Monitoring and Evaluation of National Vaccination Implementation: A Scoping Review of How Frameworks and Indicators Are Used in the Public Health Literature
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Identifying the Research Question (Stage 1)
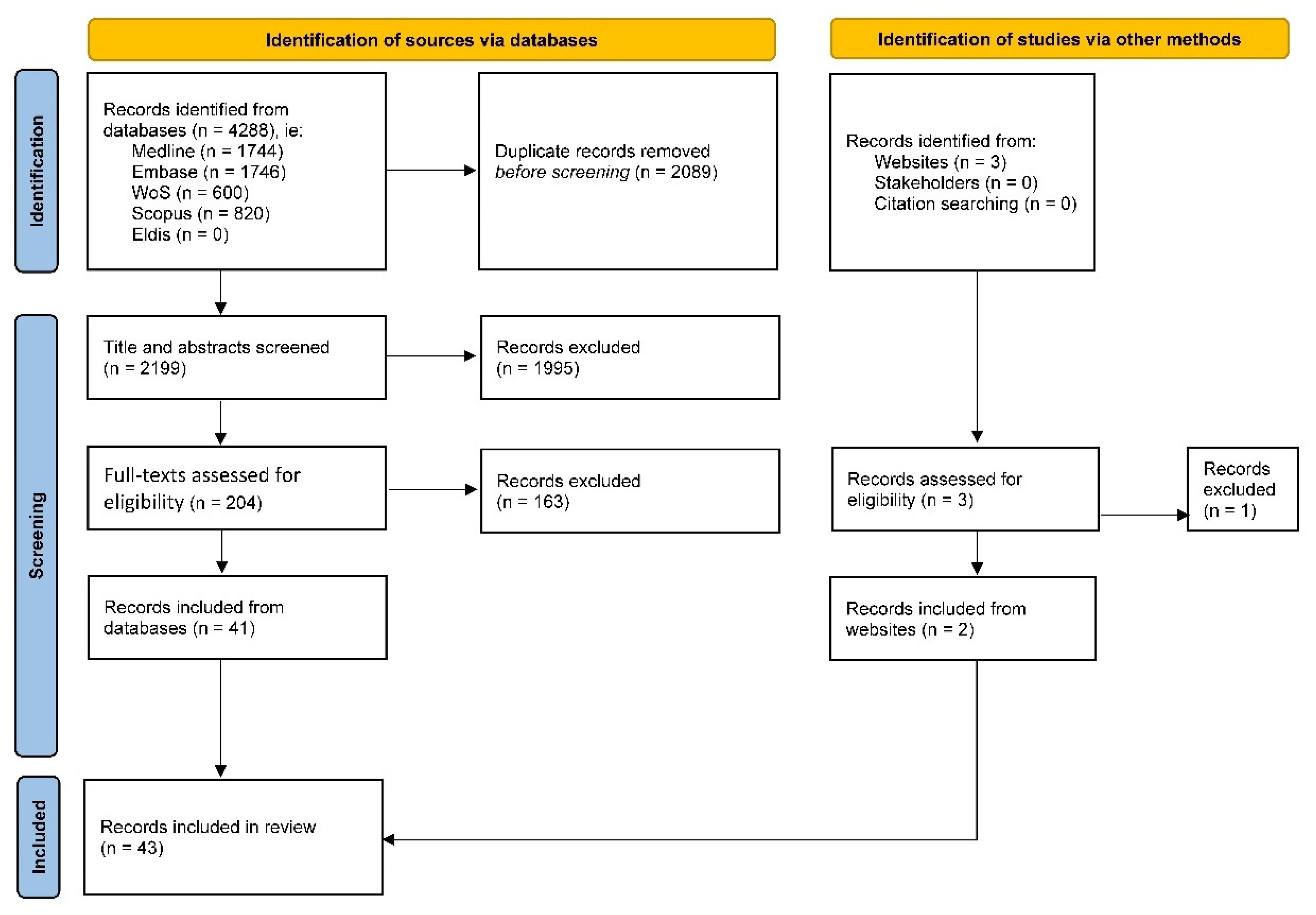
2.3. Identifying Relevant Sources (Stage 2)
2.4. Selecting Sources (Stage 3)
2.5. Extracting (Charting) Data (Stage 4)
2.6. Collating and Summarising Findings (Stage 5)
2.7. Consulting Stakeholders (Stage 6)
3. Results
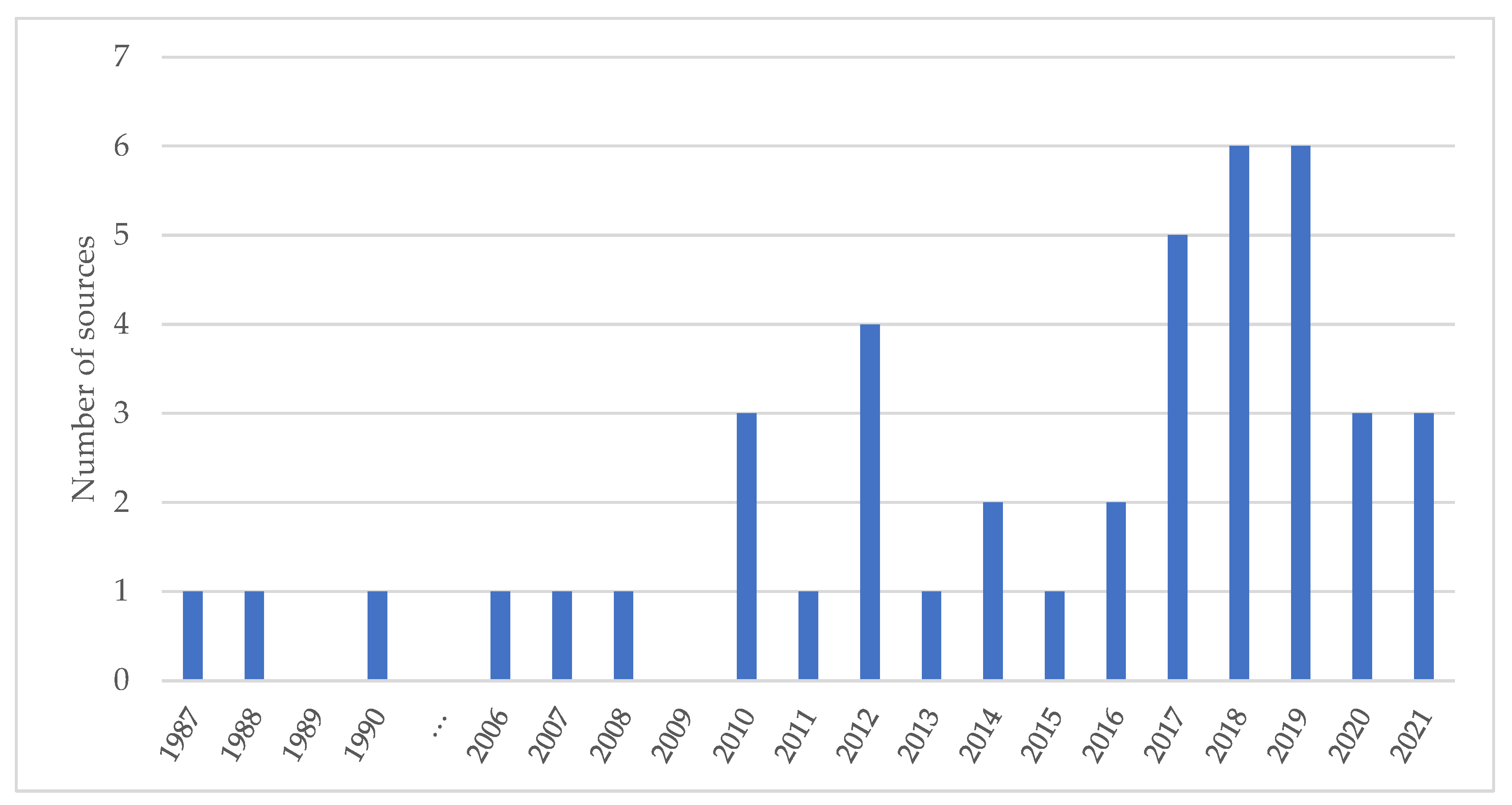
3.1. Scope of the Literature
3.2. Synthesised Findings
3.2.1. Frameworks
3.2.2. Coverage Indicators
3.2.3. Operational Indicators
3.2.4. Clinical Indicators
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. COVID-19 Strategic Preparedness and Response Plan. Monitoring and Evaluation Framework; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- ECDC. Monitoring and Evaluation Framework for COVID-19 Response Activities in the EU/EEA and the UK. 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-monitoring-and-evaluation-framework-response-activities (accessed on 22 February 2022).
- WHO. The Monitoring and Evaluation/Accountability Framework; World Health Organization: Geneva, Switzerland.
- WHO. Assessing and Improving the Accuracy of Target Population Estimates for Immunization Coverage; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- World Health Organisation. Immunization Agenda 2030: A Global Strategy to Leave No One Behind. 2020. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/strategies/ia2030 (accessed on 22 February 2022).
- IA2030. Immunization Agenda 203: Monitoring and Evaluation Framework 2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Khalil, H.; Peters, M.; Godfrey, C.M.; McInerney, P.; Soares, C.B.; Parker, D. An Evidence-Based Approach to Scoping Reviews. Worldviews Evid. Based. Nurs. 2016, 13, 118–123. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar]
- CDC. Immunization: The Basics Definition of Terms. 2018. Available online: https://www.cdc.gov/vaccines/vac-gen/imz-basics.htm (accessed on 22 February 2022).
- WHO-UNICEF. Monitoring COVID-19 Vaccination Considerations for the Collection and Use of Vaccination Data. 2021. Available online: https://apps.who.int/iris/bitstream/handle/10665/339993/WHO-2019-nCoV-vaccination-monitoring-2021.1-eng.pdf (accessed on 22 February 2022).
- Hall, V.J.; Foulkes, S.; Saei, A.; Andrews, N.; Oguti, B.; Charlett, A.; Wellington, E.; Stowe, J.; Gillson, N.; Atti, A.; et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): A prospective, multicentre, cohort study. Lancet 2021, 397, 1725–1735. [Google Scholar] [CrossRef]
- Bernal, J.L.; Andrews, N.; Gower, C.; Robertson, C.; Stowe, J.; Tessier, E.; Simmons, R.; Cottrell, S.; Roberts, R.; O’Doherty, M.; et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: Test negative case-control study. BMJ 2021, 373, n1088. [Google Scholar] [CrossRef]
- Al Awaidy, S.T.; Ezzikouri, S. Moving towards hepatitis B elimination in Gulf Health Council states: From commitment to action. J. Infect. Public Health 2020, 13, 221–227. [Google Scholar] [CrossRef]
- Hipgrave, D.B.; Maynard, J.E.; Biggs, B.A. Improving birth dose coverage of hepatitis B vaccine. Bull. World Health Organ. 2006, 84, 65–71. [Google Scholar] [CrossRef]
- Soi, C.; Shearer, J.C.; Budden, A.; Carnahan, E.; Salisbury, N.; Asiimwe, G.; Chilundo, B.; Sarma, H.; Gimbel, S.; Simuyemba, M.; et al. How to evaluate the implementation of complex health programmes in low-income settings: The approach of the Gavi Full Country Evaluations. Health Policy Plan 2020, 35 (Suppl. S2), ii35–ii46. [Google Scholar] [CrossRef]
- Dang, H.; Dao, S.; Carnahan, E.; Kawakyu, N.; Duong, H.; Nguyen, T.; Nguyen, D.; Nguyen, L.; Rivera, M.; Ngo, T.; et al. Determinants of Scale-up From a Small Pilot to a National Electronic Immunization Registry in Vietnam: Qualitative Evaluation. J. Med. Internet Res. 2020, 22, e19923. [Google Scholar] [CrossRef]
- Hutubessy, R.; Levin, A.; Wang, S.; Morgan, W.; Ally, M.; John, T.; Broutet, N. A case study using the United Republic of Tanzania: Costing nationwide HPV vaccine delivery using the WHO Cervical Cancer Prevention and Control Costing Tool. BMC Med. 2012, 10, 136. [Google Scholar] [CrossRef]
- Ijsselmuiden, C.B.; Küstner, H.G.; Barron, P.M.; Steinberg, W.J. Notification of five of the EPI target diseases in South Africa. An assessment of disease and vaccination reporting. South Afr. Med. J. 1987, 72, 311–316. [Google Scholar]
- Manyazewal, T.; Mekonnen, A.; Demelew, T.; Mengestu, S.; Abdu, Y.; Mammo, D.; Abebe, W.; Haffa, B.; Zenebe, D.; Worku, B.; et al. Improving immunization capacity in Ethiopia through continuous quality improvement interventions: A prospective quasi-experimental study. Infect. Dis. Poverty 2018, 7, 119. [Google Scholar] [CrossRef]
- Aceituno, A.M.; Stanhope, K.K.; Rebolledo, P.A.; Burke, R.M.; Revollo, R.; Iniguez, V.; Suchdev, P.S.; Leon, J.S. Using a monitoring and evaluation framework to improve study efficiency and quality during a prospective cohort study in infants receiving rotavirus vaccination in El Alto, Bolivia: The Infant Nutrition, Inflammation, and Diarrheal Illness (NIDI) study. BMC Public Health 2017, 17, 911. [Google Scholar] [CrossRef]
- Lanata, C.F.; Stroh, G.; Black, R.E.; Gonzales, H. An evaluation of Lot Quality Assurance sampling to monitor and improve immunization coverage. Int. J. Epidemiol. 1990, 19, 1086–1090. [Google Scholar] [CrossRef]
- Tuells, J. The fragile beginning of the vaccine cold chain in Spain. Gac Sanit. 2010, 24, 354–357. [Google Scholar] [CrossRef][Green Version]
- Lacapere, F.; Magloire, R.; Danovaro-Holliday, M.C.; Flannery, B.; Chamoulliet, H.; Celestin, E.P. The use of rapid coverage monitoring in the national rubella vaccination campaign, Haiti 2007-2008. J. Infect. Dis. 2011, 204 (Suppl. 2), S698–S705. [Google Scholar] [CrossRef]
- D’Ancona, F.; Gianfredi, V.; Riccardo, F.; Iannazzo, S. Immunisation Registries at regional level in Italy and the roadmap for a future Italian National Registry. Ann. Ig. 2018, 30, 77–85. [Google Scholar]
- Bianco, P.; Ieraci, R.; Comito, M.; Anzelmo, V. Controindications and adverse events of immunizations at workplace. Multidiscipunary management in workers for 2005–2010. G. Ital. Di Med. Del Lav. Ed Ergonomia 2012, 34, 631–634. (In Italian) [Google Scholar]
- Sarker, A.R.; Akram, R.; Ali, N.; Sultana, M. Coverage and factors associated with full immunisation among children aged 12-59 months in Bangladesh: Insights from the nationwide cross-sectional demographic and health survey. BMJ Open 2019, 9, e028020. [Google Scholar] [CrossRef]
- Wattiaux, A.L.; Yin, J.K.; Beard, F.; Wesselingh, S.; Cowie, B.; Ward, J.; Macartney, K. Hepatitis B immunization for indigenous adults, Australia. Bull. World Health Organ. 2016, 94, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, S.; Stephens, L.C.; Feemster, K.A.; Drew, R.J.; Eogan, M.; Butler, K.M. This choice does not just affect me. Attitudes of pregnant women toward COVID-19 vaccines: A mixed-methods study. Hum. Vaccines Immunother. 2021, 17, 3371–3376. [Google Scholar] [CrossRef] [PubMed]
- Bawa, S.; McNab, C.; Nkwogu, L.; Braka, F.; Obinya, E.; Galway, M.; Mirelman, A.J.; Hammanyero, K.I.; Safiyanu, G.; Chukwuji, M.; et al. Using the polio programme to deliver primary health care in Nigeria: Implementation research. Bull. World Health Organ. 2019, 97, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Muhamad, N.A.; Buang, S.N.; Jaafar, S.; Jais, R.; Tan, P.S.; Mustapha, N.; Aris, T.; Sulaiman, L.H.; Murad, S. Achieving high uptake of human papillomavirus vaccination in Malaysia through school-based vaccination programme. BMC Public Health 2018, 18, 1402. [Google Scholar] [CrossRef] [PubMed]
- Beard, F.H. Pertussis immunisation in pregnancy: A summary of funded Australian state and territory programs. Commun. Dis. Intell. Q Rep. 2015, 39, E329–E336. [Google Scholar] [PubMed]
- Alam, A.; Capoor, A.K.; Rao, L.V. Evaluation of adjuvanticity of promising new synthetic MDP analogues. Immunol. Lett. 1991, 27, 53–57. [Google Scholar] [CrossRef]
- Edelstein, M.; White, J.; Bukasa, A.; Saliba, V.; Ramsay, M. Triangulation of measles vaccination data in the United Kingdom of Great Britain and Northern Ireland. Bull. World Health Organ. 2019, 97, 754–763. [Google Scholar] [CrossRef]
- Walker, A.T.; Sodha, S.; Warren, W.C.; Sergon, K.; Kiptoon, S.; Ogange, J.; Ahmeda, A.H.; Eshetu, M.; Corkum, M.; Pillai, S.; et al. Forewarning of poliovirus outbreaks in the Horn of Africa: An assessment of acute flaccid paralysis surveillance and routine immunization systems in Kenya. J. Infect. Dis. 2014, 210 (Suppl. S1), S85–S90. [Google Scholar] [CrossRef]
- Özdemir, H.; Özer, A.Y. Investigating cold-chain system and efficacy of vaccines reaching the end user in Turkey and related regulations. Fabad J. Pharm. Sci. 2010, 35, 93–104. [Google Scholar]
- Cherif, D.; Ken, N.G.; Coulibaly, D.; Zengbe-Acray, P.; Ekra, K.D.; Traore, A.; Tiembré, I. Evaluation of epidemiological monitoring of post-vaccination adverse reactions in Abidjan. Sante Publique 2018, 30, 411–417. (In French) [Google Scholar]
- Carrico, R.M.; Sorrells, N.; Westhusing, K.; Wiemken, T. Monitoring of health care personnel employee and occupational health immunization program practices in the United States. Am. J. Infect. Control 2014, 42, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Ward, K.; Mugenyi, K.; Benke, A.; Luzze, H.; Kyozira, C.; Immaculate, A.; Tanifum, P.; Kisakye, A.; Bloland, P.; MacNeil, A. Enhancing Workforce Capacity to Improve Vaccination Data Quality, Uganda. Emerg. Infect. Dis. 2017, 23 (Suppl. 1), S85. [Google Scholar] [CrossRef] [PubMed]
- Loughlin, J.; Mast, T.C.; Doherty, M.C.; Wang, F.T.; Wong, J.; Seeger, J.D. Postmarketing evaluation of the short-term safety of the pentavalent rotavirus vaccine. Pediatric Infect. Dis. J. 2012, 31, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Vivekanandan, K.; Prakash, J.; Singh, G.N. Pharmacovigilance programme of India. Arch. Pharm. Pract. 2012, 3, 229–232. [Google Scholar] [CrossRef]
- OECD. First Lessons from Government Evaluations of COVID-19 Responses: A Synthesis. 2022. Available online: https://www.oecd.org/coronavirus/policy-responses/first-lessons-from-government-evaluations-of-covid-19-responses-a-synthesis-483507d6/ (accessed on 22 February 2022).
- WHO. Progress and Challenges with Achieving Universal Immunization Coverage. 2020. Available online: https://www.who.int/immunization/monitoring_surveillance/who-immuniz.pdf (accessed on 22 February 2022).
- Greene, J.C. Qualitative program evaluation. Handb. Qual. Res. 1994, 530, 544. [Google Scholar]
- WHO. Equity in Immunisation. 2022. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/vaccines-and-immunization/activities/immunization-systems/equity-in-immunization (accessed on 22 February 2022).
- The Vaccine Alliance. The Equity Goal. 2021. Available online: https://www.gavi.org/our-alliance/strategy/phase-5-2021-2025/equity-goal (accessed on 22 February 2022).
- M’Bangombe, M.; Pezzoli, L.; Reeder, B.; Kabuluzi, S.; Msyamboza, K.; Masuku, H.; Ngwira, B.; Cavailler, P.; Grandesso, F.; Palomares, A.; et al. Oral cholera vaccine in cholera prevention and control, Malawi. Bull. World Health Organ. 2018, 96, 428–435. [Google Scholar] [CrossRef]
- Teerawattananon, Y.; Anothaisintawee, T.; Pheerapanyawaranun, C.; Botwright, S.; Akksilp, K.; Sirichumroonwit, N.; Budtarad, N.; Isaranuwatchai, W. A systematic review of methodological approaches for evaluating real-world effectiveness of COVID-19 vaccines: Advising resource-constrained settings. PLoS ONE 2022, 17, e0261930. [Google Scholar] [CrossRef]
- Dutta, T.; Meyerson, B.E.; Agley, J.; Barnes, P.A.; Sherwood-Laughlin, C.; Nicholson-Crotty, J. A qualitative analysis of vaccine decision makers’ conceptualization and fostering of ‘community engagement’ in India. International. J. Equity Health 2020, 19, 185. [Google Scholar] [CrossRef]
- Guignard, A.; Praet, N.; Jusot, V.; Bakker, M.; Baril, L. Introducing new vaccines in low- and middle-income countries: Challenges and approaches. Expert Rev. Vaccines 2019, 18, 119–131. [Google Scholar] [CrossRef]
- Bonanni, P. Demographic impact of vaccination: A review. Vaccine 1999, 17 (Suppl. S3), S120–S125. [Google Scholar] [CrossRef]
| Terms | Definitions |
|---|---|
| Evaluation | The systematic assessment of an activity, project, programme, strategy, policy, topic, theme, sector, operational area or institution’s performance to determine its relevance, effectiveness, efficiency, impact, and/or sustainability [11]. |
| Framework | Shows how the programme or activity is intended to work by organising out the components of the initiative and the order or the steps needed to achieve the desired results. A framework increases understanding of the programme’s goals and objectives, defines the relationships between factors key to implementation, and articulates the internal and external elements that could affect the programme’s success. |
| Immunisation | A process by which a person becomes protected against a disease through vaccination or recovery from infection [11]. |
| Monitoring | The systematic process of collecting, analysing, and using information to track progress toward objectives and guide management decisions [10]. |
| M&E framework | A matrix compiling goal/purpose, outcomes, and outputs, along with the defined and measurable indicators with specified targets/thresholds necessary to achieve success. |
| Vaccination | The management and administration of vaccines pre-/peri-/post-vaccination to provide people with the most effective immunisation [11]. |
| Vaccine | A product, usually administered through needle injection, by mouth, or sprayed into the nose, that stimulates a person’s immune system to produce immunity to a specific disease, protecting the person from that disease [11]. |
| Criteria | Included | Excluded |
|---|---|---|
| 1. Context |
|
|
| 2. Topic |
|
|
| 3. Outcomes |
|
|
| 4. Source type |
|
|
| 5. Time-period |
|
|
| 6. Language |
|
|
| 7. Study design |
|
|
| 8. Participants |
|
|
| Lead Author, Year | Type | Country/ies | Approach | M&E Framework | Coverage Indicators | Operational Indicators | Clinical Indicators | Lessons Learnt | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Targeting/Estimation | Equity | Uptake | Service Capacity | Vaccine Supply | Human Resources | M&E Costs | Vaccine Safety | Vaccine Demand | ||||||
| Aceituno, 2017 | Article | Bolivia | Quantitative | X | X | X | X | |||||||
| Al Awaidy, 2020 | Article | multi (Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, United Arab Emirates) | Quantitative | X | X | X | ||||||||
| Alam, 2018 | Article | Bangladesh | Quantitative | X | X | |||||||||
| Ashish, 2017 | Article | Nepal | Quantitative | X | X | |||||||||
| Bawa, 2019 | Article | Nigeria | Quantitative | X | X | X | ||||||||
| Beard, 2015 | Article | Australia | Quantitative | X | X | X | X | |||||||
| Bednarczyk, 2019 | Article | US | Quantitative | X | ||||||||||
| Bernal, 2021 | Article | UK | Quantitative | X | ||||||||||
| Bhatnagar, 2016 | Article | India | Quantitative | X | ||||||||||
| Bianco, 2012 | Abstract | Italy | Quantitative | X | X | |||||||||
| Carrico, 2014 | Article | US | Mixed | X | X | |||||||||
| Checchi, 2019 | Article | UK | Quantitative | X | X | X | ||||||||
| Cherif, 2018 | Article | Ivory Coast | Quantitative | X | X | |||||||||
| Cutts, 1988 | Article | Mozambique | Quantitative | X | ||||||||||
| D’Ancona, 2018 | Article | Italy | Quantitative | X | X | |||||||||
| Dang, 2020 | Article | Viet Nam | Quantitative | X | X | |||||||||
| Edelstein, 2019 | Article | UK | Quantitative | X | X | |||||||||
| Geoghegan, 2021 | Abstract | Ireland | Qualitative | X | ||||||||||
| Hall, 2021 | Article | UK | Quantitative | X | X | |||||||||
| Hipgrave, 2006 | Article | multi (China, Indonesia, Viet Nam) | Quantitative | X | ||||||||||
| Hutubessy, 2012 | Article | Tanzania | Quantitative | X | X | |||||||||
| Ijsselmuiden, 1987 | Article | South Africa | Mixed | X | X | |||||||||
| Imoukhuede, 2007 | Article | Gambia | Quantitative | X | ||||||||||
| Lacapere, 2011 | Article | Haiti | Quantitative | X | X | X | ||||||||
| Lanata, 1990 | Article | Peru | Quantitative | X | X | |||||||||
| Loughlin, 2012 | Article | US | Quantitative | X | X | |||||||||
| Maina, 2017 | Article | Kenya | Mixed | X | X | |||||||||
| Manyazewal, 2018 | Article | Ethiopia | Mixed | X | X | X | X | X | X | X | ||||
| McCarthy, 2013 | Article | US | Quantitative | X | X | |||||||||
| Muhamad, 2018 | Article | Malaysia | Quantitative | X | X | |||||||||
| Ozdemir, 2010 | Article | Turkey | Quantitative | X | X | X | ||||||||
| Raji, 2019 | Abstract | Nigeria | Qualitative | X | ||||||||||
| Richard, 2008 | Article | Switzerland | Quantitative | X | X | |||||||||
| Sarker, 2019 | Article | Bangladesh | Quantitative | X | X | |||||||||
| Soi, 2020 | Article | multi (Bangladesh, Mozambique, Uganda, Zambia) | Quantitative | X | X | |||||||||
| Tanton, 2017 | Article | UK | Mixed | X | X | |||||||||
| Tuells, 2010 | Article | Spain | Qualitative | X | ||||||||||
| van Wijhe, 2018 | Article | Netherlands | Quantitative | X | X | X | ||||||||
| Vivekanandan, 2012 | Article | India | Quantitative | X | ||||||||||
| Walker, 2014 | Article | Kenya | Quantitative | X | X | X | X | X | ||||||
| Ward, 2017 | Article | Uganda | Quantitative | X | ||||||||||
| Watson, 2010 | Article | US | Quantitative | X | ||||||||||
| Wattiaux, 2016 | Article | Australia | Mixed | X | X | X | ||||||||
| Totals | 5 | 13 | 3 | 16 | 2 | 3 | 5 | 2 | 5 | 0 | 39 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marzouk, M.; Omar, M.; Sirison, K.; Ananthakrishnan, A.; Durrance-Bagale, A.; Pheerapanyawaranun, C.; Porncharoen, C.; Pimsarn, N.; Lam, S.T.; Ung, M.; et al. Monitoring and Evaluation of National Vaccination Implementation: A Scoping Review of How Frameworks and Indicators Are Used in the Public Health Literature. Vaccines 2022, 10, 567. https://doi.org/10.3390/vaccines10040567
Marzouk M, Omar M, Sirison K, Ananthakrishnan A, Durrance-Bagale A, Pheerapanyawaranun C, Porncharoen C, Pimsarn N, Lam ST, Ung M, et al. Monitoring and Evaluation of National Vaccination Implementation: A Scoping Review of How Frameworks and Indicators Are Used in the Public Health Literature. Vaccines. 2022; 10(4):567. https://doi.org/10.3390/vaccines10040567
Chicago/Turabian StyleMarzouk, Manar, Maryam Omar, Kanchanok Sirison, Aparna Ananthakrishnan, Anna Durrance-Bagale, Chatkamol Pheerapanyawaranun, Charatpol Porncharoen, Nopphadol Pimsarn, Sze Tung Lam, Mengieng Ung, and et al. 2022. "Monitoring and Evaluation of National Vaccination Implementation: A Scoping Review of How Frameworks and Indicators Are Used in the Public Health Literature" Vaccines 10, no. 4: 567. https://doi.org/10.3390/vaccines10040567
APA StyleMarzouk, M., Omar, M., Sirison, K., Ananthakrishnan, A., Durrance-Bagale, A., Pheerapanyawaranun, C., Porncharoen, C., Pimsarn, N., Lam, S. T., Ung, M., Mougammadou Aribou, Z., Dabak, S. V., Isaranuwatchai, W., & Howard, N. (2022). Monitoring and Evaluation of National Vaccination Implementation: A Scoping Review of How Frameworks and Indicators Are Used in the Public Health Literature. Vaccines, 10(4), 567. https://doi.org/10.3390/vaccines10040567







