullivan, S.G.; Barr, I.G.; Lambert, S.; Marshall, H. Body mass index and vaccine responses following influenza vaccination during pregnancy. Vaccine 2021, 39, 4864–4870. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.F.; Sannella, E.; Shi, J.R.; Zhu, Y.; Ikizler, M.R.; Edwards, K.M. Antibody responses after inactivated influenza vaccine in young children. Pediatric Infect. Dis. J. 2008, 27, 1004–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, K.H.; To, K.K.; Hung, I.F.; Zhang, A.J.; Chan, J.F.; Cheng, V.C.; Tse, H.; Che, X.Y.; Chen, H.; Yuen, K.Y. Differences in antibody responses of individuals with natural infection and those vaccinated against pandemic H1N1 2009 influenza. Clin. Vaccine Immunol. 2011, 18, 867–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef]
- Fox, A.; Carolan, L.; Leung, V.; Phuong, H.V.M.; Khvorov, A.; Auladell, M.; Tseng, Y.Y.; Thai, P.Q.; Barr, I.; Subbarao, K.; et al. Opposing Effects of Prior Infection versus Prior Vaccination on Vaccine Immunogenicity against Influenza A(H3N2) Viruses. Viruses 2022, 14, 470. [Google Scholar] [CrossRef]
- Dong, W.; Bhide, Y.; Sicca, F.; Meijerhof, T.; Guilfoyle, K.; Engelhardt, O.G.; Boon, L.; De Haan, C.A.; Carnell, G.; Temperton, N.; et al. Cross-Protective Immune Responses Induced by Sequential Influenza Virus Infection and by Sequential Vaccination with Inactivated Influenza Vaccines. Front. Immunol. 2018, 9, 2312. [Google Scholar] [CrossRef]
| Not Obese (n = 30) (BMI < 95th Percentile) | Obese (n = 14) (BMI ≥ 95th Percentile) | |
|---|---|---|
| Age: mean years (SD) | 13.3 (2.3) | 13.4 (1.9) |
| Gender: % male | 13/30 (43%) | 5/14 (36%) |
| Prior influenza vaccine (during last 3 years) | 13/30 (43%) | 7/14 (50%) |
| Height (cm): min, max, mean (SD) | 135–182, 161 (13) | 144–179, 163 (11) |
| Weight (kg): min, max, mean (SD) | 29–76, 52 (13) | 51–139, 90 (23) |
| BMI percentile: min, max, median, (IQR) | 1.7, 92.6, 48 (36–80) | 97.0, 99.8, 99 (98–99) |
| H3N2 | H1N1 | B(Victoria) | B(Yamagata) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Not Obese (BMI < 95th Percentile) (n = 30) | Obese (BMI ≥ 95th Percentile) (n = 14) | p Value | Not Obese (BMI < 95th Percentile) (n = 30) | Obese (BMI ≥ 95th Percentile) (n = 14) | p Value | Not Obese (BMI < 95th Percentile) (n = 30) | Obese (BMI ≥ 95th Percentile) (n = 14) | p Value | Not Obese (BMI < 95th Percentile) (n = 30) | Obese (BMI ≥ 95th Percentile) (n = 14) | p Value | |
| Baseline GMT (95% CI) | 193 | 119 | 0.30 | 80 | 88 | 0.91 | 34 | 54 | 0.24 | 34 | 44 | 0.56 |
| (124–299) | (45–312) | (48–134) | (53–148) | (23–51) | (27–108) | (20–57) | (21–94) | |||||
| 1 month post vaccination GMT (95% CI) | 864 | 672 | 0.35 | 625 | 672 | 0.96 | 266 | 289 | 0.91 | 285 | 552 | 0.15 |
| (612–1220) | (395–1144) | (415–943) | (336–1344) | (168–422) | (127–664) | (169–481) | (312–975) | |||||
| 6 months post vaccination GMT (95% CI) | 463 | 371 | 0.66 | 327 | 320 | 0.82 | 118 | 145 | 0.66 | 139 | 250 | 0.14 |
| (316–679) | (177–778) | (229–468) | (182–564) | (78–180) | (75–281) | (89–218) | (137–455) | |||||
| GMTR (95% CI) (1 m/baseline) | 4.5 | 5.7 | 0.50 | 7.8 | 7.6 | 0.80 | 7.8 | 5.4 | 0.08 | 8.4 | 12.5 | 0.39 |
| (3.1–6.6) | (2.9–11.0) | (4.6–13.2) | (2.8–20.4) | (5.4–11.4) | (2.9–10.0) | (5.1–13.7) | (5.0–31.0) | |||||
| GMTR (95% CI) (6 m/baseline) | 2.4 | 3.1 | 0.36 | 4.1 | 3.6 | 0.67 | 3.5 | 2.7 | 0.35 | 4.1 | 5.7 | 0.48 |
| (1.7–3.4) | (1.8–5.5) | (2.6–6.5) | (1.5–8.5) | (2.5–4.8) | (1.6–4.5) | (2.8–6.0) | (2.5–12.9) | |||||
| % seroconversion 1 1 m post | 18/30 (60%) | 9/14 (64%) | 1.00 | 21/30 (70%) | 9/14 (64%) | 0.74 | 27/30 (90%) | 10/14 (71%) | 0.18 | 23/30 (77%) | 10/14 (71%) | 0.72 |
| % seroprotection 2 (HI ≥ 40) | ||||||||||||
| baseline | 28/30 (93%) | 12/14 (86%) | 0.58 | 23/30 (77%) | 13/14 (93%) | 0.40 | 14/30 (47%) | 10/14 (71%) | 0.20 | 16/30 (53%) | 8/14 (57%) | 1.00 |
| 1 month post | 29/30 (97%) | 14/14 (100%) | 1.00 | 29/30 (97%) | 14/14 (100%) | 1.00 | 28/30 (93%) | 14/14 (100%) | 1.00 | 28/30 (93%) | 14/14 (100%) | 1.00 |
| 6 months post | 29/30 (97%) | 13/14 (93%) | 0.54 | 30/30 (100%) | 14/14 (100%) | 1.00 | 27/30 (90%) | 14/14 (100%) | 0.54 | 28/30 (93%) | 14/14 (100%) | 1.00 |
| % seroprotection 2 (HI ≥ 110) | ||||||||||||
| baseline | 23/30 (93%) | 7/14 (50%) | 0.10 | 16/30 (53%) | 6/14 (43%) | 0.75 | 6/30 (20%) | 3/14 (21%) | 1.00 | 7/30 (23%) | 4/14 (29%) | 0.72 |
| 1 month post | 29/30 (97%) | 14/14 (100%) | 1.00 | 26/30 (97%) | 14/14 (100%) | 1.00 | 26/30 (80%) | 11/14 (78%) | 1.00 | 23/30 (77%) | 13/14 (93%) | 0.40 |
| 6 months post | 29/30 (97%) | 11/14 (78%) | 0.09 | 25/30 (83%) | 12/14 (86%) | 1.00 | 15/30 (50%) | 6/14 (43%) | 0.75 | 19/30 (63%) | 10/14 (71%) | 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clarke, M.; Mathew, S.M.; Giles, L.C.; Pena, A.S.; Barr, I.G.; Richmond, P.C.; Marshall, H.S. A Prospective Study Investigating the Impact of Obesity on the Immune Response to the Quadrivalent Influenza Vaccine in Children and Adolescents. Vaccines 2022, 10, 699. https://doi.org/10.3390/vaccines10050699
Clarke M, Mathew SM, Giles LC, Pena AS, Barr IG, Richmond PC, Marshall HS. A Prospective Study Investigating the Impact of Obesity on the Immune Response to the Quadrivalent Influenza Vaccine in Children and Adolescents. Vaccines. 2022; 10(5):699. https://doi.org/10.3390/vaccines10050699
Chicago/Turabian StyleClarke, Michelle, Suja M. Mathew, Lynne C. Giles, Alexia S. Pena, Ian G. Barr, Peter C. Richmond, and Helen S. Marshall. 2022. "A Prospective Study Investigating the Impact of Obesity on the Immune Response to the Quadrivalent Influenza Vaccine in Children and Adolescents" Vaccines 10, no. 5: 699. https://doi.org/10.3390/vaccines10050699
APA StyleClarke, M., Mathew, S. M., Giles, L. C., Pena, A. S., Barr, I. G., Richmond, P. C., & Marshall, H. S. (2022). A Prospective Study Investigating the Impact of Obesity on the Immune Response to the Quadrivalent Influenza Vaccine in Children and Adolescents. Vaccines, 10(5), 699. https://doi.org/10.3390/vaccines10050699







