Humoral Response after Three Doses of mRNA-1273 or BNT162b2 SARS-CoV-2 Vaccines in Hemodialysis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Humoral Response Assessment
2.4. Other Variables
2.5. Outcomes
2.6. Statistical Methods
2.7. Ethical Considerations and Disclosures
3. Results
3.1. Participants
3.2. Qualitative Response
3.3. Quantitative Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronavirus Disease (COVID-19) Situation Reports. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ (accessed on 27 December 2021).
- Rodríguez-Espinosa, D.; Broseta, J.J.; Cuadrado, E.; Maduell, F. Prevalence of COVID-19 Infection in Hemodialysis Patients Detected Using Serologic Screening. J. Am. Soc. Nephrol. JASN 2020, 31, 1. [Google Scholar] [CrossRef] [PubMed]
- Broseta, J.J.; Rodriguez-Espinosa, D.; Cuadrado, E.; Guillen-Olmos, E.; Hermida, E.; Montagud-Marrahi, E.; Rodas, L.; Vera, M.; Fontsere, N.; Arias, M.; et al. SARS-CoV-2 Infection in a Spanish Cohort of CKD-5D Patients: Prevalence, Clinical Presentation, Outcomes, and De-Isolation Results. Blood Purif. 2021, 50, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Windpessl, M.; Bruchfeld, A.; Anders, H.-J.; Kramer, H.; Waldman, M.; Renia, L.; Ng, L.F.P.; Xing, Z.; Kronbichler, A. COVID-19 vaccines and kidney disease. Nat. Rev. Nephrol. 2021, 17, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Tuano, K.S.; Seth, N.; Chinen, J. Secondary immunodeficiencies: An overview. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 2021, 127, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Antoniadi, G.; Liakopoulos, V.; Kartsios, C.; Stefanidis, I. Disturbances of acquired immunity in hemodialysis patients. Semin. Dial. 2007, 20, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, T.; Pissas, G.; Antoniadi, G.; Liakopoulos, V.; Stefanidis, I. Factors affecting effectiveness of vaccination against hepatitis B virus in hemodialysis patients. World J. Gastroenterol. 2014, 20, 12018–12025. [Google Scholar] [CrossRef] [PubMed]
- Broeders, N.E.; Hombrouck, A.; Lemy, A.; Wissing, K.M.; Racapé, J.; Gastaldello, K.; Massart, A.; van Gucht, S.; Weichselbaum, L.; de Mul, A.; et al. Influenza A/H1N1 vaccine in patients treated by kidney transplant or dialysis: A cohort study. Clin. J. Am. Soc. Nephrol. 2011, 6, 2573–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Espinosa, D.; Broseta, J.J.; Bedini, J.L.; Rodríguez, N.; Maduell, F. Antibody maintenance and breakthrough infections 6 months after complete COVID-19 vaccination with the mRNA-1273 and BNT162b2 vaccines in hemodialysis patients. Clin. Kidney J. 2021, sfab282, 1–2, advanced online publication. [Google Scholar] [CrossRef]
- Broseta, J.J.; Rodríguez-Espinosa, D.; Soruco, E.; Maduell, F. Weekly seroconversion rate of the mRNA-1273 SARS-CoV-2 vaccine in haemodialysis patients. Nephrol. Dial. Transplant. 2021, 36, 1754–1755. [Google Scholar] [CrossRef]
- Broseta, J.J.; Rodríguez-Espinosa, D.; Rodríguez, N.; Mosquera, M.d.M.; Marcos, M.Á.; Egri, N.; Pascal, M.; Soruco, E.; Bedini, J.L.; Bayés, B.; et al. Humoral and Cellular Responses to mRNA-1273 and BNT162b2 SARS-CoV-2 Vaccines Administered to Hemodialysis Patients. Am. J. Kidney Dis. 2021, 78, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Bayart, J.-L.; Mullier, F.; Elsen, M.; Eucher, C.; van Eeckhoudt, S.; Roy, T.; Wieers, G.; Laurent, C.; Dogné, J.-M.; et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg. Microbes Infect. 2021, 10, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Broseta, J.J.; Rodríguez-Espinosa, D.; Bedini, J.L.; Rodríguez, N.; Maduell, F. Antibody maintenance 3 months after complete messenger RNA COVID-19 vaccination in haemodialysis patients. Nephrol. Dial. Transplant. 2021, 36, 2340–2341. [Google Scholar] [CrossRef] [PubMed]
- Wand, O.; Nacasch, N.; Fadeela, A.; Shashar, M.; Grupper, A.; Benchetrit, S.; Erez, D.; Shitrit, P.; Cohen-Hagai, K. Humoral response and breakthrough infections with SARS-CoV-2 B.1.617.2 variant in vaccinated maintenance hemodialysis patients. J. Nephrol. 2022, 1–9, advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Espi, M.; Charmetant, X.; Barba, T.; Mathieu, C.; Pelletier, C.; Koppe, L.; Chalencon, E.; Kalbacher, E.; Mathias, V.; Ovize, A.; et al. A prospective observational study for justification, safety, and efficacy of a third dose of mRNA vaccine in patients receiving maintenance hemodialysis. Kidney Int. 2021, 101, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Davidovic, T.; Schimpf, J.; Abbassi-Nik, A.; Stockinger, R.; Sprenger-Mähr, H.; Lhotta, K.; Zitt, E. Waning humoral response 6 months after SARS-CoV-2 vaccination with the mRNA-BNT162b2 vaccine in hemodialysis patients: Time for a boost. Kidney Int. 2021, 100, 1334–1335. [Google Scholar] [CrossRef]
- Bachelet, T.; Bourdenx, J.P.; Martinez, C.; Mucha, S.; Martin-Dupont, P.; Perier, V.; Pommereau, A. Humoral response after SARS-CoV-2 mRNA vaccines in dialysis patients: Integrating anti-SARS-CoV-2 Spike-Protein-RBD antibody monitoring to manage dialysis centers in pandemic times. PLoS ONE 2021, 16, e0257646. [Google Scholar] [CrossRef] [PubMed]
- Bensouna, I.; Caudwell, V.; Kubab, S.; Acquaviva, S.; Pardon, A.; Vittoz, N.; Bozman, D.-F.; Hanafi, L.; Faucon, A.-L.; Housset, P. SARS-CoV-2 Antibody Response After a Third Dose of the BNT162b2 Vaccine in Patients Receiving Maintenance Hemodialysis or Peritoneal Dialysis. Am. J. Kidney Dis. 2021, 79, 185–192.e1. [Google Scholar] [CrossRef] [PubMed]
- Robert, T.; Lano, G.; Giot, M.; Fourié, T.; de Lamballeri, X.; Jehel, O.; Bouchouareb, D.; Brunet, P.; Ninove, L.; Burtey, S. Humoral response after SARS-CoV-2 vaccination in patients undergoing maintenance haemodialysis: Loss of immunity, third dose and non-responders. Nephrol. Dial. Transplant. 2021, 37, 390–392. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, F.P.; Figiel, L.; Ricken, J.; Still, H.; Korte, C.; Plassmann, G.; von Landenberg, P. Evolution of SARS-COV-2-neutralizing antibodies after two standard dose vaccinations, risk factors for non-response and effect of a third dose booster vaccination in non-responders on hemodialysis. A prospective multi-centre cohort study. J. Clin. Med. 2021, 10, 5113. [Google Scholar] [CrossRef] [PubMed]
- Ducloux, D.; Colladant, M.; Chabannes, M.; Yannaraki, M.; Courivaud, C. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021, 100, 702–704. [Google Scholar] [CrossRef]
- Dekervel, M.; Henry, N.; Torreggiani, M.; Pouteau, L.-M.; Imiela, J.-P.; Mellaza, C.; Garnier, A.-S.; Dujardin, A.; Asfar, M.; Ducancelle, A.; et al. Humoral response to a third injection of BNT162b2 vaccine in patients on maintenance haemodialysis. Clin. Kidney J. 2021, 14, 2349–2355. [Google Scholar] [CrossRef]
- Clarke, C.; Prendecki, M.; Dhutia, A.; Ali, M.A.; Sajjad, H.; Shivakumar, O.; Lightstone, L.; Kelleher, P.; Pickering, M.C.; Thomas, D.; et al. High Prevalence of Asymptomatic COVID-19 Infection in Hemodialysis Patients Detected Using Serologic Screening. J. Am. Soc. Nephrol. 2020, 31, 1969–1975. [Google Scholar] [CrossRef]
- Li, J.; Xu, G. Lessons from the Experience in Wuhan to Reduce Risk of COVID-19 Infection in Patients Undergoing Long-Term Hemodialysis. Clin. J. Am. Soc. Nephrol. CJASN 2020, 15, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Espinosa, D.; Montagud-Marrahi, E.; Cacho, J.; Arana, C.; Taurizano, N.; Hermida, E.; del Risco-Zevallos, J.; Casals, J.; Rosario, A.; Cuadrado-Payán, E.; et al. Incidence of severe breakthrough SARS-CoV-2 infections in vaccinated kidney transplant and haemodialysis patients. J. Nephrol. 2022, 1–10, advance online publication. [Google Scholar] [CrossRef] [PubMed]
- Altieri, P.; Sau, G.; Cao, R.; Barracca, A.; Menneas, A.; Micchittu, B.; Cabiddu, G.; Esposito, P.; Pani, A. Immunosuppressive treatment in dialysis patients. Nephrol. Dial. Transplant. 2002, 17 (Suppl. S8), 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jassal, S.V.; Lok, C.E.; Walele, A.; Bargman, J.M. Continued transplant immunosuppression may prolong survival after return to peritoneal dialysis: Results of a decision analysis. Am. J. Kidney Dis. 2002, 40, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, J.; Tonnus, W.; Paliege, A.; Rettig, R.; Steglich, A.; Gembardt, F.; Kessel, F.; Kröger, H.; Arndt, P.; Sradnick, J.; et al. Cellular and Humoral Immune Responses after 3 Doses of BNT162b2 mRNA SARS-CoV-2 Vaccine in Kidney Transplant. Transplantation 2021, 105, E267–E269. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.P.; Hassler, H.B.; Wang, Z.; Miura, S.; Singh, J.; Kumar, S.; Ruddle, N.H.; Galvani, A.P.; Dornburg, A. The durability of immunity against reinfection by SARS-CoV-2: A comparative evolutionary study. Lancet Microbe 2021, 2, e666–e675. [Google Scholar] [CrossRef]
- Mair, M.J.; Berger, J.M.; Berghoff, A.S.; Starzer, A.M.; Ortmayr, G.; Puhr, H.C.; Steindl, A.; Perkmann, T.; Haslacher, H.; Strassl, R.; et al. Humoral Immune Response in Hematooncological Patients and Health Care Workers Who Received SARS-CoV-2 Vaccinations. JAMA Oncol. 2022, 8, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Fendler, A.; Shepherd, S.T.C.; Au, L.; Wilkinson, K.A.; Wu, M.; Schmitt, A.M.; Tippu, Z.; Farag, S.; Rogiers, A.; Harvey, R.; et al. Immune responses following third COVID-19 vaccination are reduced in patients with hematological malignancies compared to patients with solid cancer. Cancer Cell 2022, 40, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Achtnichts, L.; Jakopp, B.; Oberle, M.; Nedeltchev, K.; Fux, C.A.; Sellner, J.; Findling, O. Humoral Immune Response after the Third SARS-CoV-2 mRNA Vaccination in CD20 Depleted People with Multiple Sclerosis. Vaccines 2021, 9, 1470. [Google Scholar] [CrossRef] [PubMed]
- Bracci, L.; Schiavoni, G.; Sistigu, A.; Belardelli, F. Immune-based mechanisms of cytotoxic chemotherapy: Implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014, 21, 15–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, A.; Caimi, B.; Franzetti, M.; Bergna, A.; Velleca, R.; Gatti, A.; Rossi, P.L.; D’Orso, M.; Pregliasco, F.; Balotta, C.; et al. Durability of Humoral Responses after the Second Dose of mRNA BNT162b2 Vaccine in Residents of a Long Term Care Facility. Vaccines 2022, 10, 446. [Google Scholar] [CrossRef] [PubMed]
- Papadokostaki, E.; Tentolouris, A.; Anastasiou, I.A.; Psichogiou, M.; Iliaki, E.; Eleftheriadou, I.; Hatzakis, A.; Tentolouris, N. Immunogenicity of SARS-CoV-2 BNT162b2 Vaccine in People with Diabetes: A Prospective Observational Study. Vaccines 2022, 10, 382. [Google Scholar] [CrossRef] [PubMed]
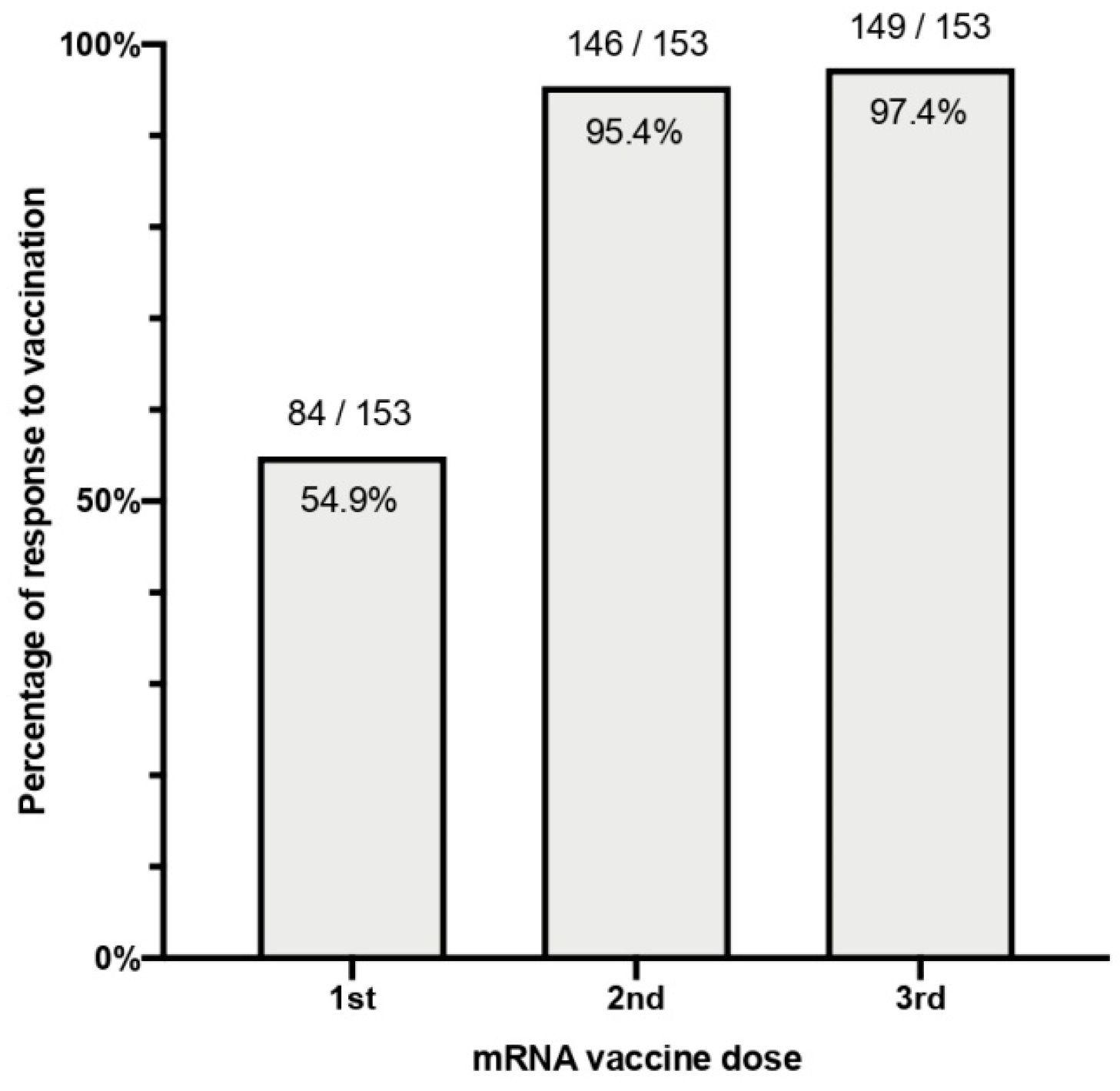
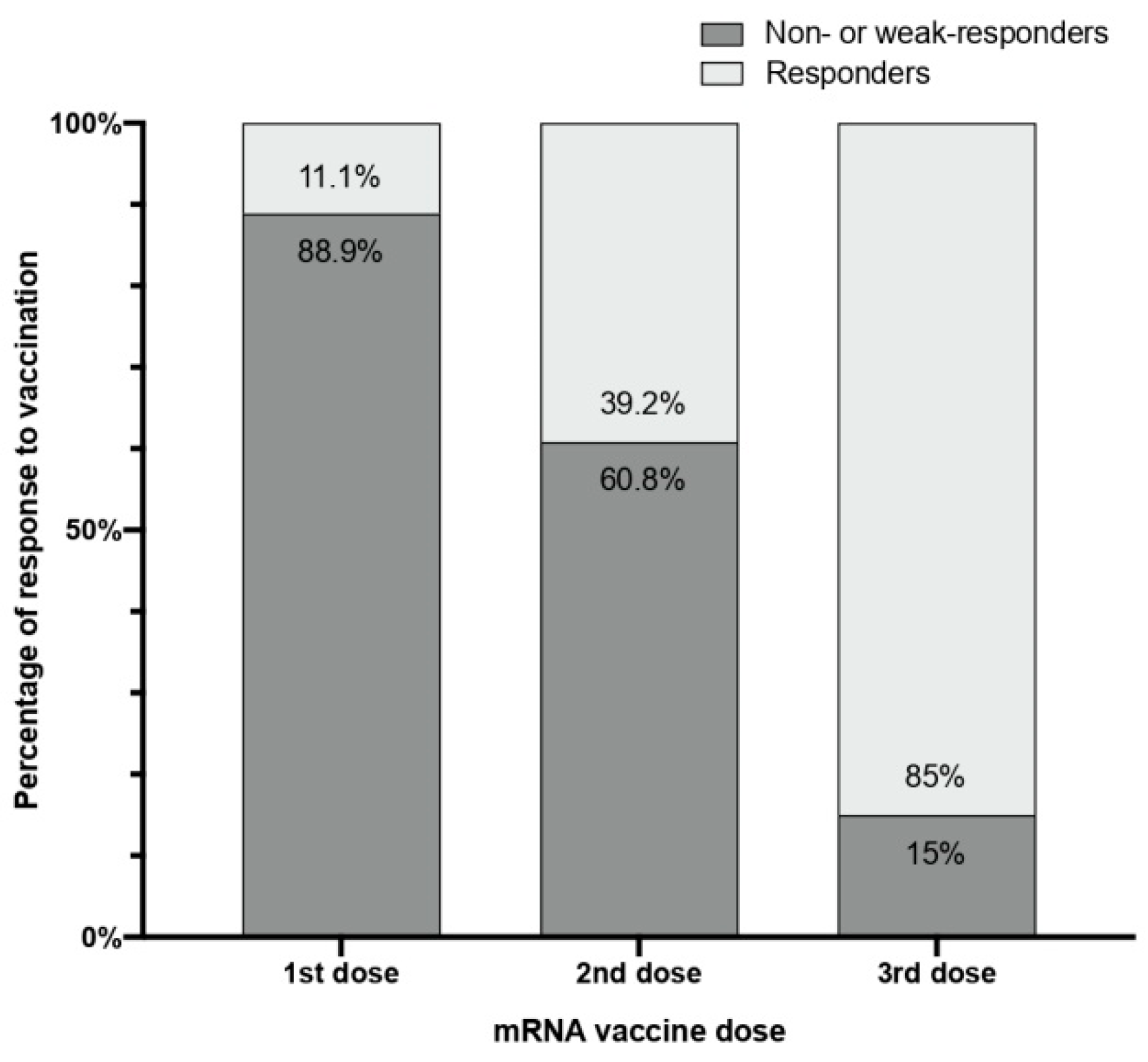
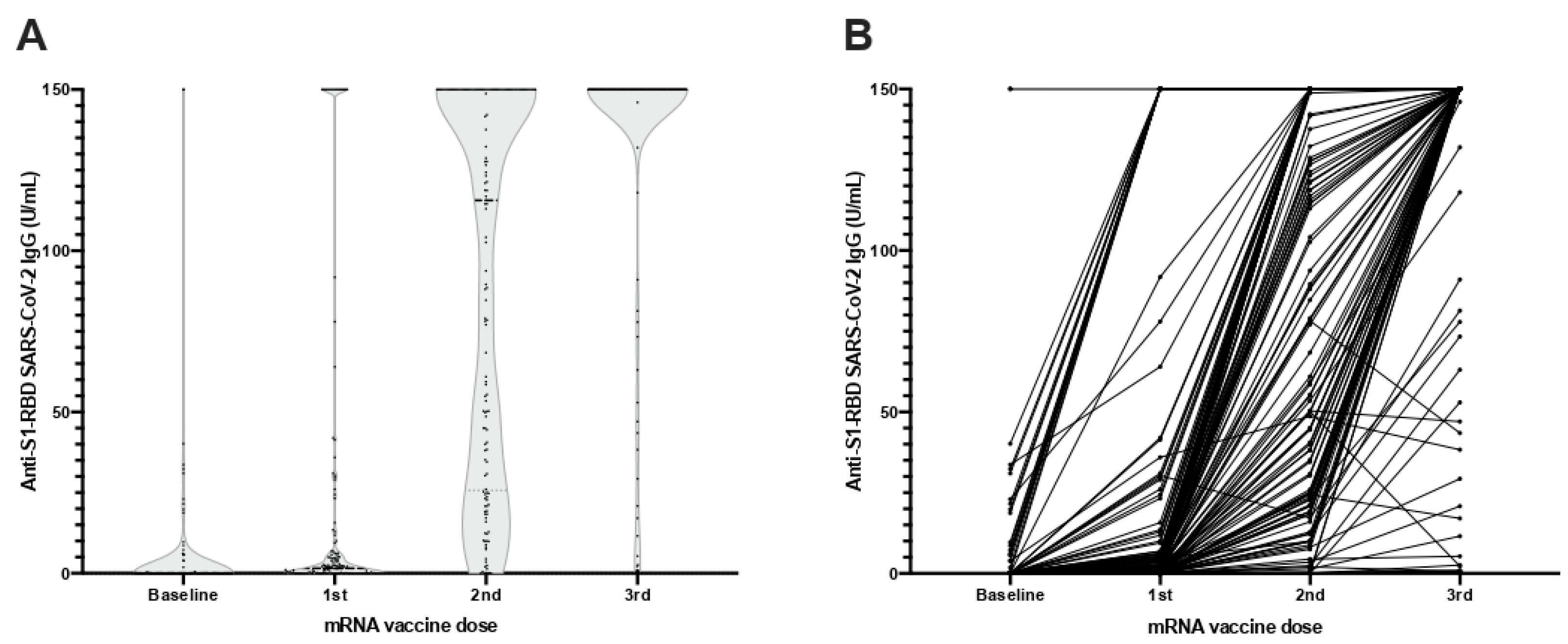
| N | Sex | Age | Vaccine | ESKD Cause | Dialysis Vintage (Months) | Immunosuppressive Treatment | Comorbidities | Previous Humoral Response |
|---|---|---|---|---|---|---|---|---|
| 1 | Male | 88 | BNT162b2 | CNI toxicity | 58 | Tacrolimus | Liver Transplant | No response after two doses |
| 2 | Male | 65 | BNT162b2 | DKD | 40 | No | POEMS syndrome Obesity, Diabetes | No response after two doses |
| 3 | Female | 82 | BNT162b2 | Unknown | 32 | Cyclophosphamide | Multiple myeloma | Response lost 3 months after the two doses |
| 4 | Male | 54 | mRNA-1273 | Unknown | 114 | Tacrolimus and Mycophenolic acid | Liver transplant Colon cancer HIV, HBV | No response after two doses |
| Variable | Total n = 153 | Non- or Weak Responders (<150 U/mL) n = 23 (15%) | Responders (≥150 U/mL) n = 130 (85%) | p-Value | Odds Ratio 1 (95% Confidence Interval) |
|---|---|---|---|---|---|
| Age > 75 years | 74 (48.4%) | 12 (52.2%) | 62 (47.7%) | 0.69 | 1.2 (0.49–2.9) |
| Male sex | 83 (54.2% | 12 (52.2%) | 71 (54.6%) | 0.83 | 1.1 (0.45–2.68) |
| Dialysis vintage (over the 50th percentile) | 75 (49%) | 13 (56.5%) | 62 (47.7%) | 0.44 | 1.43 (0.58–3.48) |
| BNT162b2 vaccine | 71 (46.4%) | 16 (69.6%) | 55 (42.3%) | 0.02 | 3.12 (1.2–8.1) |
| Overweight | 45 (29.4%) | 7 (30.4%) | 38 (29.2%) | 0.97 | 1.06 (0.4–2.7) |
| Obesity | 27 (17.6%) | 3 (13%) | 24 (18.5%) | 0.53 | 0.66 (0.18–2.4) |
| Diabetes | 55 (36.2%) | 7 (30.4%) | 48 (37.2%) | 0.53 | 0.74 (0.28–1.92) |
| Immunosuppressive therapy | 11 (7.2%) | 5 (21.7%) | 6 (4.6%) | 0.01 | 5.74 (1.59–20.83) |
| Active cancer | 5 (3.3%) | 3 (13%) | 2 (1.6%) | 0.03 | 9.52 (1.5–58.82) |
| HIV chronic infection | 4 (2.6%) | 1 (4.3%) | 3 (2.3%) | 0.49 | 1.9 (0.19–19.23) |
| HBV chronic infection | 6 (3.9%) | 1 (4.3%) | 5 (3.9%) | 1 | 1.13 (0.13–10.1) |
| Previous SARS-CoV-2 infection | 20 (13.1%) | 1 (4.3%) | 19 (14.6%) | 0.18 | 3.77 (0.48–29.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Broseta, J.J.; Rodríguez-Espinosa, D.; Cuadrado, E.; Rodríguez, N.; Bedini, J.L.; Maduell, F. Humoral Response after Three Doses of mRNA-1273 or BNT162b2 SARS-CoV-2 Vaccines in Hemodialysis Patients. Vaccines 2022, 10, 522. https://doi.org/10.3390/vaccines10040522
Broseta JJ, Rodríguez-Espinosa D, Cuadrado E, Rodríguez N, Bedini JL, Maduell F. Humoral Response after Three Doses of mRNA-1273 or BNT162b2 SARS-CoV-2 Vaccines in Hemodialysis Patients. Vaccines. 2022; 10(4):522. https://doi.org/10.3390/vaccines10040522
Chicago/Turabian StyleBroseta, José Jesús, Diana Rodríguez-Espinosa, Elena Cuadrado, Néstor Rodríguez, José Luis Bedini, and Francisco Maduell. 2022. "Humoral Response after Three Doses of mRNA-1273 or BNT162b2 SARS-CoV-2 Vaccines in Hemodialysis Patients" Vaccines 10, no. 4: 522. https://doi.org/10.3390/vaccines10040522
APA StyleBroseta, J. J., Rodríguez-Espinosa, D., Cuadrado, E., Rodríguez, N., Bedini, J. L., & Maduell, F. (2022). Humoral Response after Three Doses of mRNA-1273 or BNT162b2 SARS-CoV-2 Vaccines in Hemodialysis Patients. Vaccines, 10(4), 522. https://doi.org/10.3390/vaccines10040522







