Abstract
The coronavirus virus disease 2019 (COVID-19) pandemic has impacted the global healthcare system. In Thailand, the first and most available vaccines were inactivated and viral vector vaccines. We reported the impact of those vaccines in preventing severe disease and death in kidney transplant recipients. This retrospective study comprised 45 kidney transplant recipients with COVID-19 infection, classified by vaccination status. Outcomes of interest were death, pneumonia, and allograft dysfunction. There were 23 patients in vaccinated group and 22 patients in unvaccinated group. All baseline characteristics were similar except mean age was older in vaccinated group, 55 vs. 48 years. Total 11 patients (24%) died (13% vaccinated vs. 36% unvaccinated RR, 0.56; 95% CI, 0.29–0.83; p = 0.03). Multivariate analysis showed that vaccination significantly decrease mortality (odds ratio, 0.54; 95% CI, 0.10–0.94; p = 0.03). Pneumonia developed equally in both groups (70%). There was a trend toward less oxygen requirement as well as ventilator requirement in vaccinated group. The rate of allograft dysfunction was similar (47%). Inactivated and viral vector COVID-19 vaccines have beneficial effect on mortality reduction in kidney transplant recipients. Even partial vaccination can exert some protection against death. However, full vaccination should be encouraged to achieve better prevention.
1. Introduction
The novel coronavirus causing severe acute respiratory distress syndrome (COVID-19) pandemic has impacted the global healthcare system. According to the burden of the disease and the lack of effective treatments, the strategy was focused on vaccine development. Recent studies in general population showed the efficacy of vaccination in preventing COVID-19 infection and also a reduction in severity and mortality, by inducing humoral and cellular immune response [1,2,3,4,5,6]. Immune response after vaccination in transplant recipients is markedly lower than immunocompetent counterpart [7]. Studies of humoral immune response after variety of vaccination platform showed negligible antibody seroconversion after inactivated vaccine [8]. Studies on viral vector and mRNA vaccine showed higher seroconversion after mRNA vaccine than after viral vector vaccine. On the other hand, cell-mediated immune response can be achieved after all types of vaccine [9,10,11,12,13]. The above-mentioned studies did not report clinical outcome. In addition to lower immune response after vaccination, the severity and mortality rate are much higher in unvaccinated kidney transplant recipients than general population (19–38% vs. 2–3%) [14,15,16,17]. Messenger ribonucleic acid (mRNA) vaccine have shown to reduce severity and mortality significantly in kidney transplant recipients [18]. There were scant reports on the efficacy of inactivated vaccine or viral vector vaccine in transplant recipients. Initially, mRNA vaccines were available in developed countries, but the first and most available vaccines in Thailand were inactivated and later on, viral vector vaccine. When vaccines became available, the amount were limited so prioritization was introduced to vaccinate people who were believed to be at high risk for developing severe disease and thus received highest benefit from vaccination. Unfortunately, kidney transplant recipients did not receive priority and vaccine hesitancy was prevalent among some patients [19]. With above reasons, only minority of kidney transplant recipients received vaccination during the study period. This study aims to demonstrate the impact of inactivated and viral vector vaccines on severity and mortality reduction in COVID-19 infected kidney transplant recipients.
2. Methods
2.1. Study Design and Participants
This is a retrospective cohort study of kidney transplant patients at Ramathibodi Excellent Center for Organ Transplantation who were diagnosed with COVID-19 infection between January 2021 and October 2021. All adult recipients, aged 18 years or older, who received either living or deceased donor kidney transplantation were eligible for the study. Patients with symptoms suggestive of having COVID-19 infection or asymptomatic patients who had contacted with COVID-19 cases were tested. Diagnosis was confirmed by real-time polymerase chain reaction (RT-PCR) for severe acute respiratory syndrome coronavirus 2 (SARS-CoV2). The study protocol was approved by the Institutional Human Research Ethics Committee of Faculty of Medicine Ramathibodi Hospital, Mahidol University [MURA 2021/895]. The patients were categorized by vaccination status. COVID-19 vaccines available in Thailand at the time of this study were CoronaVac inactivated vaccine (Sinovac Biotech) and ChAdOx1 nCoV-19 adenoviral vector vaccine (Vaxzevria, Oxford-AstraZeneca). Inactivated vaccines were given 4 weeks apart [20] while viral vector vaccines were given 12 weeks apart [21]. Patients who received 2 doses of either vaccine were considered fully vaccinated and those who received only 1 dose were considered partially vaccinated.
2.2. Outcomes
The primary objective of this study was to assess the impact of inactivated and viral vector vaccines in mortality reduction in COVID-19 infected kidney transplant recipients. The observation endpoint was defined when the patients were discharged from the hospital, or hotel-based self-care program. Secondary outcomes included severity of COVID-19 disease, complication, oxygen and ventilator support requirement, and acute kidney injury (AKI) during the hospital course. AKI was defined and staged by using Kidney Disease: Improving Global Outcomes (KDIGO) [22].
2.3. Management during COVID-19 Infection
According to the local standard of care for COVID-19 infection, patients have been classified by the severity and risk factors of severe disease. Severe cases were admitted to the hospital and those who had mild symptoms or asymptomatic were assigned to hotel-based self-care. Kidney transplant recipients were always classified as high risk of severe disease due to immunosuppressive state, thus they all received antiviral agent, favipiravir, as soon as the RT-PCR was confirmed. If the patients required oxygen therapy, they were prescribed dexamethasone 6–20 mg daily according to the treating physicians. Immunomodulatory agents or hemoperfusion was considered in case of severe pneumonia. The immunosuppressive medication was adjusted according to the severity of disease. If the patients develop any symptoms, anti-proliferative agents were discontinued and calcineurin inhibitors (CNIs) were reduced toward the low therapeutic level. Corticosteroids were continued or resumed if steroid-free regimens were used. If there is an indication for methylprednisolone or dexamethasone, prednisolone was substituted.
2.4. Statistical Analysis
Baseline characteristics were described as frequency (percent) for categorical data. Chi-square test was used for comparison between groups. Continuous measurements were reported as mean (standard deviation) for normal, and median (interquartile range) for non-normal distribution. Depending on data distribution, independent t-test and Mann-Whitney test were used to compare their differences [23].
The primary outcome was mortality and evaluated by logistic regression analysis as relative risk ratio (RR) of death between groups with 95% confident intervals (CIs), and also reported in Kaplan-Meier time-to-event analysis using log-rank test [24]. We also reported secondary outcomes as a relative risk ratio with 95% CIs. Univariate and multivariate logistic regression analyses were used to identify factors that may affect mortality (vaccination status, recipient age, type of donor, diabetes, obesity and sepsis). All statistical analyses were performed with IBM SPSS software, version 23.
3. Results
3.1. Patients
During the study period, there were 45 kidney transplant recipients with confirmed COVID-19 infection. Twenty-three patients (51%) received COVID-19 vaccine. The median post-transplant time was 5.4 years and mean baseline renal function assessed by estimated glomerular filtration rate (eGFR) was 62.1 mL/min/1.73 m2. The baseline characteristics were shown in Table 1.

Table 1.
Baseline characteristics.
Of the 23 patients, 18 (71%) received ChAdOx1 nCoV-19 vaccine, of which only 2 patients were fully vaccinated with 2 doses. The remaining 5 patients (29%) were fully vaccinated with 2 doses of CoronaVac vaccine. Infection was diagnosed with median of 49 days after the last dose of vaccination (mean of 37 days in patients who received only one dose of vaccine and 71 days in those who received two doses). The mean age of patients in vaccinated group was higher than unvaccinated group, 55 vs. 48 years, respectively (p = 0.04).
3.2. Hospital Course
Ninety-five percent of COVID-19 infection in kidney transplant recipients were symptomatic. Pneumonia was found in 70% of patients in both groups (p = 0.89). Gastrointestinal symptoms were found in only 5% of the patients. Twelve patients (52%) in vaccinated group required oxygen therapy, while 16 patients (71%) in unvaccinated group needed oxygen supplement (p = 0.10). More patients in unvaccinated group required mechanical ventilator support (19% vs. 9%; p = 0.48). All patients received favipiravir as antiviral agent for 5–10 days and nearly half required corticosteroids (either dexamethasone or methylprednisolone) for the treatment of COVID-19. Biologic immunomodulatory agents or hemoperfusion were required in a few patients. Immunosuppressive management was the same in both groups, i.e., continue CNIs, discontinue mycophenolate in the majority of the patients (Table 2).

Table 2.
Hospital course of COVID-19 infection in kidney transplant recipients.
3.3. Clinical Outcomes
The mortality rate was 24.4% overall and significantly lower in vaccinated group than unvaccinated group (Figure 1), 13% vs. 36% (RR, 0.56; 95% CI, 0.29–0.83; p = 0.03). There was no mortality in patients who received 2 doses of vaccine, either inactivated (n = 5) or viral vector (n = 2) vaccine. Even with partial vaccination (only 1 dose of vaccine), there was a trend toward mortality reduction compared with no vaccination, 19% and 36%, respectively (p = 0.08). There was no significant difference in other complications such as bacterial pneumonia, invasive pulmonary aspergillosis, pulmonary embolism, or septicemia (Table 3).
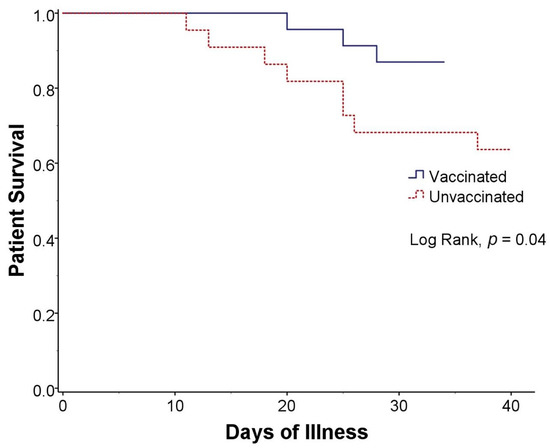
Figure 1.
Kaplan-Meier curve of survival in kidney transplant recipients with COVID-19 infection who were vaccinated (blue solid line) and unvaccinated (red dashed line).

Table 3.
Outcomes of COVID-19 infection in kidney transplant recipients classified by vaccination status.
Univariate analysis revealed that diabetes and vaccination affected mortality. Multivariate analysis showed that diabetes significantly increase mortality (odds ratio, 5.37; 95% CI, 1.07–26.95; p = 0.04) whereas vaccination decrease mortality (odds ratio, 0.54; 95% CI, 0.10–0.94; p = 0.03, Table 4). The incidence of allograft dysfunction in COVID-19 infected kidney transplant recipients was 47%, similar between groups (p = 0.64). Most allograft dysfunctions were Acute Kidney Injury Network (AKIN) stage 1, caused by prerenal which improved after intravenous hydration. Only one patient in each group (4%) requires intermittent hemodialysis for kidney replacement therapy.

Table 4.
Univariate and multivariate analysis of factors associated mortality in COVID-19 infected kidney transplant recipients.
4. Discussion
Our study showed overall mortality of COVID-19 infection of 24.4% in kidney transplant recipients, similar to previous reports ranging from 18–37% [14,15,16,17]. Kidney transplant recipients had a much greater mortality rate than the overall population, especially those with diabetes. Studies have shown correlation between age and severity of disease, i.e., the higher the age of the patients, the higher the mortality [25,26]. In our study, the vaccinated group was older than unvaccinated group but lower mortality rate. After adjusting with multivariate analysis, this study demonstrated that inactivated and viral vector vaccines can significantly reduce mortality in COVID-19 infected kidney transplant recipients. In our study, no patients who received 2 doses of vaccine succumbed to the disease and patients who received only one dose of vaccine had mortality rate of 19% which was lower than 36% in unvaccinated group albeit not statistically significant. Our data suggested that kidney transplant recipients should be fully vaccinated as soon as possible to acquire the full benefit. There were reports showing that longer interval (12 weeks) between first and second dose of viral vector vaccine produced better immune response [6,27]. Our study showed that the mean interval from vaccination in patients who received only one dose of vaccine to the time of confirmed diagnosis of COVID-19 infection was 37 days. In situation of infection outbreak, shorten the interval between the 2 doses may be a reasonable practice to provide better protection for the patients. Another strategy is to use vaccine platform with short interval between doses such as inactivated or mRNA vaccine.
Management of COVID-19 infection in kidney transplant recipients was similar to that for general population except antiviral agent. They all received antiviral agent, favipiravir, due to their immunosuppressive state and high risk for disease progression. The intensity of corticosteroids was adjusted according to the oxygen therapy requirement. In our study, 45% required dexamethasone or methylprednisolone. At Ramathibodi Hospital, we manage immunosuppressive medication following the ERA-EDTA DESCARTES expert opinion [28], which is similar to other reports [15,29,30]. For antimetabolite, mycophenolate was mostly withheld during COVID-19 infection and resumed 1–2 weeks after infection resolved. CNIs were continued the same dose as prior to infection. Only 7% of the cohort had to withdraw CNIs due to severe diseases i.e., requirement of mechanical ventilation, hemodynamic instability, or complicated with opportunistic infection. Thus, the rate of allograft rejection after COVID-19 infection is a challenging issue in managing this group of patients.
Not only the mortality, the COVID-19 infected kidney transplant recipients developed pneumonia more often than the general population. Our study showed the incidence of pneumonia in kidney transplant recipients at 70%, while Pongpirul et al. [31] reported 39% in general population. Inactivated and viral vector vaccines did not reduce the incidence of pneumonia which may be from a very low number of fully vaccinated patients, but there was a trend toward less oxygen requirement in vaccinated patients (52% vs. 71%, p = 0.10) as well as less ventilator requirement (9% vs. 19%, p = 0.48). This implied that vaccination could reduce the severity of the disease. Meshram et al. [32] reported 4 kidney transplant recipients developing COVID-19 infection after 2 doses of ChAdOx1 nCoV-19, viral vector vaccines. One patient died due to acute respiratory distress syndrome (ARDS) and 2 patients required mechanical ventilation. Our study demonstrated the benefit of inactivated and viral vector vaccines in terms of decreased mortality and severity.
Recent studies showed a suboptimal humoral immune response to mRNA COVID-19 vaccine among kidney transplant recipients measured by anti-spike antibody [9,10,11,13,33]. Study by Bruminhent et al. [8] on the effect of immune response in patients receiving inactivated vaccine showed that humoral immune response was almost negligible after 2 doses of vaccine. On the other hand, cell mediated immune response could be achieved at the level almost the same as normal population. For viral vector vaccine, cell mediated immune response could be measured to be stimulated after just one dose of vaccine but humoral immune response was poor [34]. Recent data showed a good efficacy of the third dose of mRNA vaccine which improved humoral immune response in kidney transplant recipients from 50% to 70% [35]. However, the efficacy of booster dose with mRNA vaccine after other forms (inactivated or viral vector) of COVID-19 vaccines is unknown and required further study. Although in real life, we did not measure the immune response in our patients, it was assumed that the response would be the same and there was benefit of vaccination in reducing mortality significantly. This underscores the importance of cellular immune response in fighting viral infection.
Renal allograft dysfunction occurred in 47% of cases. The rate of allograft dysfunction did not differ between groups and only 10% of those required renal replacement therapy. The incidences were similar to the previous studies which reported ranging 40–75% and 6.6–46%, respectively [14,15].
The limitation of this study is the small number of vaccinated subjects included in the study. In Thailand, due to vaccine shortages during the study period, vaccination initially prioritize given to population 60 years or older and those with some specified comorbidity. Transplant recipients did not receive priority for vaccination. This may limit the number of kidney transplant recipients receiving vaccination and make a demonstration of vaccine efficacy in reducing severity other than death difficult due to a small number of vaccinated transplant recipients.
5. Conclusions
The mortality and severity of COVID-19 infection among kidney transplant recipients were significantly higher than the general population. Inactivated and viral vector COVID-19 vaccines have beneficial effect on mortality reduction in kidney transplant recipients. Even partial vaccination can exert some protection against death. However, to achieve better prevention, full vaccination should be encouraged for all kidney transplant recipients.
Author Contributions
N.S., S.T., S.K. and P.W. designed the study. N.S., S.T. and P.W. collected and analyzed data. N.S. and P.W. drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study protocol was approved by the Institutional Human Research Ethics Committee of Faculty of Medicine Ramathibodi Hospital, Mahidol University [MURA 2021/895].
Informed Consent Statement
As this study is a retrospective cohort study, no informed consent was needed from the subjects involved in the study.
Data Availability Statement
Data are available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AKIN | Acute Kidney Injury Network |
| CI | confidence interval |
| CNIs | calcineurin inhibitors |
| COVID-19 | coronavirus virus disease 2019 |
| eGFR | estimated glomerular filtration rate |
| IQR | interquartile range |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| mRNA | messenger ribonucleic acid |
| mTOR | mammalian target of rapamycin |
| RR | relative risk ratio |
| RT-PCR | real-time polymerase chain reaction |
| SARS-CoV2 | severe acute respiratory syndrome coronavirus 2 |
| SD | standard deviation |
References
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the mRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef]
- Rotondo, J.C.; Martini, F.; Maritati, M.; Mazziotta, C.; Di Mauro, G.; Lanzillotti, C.; Barp, N.; Gallerani, A.; Tognon, M.; Contini, C. SARS-CoV-2 Infection: New Molecular, Phylogenetic, and Pathogenetic Insights. Efficacy of Current Vaccines and the Potential Risk of Variants. Viruses 2021, 13, 1687. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Perkins, G.B.; Tunbridge, M.; Salehi, T.; Chai, C.S.; Kireta, S.; Johnston, J.; Penko, D.; Nitschke, J.; Yeow, A.; Al-Delfi, Z.; et al. Concurrent vaccination of kidney transplant recipients and close household cohabitants against COVID-19. Kidney Int. 2022. [Google Scholar] [CrossRef] [PubMed]
- Bruminhent, J.; Setthaudom, C.; Chaumdee, P.; Boongird, S.; Kiertiburanakul, S.; Malathum, K.; Nongnuch, A.; Phuphuakrat, A.; Jirasiritham, S.; Janphram, C.; et al. SARS-CoV-2-specific humoral and cell-mediated immune responses after immunization with inactivated COVID-19 vaccine in kidney transplant recipients (CVIM 1 study). Am. J. Transplant. 2021, 22, 813–822. [Google Scholar] [CrossRef]
- Benotmane, I.; Gautier-Vargas, G.; Cognard, N.; Olagne, J.; Heibel, F.; Braun-Parvez, L.; Martzloff, J.; Perrin, P.; Moulin, B.; Fafi-Kremer, S.; et al. Low immunization rates among kidney transplant recipients who received 2 doses of the mRNA-1273 SARS-CoV-2 vaccine. Kidney Int. 2021, 99, 1498–1500. [Google Scholar] [CrossRef]
- Benotmane, I.; Gautier-Vargas, G.; Cognard, N.; Olagne, J.; Heibel, F.; Braun-Parvez, L.; Martzloff, J.; Perrin, P.; Moulin, B.; Fafi-Kremer, S.; et al. Weak anti-SARS-CoV-2 antibody response after the first injection of an mRNA COVID-19 vaccine in kidney transplant recipients. Kidney Int. 2021, 99, 1487–1489. [Google Scholar] [CrossRef]
- Korth, J.; Jahn, M.; Dorsch, O.; Anastasiou, O.; Sorge-Hädicke, B.; Eisenberger, U.; Gäckler, A.; Dittmer, U.; Witzke, O.; Wilde, B.; et al. Impaired Humoral Response in Renal Transplant Recipients to SARS-CoV-2 Vaccination with BNT162b2 (Pfizer-BioNTech). Viruses 2021, 13, 756. [Google Scholar] [CrossRef] [PubMed]
- Malinis, M.; Cohen, E.; Azar, M.M. Effectiveness of SARS-CoV-2 vaccination in fully vaccinated solid organ transplant recipients. Am. J. Transplant. 2021, 21, 2916–2918. [Google Scholar] [CrossRef] [PubMed]
- Miele, M.; Busà, R.; Russelli, G.; Sorrentino, M.C.; Di Bella, M.; Timoneri, F.; Mularoni, A.; Panarello, G.; Vitulo, P.; Conaldi, P.G.; et al. Impaired anti-SARS-CoV-2 humoral and cellular immune response induced by Pfizer-BioNTech BNT162b2 mRNA vaccine in solid organ transplanted patients. Am. J. Transplant. 2021, 21, 2919–2921. [Google Scholar] [CrossRef] [PubMed]
- Akalin, E.; Azzi, Y.; Bartash, R.; Seethamraju, H.; Parides, M.; Hemmige, V.; Ross, M.; Forest, S.; Goldstein, Y.D.; Ajaimy, M.; et al. Covid-19 and Kidney Transplantation. N. Engl. J. Med. 2020, 382, 2475–2477. [Google Scholar] [CrossRef] [PubMed]
- Cristelli, M.P.; Viana, L.A.; Dantas, M.T.; Martins, S.B.; Fernandes, R.; Nakamura, M.R.; Santos, D.W.; Taddeo, J.B.; Azevedo, V.F.; Foresto, R.D.; et al. The Full Spectrum of COVID-19 Development and Recovery among Kidney Transplant Recipients. Transplantation 2021, 105, 1433–1444. [Google Scholar] [CrossRef]
- Danziger-Isakov, L.; Blumberg, E.A.; Manuel, O.; Sester, M. Impact of COVID-19 in solid organ transplant recipients. Am. J. Transplant. 2021, 21, 925–937. [Google Scholar] [CrossRef]
- Requião-Moura, L.R.; de Sandes-Freitas, T.V.; Viana, L.A.; Cristelli, M.P.; de Andrade, L.G.M.; Garcia, V.D.; de Oliveira, C.M.C.; Esmeraldo, R.D.M.; Filho, M.A.; Pacheco-Silva, A.; et al. High mortality among kidney transplant recipients diagnosed with coronavirus disease 2019: Results from the Brazilian multicenter cohort study. PLoS ONE 2021, 16, e0254822. [Google Scholar] [CrossRef]
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Yoda, T.; Suksatit, B.; Tokuda, M.; Katsuyama, H. Analysis of People’s Attitude toward COVID-19 Vaccine and Its Information Sources in Thailand. Cureus 2022, 14, 22215. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Interim Recommendations for Use of the Inactivated COVID-19 Vaccine, CoronaVac, Developed by Sinovac: Interim Guidance, First Issued 24 May 2021, Updated 21 October 2021, Updated 15 March 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- World Health Organization (WHO). Interim Recommendations for Use of the ChAdOx1-S [Recombinant] Vaccine against COVID-19 (AstraZeneca COVID-19 Vaccine AZD1222, SII Covishield, SK Bioscience); World Health Organization (WHO): Geneva, Switzerland, 2021. [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Section 2: AKI Definition. Kidney Int. Suppl. 2012, 2, 19–36. [Google Scholar] [CrossRef] [Green Version]
- Mishra, P.; Pandey, C.M.; Singh, U.; Keshri, A.; Sabaretnam, M. Selection of appropriate statistical methods for data analysis. Ann. Card. Anaesth. 2019, 22, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Kishore, J.; Goel, M.; Khanna, P. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010, 1, 274–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonanad, C.; García-Blas, S.; Tarazona-Santabalbina, F.; Sanchis, J.; Bertomeu-González, V.; Fácila, L.; Ariza, A.; Núñez, J.; Cordero, A. The Effect of Age on Mortality in Patients With COVID-19: A Meta-Analysis With 611,583 Subjects. J. Am. Med. Dir. Assoc. 2020, 21, 915–918. [Google Scholar] [CrossRef] [PubMed]
- Trecarichi, E.M.; Mazzitelli, M.; Serapide, F.; Pelle, M.C.; Tassone, B.; Arrighi, E.; Perri, G.; Fusco, P.; Scaglione, V.; Davoli, C.; et al. Clinical characteristics and predictors of mortality associated with COVID-19 in elderly patients from a long-term care facility. Sci. Rep. 2020, 10, 20834. [Google Scholar] [CrossRef]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Maggiore, U.; Abramowicz, D.; Crespo, M.; Mariat, C.; Mjoen, G.; Peruzzi, L.; Sever, M.S.; Oniscu, G.C.; Hilbrands, L.; Watschinger, B. How should I manage immunosuppression in a kidney transplant patient with COVID-19? An ERA-EDTA DESCARTES expert opinion. Nephrol. Dial. Transplant. 2020, 35, 899–904. [Google Scholar] [CrossRef]
- Angelico, R.; Blasi, F.; Manzia, T.; Toti, L.; Tisone, G.; Cacciola, R. The Management of Immunosuppression in Kidney Transplant Recipients with COVID-19 Disease: An Update and Systematic Review of the Literature. Medicina 2021, 57, 435. [Google Scholar] [CrossRef]
- López, V.; Vázquez, T.; Alonso-Titos, J.; Cabello, M.; Alonso, A.; Beneyto, I.; Crespo, M.; Díaz-Corte, C.; Franco, A.; González-Roncero, F.; et al. Recommendations on management of the SARS-CoV-2 coronavirus pandemic (COVID-19) in kidney transplant patients. Nefrología 2020, 40, 265–271. [Google Scholar] [CrossRef]
- Pongpirul, W.A.; Wiboonchutikul, S.; Charoenpong, L.; Panitantum, N.; Vachiraphan, A.; Uttayamakul, S.; Pongpirul, K.; Manosuthi, W.; Prasithsirikul, W. Clinical course and potential predictive factors for pneumonia of adult patients with Coronavirus Disease 2019 (COVID-19): A retrospective observational analysis of 193 confirmed cases in Thailand. PLoS Negl. Trop. Dis. 2020, 14, e0008806. [Google Scholar] [CrossRef]
- Meshram, H.S.; Kute, V.B.; Shah, N.; Chauhan, S.; Navadiya, V.V.; Patel, A.H.; Patel, H.V.; Engineer, D.; Banerjee, S.; Rizvi, J.; et al. COVID-19 in Kidney Transplant Recipients Vaccinated With Oxford-AstraZeneca COVID-19 Vaccine (Covishield): A Single-center Experience From India. Transplantation 2021, 105, e100–e103. [Google Scholar] [CrossRef]
- Prasad, N.; Yadav, B.; Singh, M.; Gautam, S.; Bhadauria, D.; Patel, M.; Kushwaha, R.; Yadav, D.; Singh, A.; Yachha, M.; et al. Humoral Immune Response of SARS-CoV-2 Infection and Anti-SARS-CoV-2 Vaccination in Renal Transplant Recipients. Vaccines 2022, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.; Klemis, V.; Schub, D.; Schneitler, S.; Reichert, M.C.; Wilkens, H.; Sester, U.; Sester, M.; Mihm, J. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am. J. Transplant. 2021, 21, 3990–4002. [Google Scholar] [CrossRef] [PubMed]
- Masset, C.; Kerleau, C.; Garandeau, C.; Ville, S.; Cantarovich, D.; Hourmant, M.; Kervella, D.; Houzet, A.; Guillot-Gueguen, C.; Guihard, I.; et al. A third injection of the BNT162b2 mRNA COVID-19 vaccine in kidney transplant recipients improves the humoral immune response. Kidney Int. 2021, 100, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).