Global Pandemic Preparedness: Optimizing Our Capabilities and the Influenza Experience
Abstract
:1. Introduction
2. The 100-Day Pandemic Response Ambition
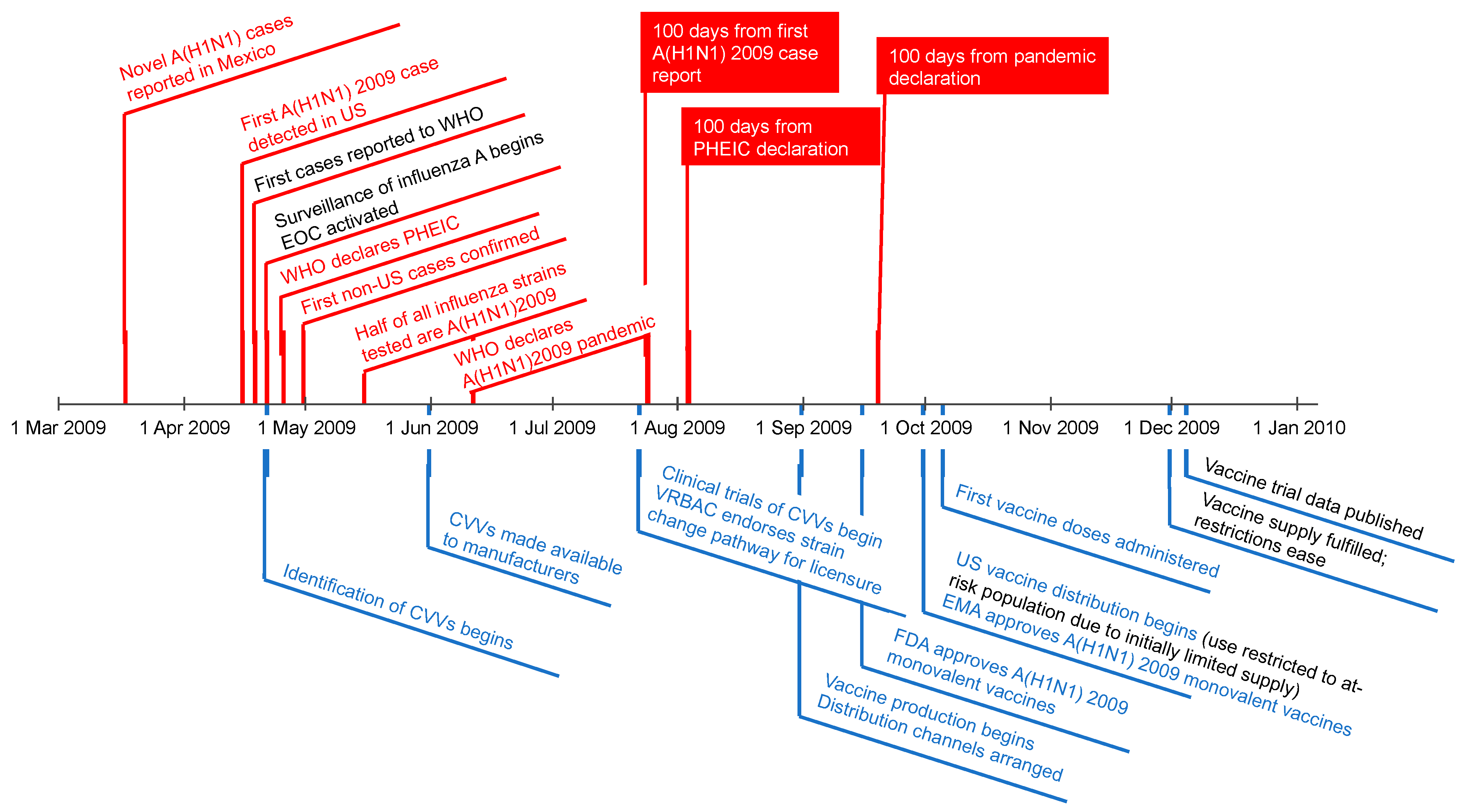
3. A(H1N1) 2009 Pandemic Response Timelines as a Model
4. Improvements in Influenza Pandemic Preparedness and Response since 2009
5. New and Emerging Vaccine Technologies
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 20 January 2022).
- Zimmer, C.; Corum, J.; Wee, S.-L.; Kristoffersen, M. New York Times Coronavirus Vaccine Tracker. Available online: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html (accessed on 14 February 2022).
- Ball, P. The lightning-fast quest for COVID vaccines—And what it means for other diseases. Nature 2021, 589, 16–18. [Google Scholar] [CrossRef] [PubMed]
- Dolgin, E. The tangled history of mRNA vaccines. Nature 2021, 597, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Nestić, D.; Božinović, K.; Pehar, I.; Wallace, R.; Parker, A.L.; Majhen, D. The Revolving Door of Adenovirus Cell Entry: Not All Pathways Are Equal. Pharmaceutics 2021, 13, 1585. [Google Scholar] [CrossRef]
- CureVac to Shift Focus of COVID-19 Vaccine Development to Second-Generation mRNA Technology. Available online: https://www.curevac.com/en/2021/10/12/curevac-to-shift-focus-of-covid-19-vaccine-development-to-second-generation-mrna-technology/ (accessed on 30 November 2021).
- Vrba, S.M.; Kirk, N.M.; Brisse, M.E.; Liang, Y.; Ly, H. Development and Applications of Viral Vectored Vaccines to Combat Zoonotic and Emerging Public Health Threats. Vaccines 2020, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Percival, K.M. The Present and Future of Flu Vaccine Production Technologies. Available online: https://www.healio.com/news/infectious-disease/20210917/the-present-and-future-of-flu-vaccine-production-technologies (accessed on 2 December 2021).
- World Health Organization. Global Influenza Surveillance and Response System (GISRS). Available online: https://www.who.int/initiatives/global-influenza-surveillance-and-response-system (accessed on 30 November 2021).
- HM Government. National Risk Register 2020. Available online: https://www.gov.uk/government/publications/national-risk-register-2020 (accessed on 30 November 2021).
- Centers for Disease Control and Prevention. Summary of Influenza Risk Assessment Tool (IRAT) Results. Available online: https://www.cdc.gov/flu/pandemic-resources/monitoring/irat-virus-summaries.htm#chart-scores (accessed on 30 November 2021).
- World Health Organization. Tool for Influenza Pandemic Risk Assessment (TIPRA). Available online: https://apps.who.int/iris/handle/10665/250130 (accessed on 14 February 2022).
- World Health Organization. Influenza at the Human-Animal Interface Monthly Risk Assessment Summary. Available online: https://www.who.int/teams/global-influenza-programme/avian-influenza/monthly-risk-assessment-summary (accessed on 14 February 2022).
- World Health Organization. Pandemic Influenza Preparedness (PIP) Framework. Available online: https://www.who.int/initiatives/pandemic-influenza-preparedness-framework (accessed on 13 December 2021).
- GISAID Initiative. GISAID. Available online: https://www.gisaid.org (accessed on 5 April 2022).
- Khorramdelazad, H.; Kazemi, M.H.; Najafi, A.; Keykhaee, M.; Zolfaghari Emameh, R.; Falak, R. Immunopathological similarities between COVID-19 and influenza: Investigating the consequences of Co-infection. Microb. Pathog. 2021, 152, 104554. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. The 2009 H1N1 Pandemic: Summary Highlights, April 2009–April 2010. Available online: https://www.cdc.gov/h1n1flu/cdcresponse.htm (accessed on 30 November 2021).
- World Health Organization. Evolution of a Pandemic: A(H1N1) 2009. April 2009–March 2010; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Center for Infectious Disease Research and Policy. The IVR Initiative: Influenza Vaccines Roadmap. Available online: https://ivr.cidrap.umn.edu (accessed on 14 February 2022).
- Moore, K.A.; Ostrowsky, J.T.; Kraigsley, A.M.; Mehr, A.J.; Bresee, J.S.; Friede, M.H.; Gellin, B.G.; Golding, J.P.; Hart, P.J.; Moen, A.; et al. A Research and Development (R&D) roadmap for influenza vaccines: Looking toward the future. Vaccine 2021, 39, 6573–6584. [Google Scholar] [CrossRef]
- Pandemic Prepardness Partnership. 100 Days Mission to Respond to Future Pandemic Threats: Reducing the Impact of Future Pandemics by Making Diagnostics, Therapeutics and Vaccines Available within 100 Days—A Report to the G7 by the Pandemic Preparedness Partnership. Available online: https://www.gov.uk/government/publications/100-days-mission-to-respond-to-future-pandemic-threats (accessed on 19 December 2021).
- G7 Scientific Advisors. 100 Days Mission: First Implementation Report. Available online: https://www.gov.uk/government/publications/100-days-mission-first-implementation-report (accessed on 19 December 2021).
- Lander, E.S.; Sullivan, J.J. American Pandemic Prepardness: Transforming Our Capabilities; The White House: Washington, DC, USA, 2021.
- Developing Countries Vaccine Manufacturers Network. About DCVMN. Available online: http://www.dcvmn.org/About-DCVMN (accessed on 7 April 2022).
- Jadhav, S.; Dhere, R.; Yeolekar, L.; Gautam, M. Influenza Vaccine Production Capacity Building in Developing Countries: Example of the Serum Institute of India. Procedia Vaccinol. 2010, 2, 166–171. [Google Scholar] [CrossRef]
- Maxmen, A. South African scientists copy Moderna’s COVID vaccine. Nature 2022, 602, 372–373. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Outbreak of swine-origin influenza A (H1N1) virus infection—Mexico, March–April 2009. MMWR Morb. Mortal. Wkly. Rep. 2009, 58, 467–470. [Google Scholar]
- Centers for Disease Control and Prevention. 2009 H1N1 Pandemic Timeline. Available online: https://www.cdc.gov/flu/pandemic-resources/2009-pandemic-timeline.html (accessed on 14 February 2022).
- US Food and Drug Administration. Influenza A (H1N1) 2009 Monovalent. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/influenza-h1n1-2009-monovalent (accessed on 1 December 2021).
- European Medicines Agency. Pandemic Report and Lessons Learned: Outcome of the European Medicines Agency’s Activities during the 2009 (H1N1) Flu Pandemic; European Medicines Agency: London, UK, 2011.
- Rockman, S.; Laurie, K.; Barr, I. Pandemic Influenza Vaccines: What did We Learn from the 2009 Pandemic and are We Better Prepared Now? Vaccines 2020, 8, 211. [Google Scholar] [CrossRef]
- Australian Government Department of Health and Ageing. Review of Australia’s Health Sector Response to Pandemic (H1N1) 2009: Lessons Identified; Australian Government: Canberra, ACT, Australia, 2011.
- Schild, G.C.; Wood, J.M.; Newman, R.W. A single-radial-immunodiffusion technique for the assay of influenza haemagglutinin antigen. Proposals for an assay method for the haemagglutinin content of influenza vaccines. Bull. World Health Organ. 1975, 52, 223–231. [Google Scholar] [PubMed]
- US Department of Health and Human Services. Pandemic Influenza Plan: 2017 Update; US Department of Health and Human Services: Washington, DC, USA, 2017.
- Mereckiene, J.; Cotter, S.; Weber, J.T.; Nicoll, A.; D’Ancona, F.; Lopalco, P.L.; Johansen, K.; Wasley, A.M.; Jorgensen, P.; Lévy-Bruhl, D.; et al. Influenza A(H1N1)pdm09 vaccination policies and coverage in Europe. Eurosurveillance 2012, 17, 20064. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, P.; Wasley, A.; Mereckiene, J.; Cotter, S.; Weber, J.T.; Brown, C.S. Unequal access to vaccines in the WHO European Region during the A(H1N1) influenza pandemic in 2009. Vaccine 2013, 31, 4060–4062. [Google Scholar] [CrossRef] [PubMed]
- Convention on Biological Diversity. About the Nagoya Protocol. Available online: https://www.cbd.int/abs/about/ (accessed on 14 February 2022).
- European Medicines Agency. Vaccines for Pandemic Influenza. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/pandemic-influenza/vaccines-pandemic-influenza (accessed on 2 December 2021).
- Assistant Secretary for Prepardness and Response; US Department of Health and Human Services. National Influenza Vaccine Modernization Strategy, 2020–2030. Available online: https://www.phe.gov/Preparedness/planning/nivms/Pages/default.aspx (accessed on 2 December 2021).
- Centers for Disease Control and Prevention. Seasonal Influenza Vaccine Supply for the U.S. 2021–2022 Influenza Season. Available online: https://www.cdc.gov/flu/prevent/vaxsupply.htm (accessed on 2 December 2021).
- Wood, J.M.; Weir, J.P. Standardisation of inactivated influenza vaccines-Learning from history. Influenza Other Respir. Viruses 2018, 12, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Minor, P.D. Assaying the Potency of Influenza Vaccines. Vaccines 2015, 3, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Bodle, J.; Verity, E.E.; Ong, C.; Vandenberg, K.; Shaw, R.; Barr, I.G.; Rockman, S. Development of an enzyme-linked immunoassay for the quantitation of influenza haemagglutinin: An alternative method to single radial immunodiffusion. Influenza Other Respir. Viruses 2013, 7, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Hardy, S.; Eichelberger, M.; Griffiths, E.; Weir, J.P.; Wood, D.; Alfonso, C. Confronting the next pandemic--workshop on lessons learned from potency testing of pandemic (H1N1) 2009 influenza vaccines and considerations for future potency tests, Ottawa, Canada, July 27–29, 2010. Influenza Other Respir. Viruses 2011, 5, 438–442. [Google Scholar] [CrossRef]
- Atmar, R.L.; Keitel, W.A. Adjuvants for pandemic influenza vaccines. Curr. Top. Microbiol. Immunol. 2009, 333, 323–344. [Google Scholar] [CrossRef]
- Pawelec, G.; McElhaney, J. Recent advances in influenza vaccines. F1000Research 2020, 9, 305. [Google Scholar] [CrossRef]
- World Health Organization. Global Action Plan for Influenza Vaccines: Global Progress Report 2006–2016; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Sparrow, E.; Wood, J.G.; Chadwick, C.; Newall, A.T.; Torvaldsen, S.; Moen, A.; Torelli, G. Global production capacity of seasonal and pandemic influenza vaccines in 2019. Vaccine 2021, 39, 512–520. [Google Scholar] [CrossRef]
- Biomedical Advanced Research and Development Authority. Influenza & Emerging Infectious Diseases (IEID) Medical Countermeasures. Available online: https://www.medicalcountermeasures.gov/barda/influenza-and-emerging-infectious-diseases/ (accessed on 2 December 2021).
- Public Health England. New and Emerging Respiratory Virus Threats Advisory Group. Available online: https://www.gov.uk/government/groups/new-and-emerging-respiratory-virus-threats-advisory-group (accessed on 3 December 2021).
- European Commission. European Health Emergency Preparedness and Response Authority (HERA): Getting Ready for Future Health Emergencies. Available online: https://ec.europa.eu/commission/presscorner/detail/en/ip_21_4672 (accessed on 19 December 2021).
- Dolgin, E. mRNA flu shots move into trials. Nat. Rev. Drug Discov. 2021, 20, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Zhang, J.; Ly, H. Advances in Development and Application of Influenza Vaccines. Front. Immunol. 2021, 12, 711997. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C. First COVID-19 DNA vaccine approved, others in hot pursuit. Nat. Biotechnol. 2021, 39, 1479–1482. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.Y.; Izzard, L.; Hurt, A.C. A Review of DNA Vaccines Against Influenza. Front. Immunol. 2018, 9, 1568. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.S.; Prazeres, D.M.F.; Azevedo, A.M.; Marques, M.P.C. mRNA vaccines manufacturing: Challenges and bottlenecks. Vaccine 2021, 39, 2190–2200. [Google Scholar] [CrossRef]
- Lee, S.; Ryu, J.H. Influenza Viruses: Innate Immunity and mRNA Vaccines. Front. Immunol. 2021, 12, 710647. [Google Scholar] [CrossRef]
- Nuwarda, R.F.; Alharbi, A.A.; Kayser, V. An Overview of Influenza Viruses and Vaccines. Vaccines 2021, 9, 1032. [Google Scholar] [CrossRef]
- Kis, Z.; Shattock, R.; Shah, N.; Kontoravdi, C. Emerging Technologies for Low-Cost, Rapid Vaccine Manufacture. Biotechnol. J. 2019, 14, 1–2. [Google Scholar] [CrossRef]
- Kis, Z.; Rizvi, Z. How to Make Enough Vaccine for the World in One Year. Available online: https://www.citizen.org/article/how-to-make-enough-vaccine-for-the-world-in-one-year/ (accessed on 16 February 2022).
- Mao, Q.; Xu, M.; He, Q.; Li, C.; Meng, S.; Wang, Y.; Cui, B.; Liang, Z.; Wang, J. COVID-19 vaccines: Progress and understanding on quality control and evaluation. Signal Transduct. Target. Ther. 2021, 6, 199. [Google Scholar] [CrossRef]
| Vaccine Platform | COVID-19 Vaccine Status * | Influenza Vaccine Status * |
|---|---|---|
| Established | ||
| Egg-based, inactivated split virion or protein subunit | None in human trials | Licensed seasonal |
| Egg based inactivated split virion or protein subunit, adjuvanted | None in human trials | Licensed pandemic and seasonal |
| Egg-based live attenuated | None in human trials | Licensed seasonal |
| Cell culture–based, purified protein subunit | Licensed | Licensed seasonal |
| Cell culture–based, purified protein subunit, adjuvanted | Licensed | Licensed pandemic |
| r-Protein subunit | Licensed | Licensed pandemic and seasonal |
| New/emerging | ||
| mRNA | Licensed | Phase 1/2 |
| sa-mRNA | Phase 1 | Preclinical |
| Viral vector | Licensed | Preclinical |
| Combination (influenza/COVID) | COVID + seasonal influenza in phase 1/2 | |
| DNA | Licensed | None in human trials |
| Platform | Source and Prepare (after Strain Selection) | Propagation, Harvest, and Inactivation | Splitting, Purification, and Filtration | Bulk Production | In-Process and DS Testing | Formulation and Filtration | Filling | Inspection, Labelling, and Packaging | Final Product Release Testing and QA Review |
|---|---|---|---|---|---|---|---|---|---|
| IIV and purified surface antigen | WHO provides wild-type virus to the reassortment laboratories. CVV made available to manufacturer Synthetic seed prepared using genetic sequence shared on publicly accessible database (e.g., GISAID) | Incubate in hens’ eggs or mammalian cells for virus replication Harvest fluid containing virus Virus inactivation (some processes position virus inactivation just before final filtration) | Splitting/disruption of virus depending on specific vaccine (except for whole virion vaccine) Bulk antigen purification (ultra centrifugation on saccharose gradient, filtration, or alternate separation steps | Sterile filtration | QC DS release testing including: Potency, sterility, purity and impurities | Dilution and sterile filtration Mix with adjuvant (if applicable) QC | Filling into vials, syringes or other administration form (e.g., sprayers) | Automated, semi-automated, or manual visual inspection of the filled material Labelling and packaging (country or region specific) | Internal QC product release assays, including: Potency, sterility, purity and impurities For bulk, formulated bulk, and fill and pack steps: Deviation investigation, QA review, and closure Internal manufacturing and QA batch dossier review and final release Additional packaging, as required Submission of BPR to external regulatory agency(ies). External regulatory agency(ies)’ testing and release of product |
| Live attenuated influenza virus | Genetic sequence provided by WHO or GISAID (wild-type viruses for IVPP not usually shipped) Manufacturer initiates virus reassortment by reverse genetics; propagated in eggs to produce CVV | Incubate in hens’ eggs for virus to replicate Harvest fluid containing virus | Clarification and concentration Sterile filtration (if possible) Freezing | QC DS release testing including: Potency, bioburden, sterility, purity and impurities | Dilution and sterile filtration QC | ||||
| r-Protein subunit | Combine gene with baculovirus to make recombinant HA Plasmid construction | Engineering cell expression: inoculate cultured mammalian cells to replicate HA | Clarification, centrifugation, chromatography | Bulk antigen is sterile filtered, collected, and frozen | QC Potency, sterility, purity and impurities | Mix with adjuvant (if applicable) or extemporaneous addition of adjuvant | |||
| Viral vector vaccine | Genomic sequence Cell banks and virus seed stocks | Cell culture Transfection (into viral DNA into cells) Virus infection, viral vector production | Virus propagation Viral vector purification Ultracentrifugation, chromatography, purification solutions | QC Potency, sterility, purity and impurities | Ultrafiltration Viral vector QC | ||||
| mRNA vaccine | Manufacture DNA template, insert into plasmid DNA In vitro transcription | Transcribe mRNA Degrade by DNase step Addition of the cap | High pressure LC Chromatography, adjust concentration, filtration, freezing | QC Potency, sterility | LNP formulation Sterile filtration QC | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rockman, S.; Taylor, B.; McCauley, J.W.; Barr, I.G.; Longstaff, R.; Bahra, R. Global Pandemic Preparedness: Optimizing Our Capabilities and the Influenza Experience. Vaccines 2022, 10, 589. https://doi.org/10.3390/vaccines10040589
Rockman S, Taylor B, McCauley JW, Barr IG, Longstaff R, Bahra R. Global Pandemic Preparedness: Optimizing Our Capabilities and the Influenza Experience. Vaccines. 2022; 10(4):589. https://doi.org/10.3390/vaccines10040589
Chicago/Turabian StyleRockman, Steven, Beverly Taylor, John W. McCauley, Ian G. Barr, Ray Longstaff, and Ranbir Bahra. 2022. "Global Pandemic Preparedness: Optimizing Our Capabilities and the Influenza Experience" Vaccines 10, no. 4: 589. https://doi.org/10.3390/vaccines10040589
APA StyleRockman, S., Taylor, B., McCauley, J. W., Barr, I. G., Longstaff, R., & Bahra, R. (2022). Global Pandemic Preparedness: Optimizing Our Capabilities and the Influenza Experience. Vaccines, 10(4), 589. https://doi.org/10.3390/vaccines10040589






