Safety and Efficacy of the Common Vaccines against COVID-19
Abstract
:1. Introduction
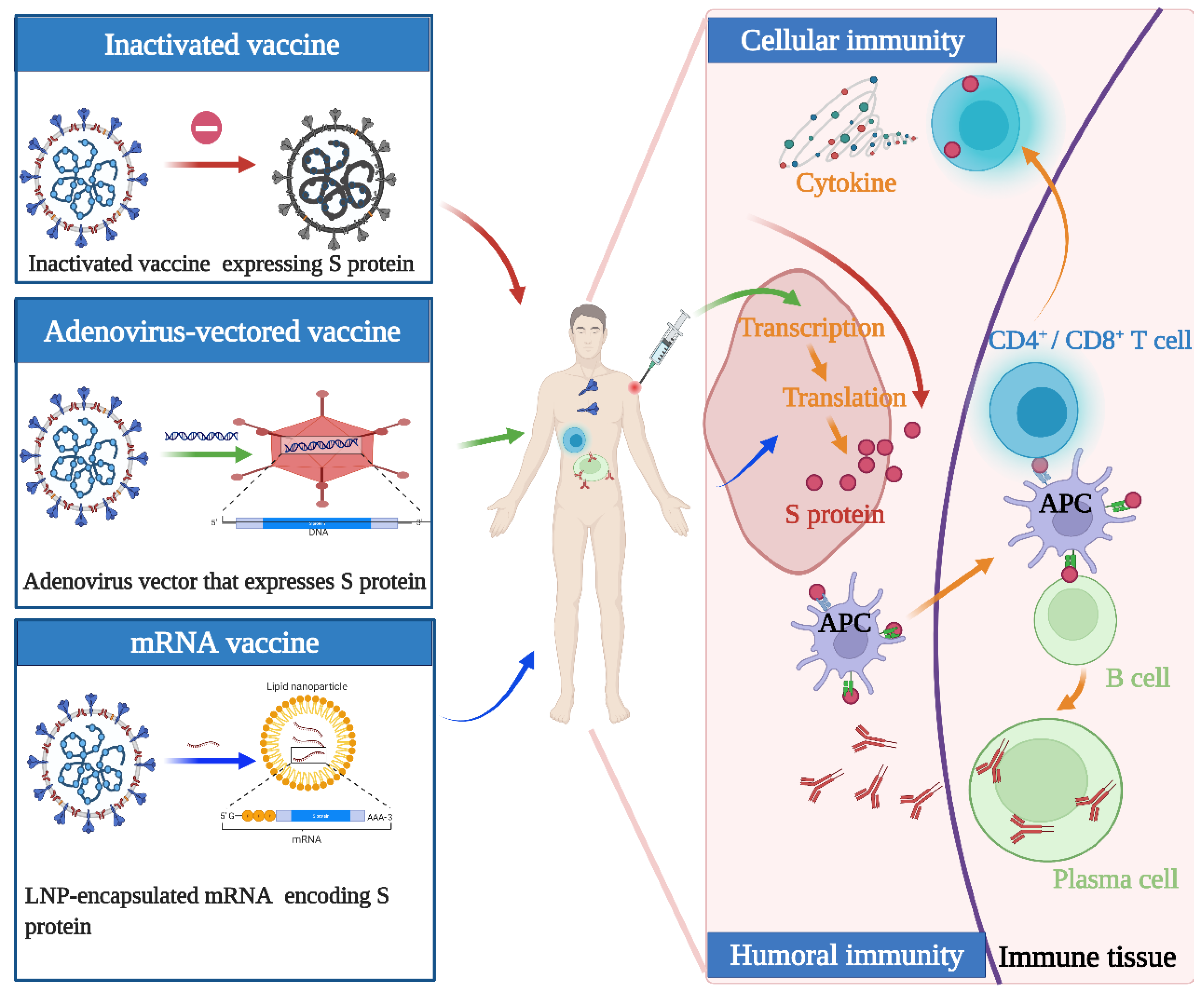
2. Concept of Vaccine Design and Mechanism of Action
3. Types and Differences of Vaccines
4. Safety
4.1. Safety of Inactivated Vaccine CoronaVac
4.2. Safety of Adenoviral Vector Vaccine Convidecia
4.3. Safety of the mRNA Vaccine BNT162b2
4.4. Comprehensive Safety Evaluation
5. Effectiveness and Immunogenicity
5.1. Effectiveness and Immunogenicity of the Inactivated Vaccine CoronaVac
5.2. Effectiveness and Immunogenicity of Adenovirus-Vectored Vaccine Convidecia
5.3. Effectiveness and Immunogenicity of the mRNA Vaccine BNT162b2
5.4. Comprehensive Effectiveness Evaluation
6. Prime-Boost Strategies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Okell, L.C.; Verity, R.; Watson, O.J.; Mishra, S.; Walker, P.; Whittaker, C.; Katzourakis, A.; Donnelly, C.A.; Riley, S.; Ghani, A.C.; et al. Have deaths from COVID-19 in Europe plateaued due to herd immunity? Lancet 2020, 395, e110–e111. [Google Scholar] [CrossRef]
- Anderson, R.M.; Vegvari, C.; Truscott, J.; Collyer, B.S. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. Lancet 2020, 396, 1614–1616. [Google Scholar] [CrossRef]
- Angulo, F.J.; Finelli, L.; Swerdlow, D.L. Reopening Society and the Need for Real-Time Assessment of COVID-19 at the Community Level. JAMA 2020, 323, 2247–2248. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.G.M.; Corder, R.M.; King, J.G.; Langwig, K.E.; Souto-Maior, C.; Carneiro, J.; Gonçalves, G.; Penha-Gonçalves, C.; Ferreira, M.U.; Aguas, R. Individual variation in susceptibility or exposure to SARS-CoV-2 lowers the herd immunity threshold. medRxiv 2020, 540, 111063. [Google Scholar] [CrossRef] [PubMed]
- Holvast, A.; Huckriede, A.; Wilschut, J.; Horst, G.; De Vries, J.J.; Benne, C.A.; Kallenberg, C.G.; Bijl, M. Safety and efficacy of influenza vaccination in systemic lupus erythematosus patients with quiescent disease. Ann. Rheum. Dis. 2006, 65, 913–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zolla-Pazner, S.; Michael, N.L.; Kim, J.H. A tale of four studies: HIV vaccine immunogenicity and efficacy in clinical trials. Lancet HIV 2021, 8, e449–e452. [Google Scholar] [CrossRef]
- Kanimozhi, G.; Pradhapsingh, B.; Singh Pawar, C.; Khan, H.A.; Alrokayan, S.H.; Prasad, N.R. SARS-CoV-2: Pathogenesis, Molecular Targets and Experimental Models. Front. Pharmacol. 2021, 12, 638334. [Google Scholar] [CrossRef]
- Prates, E.T.; Garvin, M.R.; Pavicic, M.; Jones, P.; Shah, M.; Demerdash, O.; Amos, B.K.; Geiger, A.; Jacobson, D. Potential Pathogenicity Determinants Identified from Structural Proteomics of SARS-CoV and SARS-CoV-2. Mol. Biol. Evol. 2021, 38, 702–715. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, Y.; Liu, K.; Li, Y.; Lu, Q.; Wang, Q.; Zhang, Y.; Wang, L.; Liao, H.; Zheng, A.; et al. The molecular basis for SARS-CoV-2 binding to dog ACE2. Nat. Commun. 2021, 12, 4195. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gao, G.F. WHO Targets for Vaccines against COVID-19. Nat. Rev. Immunol. 2021, 21, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S. History of vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Background Document on the Inactivated Vaccine Sinovac-CoronaVac against COVID-19. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-Sinovac-CoronaVac-background-2021.1 (accessed on 10 January 2022).
- World Health Organization. Background Document on the Inactivated COVID-19 Vaccine BIBP Developed by China National Biotec Group (CNBG), Sinopharm. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE_recommendation-BIBP-background-2021.1 (accessed on 10 January 2022).
- World Health Organization. Background Document on the AZD1222 Vaccine against COVID-19 Developed by Oxford University and AstraZeneca. Available online: https://www.who.int/publications/i/item/background-document-on-the-azd1222-vaccine-against-covid-19-developed-by-oxford-university-and-astrazeneca (accessed on 10 January 2022).
- World Health Organization. Background Document on the mRNA-1273 Vaccine (Moderna) against COVID-19. Available online: https://www.who.int/publications/i/item/background-document-on-the-mrna-1273-vaccine-(moderna)-against-covid-19 (accessed on 10 January 2022).
- World Health Organization. Background Document on the mRNA Vaccine BNT162b2 (Pfizer-BioNTech) against COVID-19. Available online: https://www.who.int/publications/i/item/background-document-on-mrna-vaccine-bnt162b2-(pfizer-biontech)-against-covid-19 (accessed on 10 January 2022).
- World Health Organization. Background Document on the Janssen Ad26.COV2. S (COVID-19) Vaccine. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-Ad26.COV2.S-background-2021.1 (accessed on 10 January 2022).
- Li, J.X.; Zhu, F.C. Inactivated SARS-CoV-2 vaccine (BBV152)-induced protection against symptomatic COVID-19. Lancet 2021, 398, 2134–2135. [Google Scholar] [CrossRef]
- Chua, B.Y.; Wong, C.Y.; Mifsud, E.J.; Edenborough, K.M.; Sekiya, T.; Tan, A.C.; Mercuri, F.; Rockman, S.; Chen, W.; Turner, S.J.; et al. Inactivated Influenza Vaccine That Provides Rapid, Innate-Immune-System-Mediated Protection and Subsequent Long-Term Adaptive Immunity. mBio 2015, 6, e01024-15. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Zhong, G.; Zhang, J.; Shuai, L.; Zhang, Z.; Wen, Z.; Wang, B.; Zhao, Z.; Song, X.; Chen, Y.; et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 4081. [Google Scholar] [CrossRef]
- Roy, C.J.; Ault, A.; Sivasubramani, S.K.; Gorres, J.P.; Wei, C.J.; Andersen, H.; Gall, J.; Roederer, M.; Rao, S.S. Aerosolized adenovirus-vectored vaccine as an alternative vaccine delivery method. Respir. Res. 2011, 12, 153. [Google Scholar] [CrossRef] [Green Version]
- Buchbinder, S.P.; McElrath, M.J.; Dieffenbach, C.; Corey, L. Use of adenovirus type-5 vectored vaccines: A cautionary tale. Lancet 2020, 396, e68–e69. [Google Scholar] [CrossRef]
- Humphreys, I.R.; Sebastian, S. Novel viral vectors in infectious diseases. Immunology 2018, 153, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Vogel, F.R.; Sarver, N. Nucleic acid vaccines. Clin. Microbiol. Rev. 1995, 8, 406–410. [Google Scholar] [CrossRef]
- Park, J.W.; Lagniton, P.N.P.; Liu, Y.; Xu, R.H. mRNA vaccines for COVID-19: What, why and how. Int. J. Biol. Sci. 2021, 17, 1446–1460. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.R.F.; Patel, A.; Ramos, S.; Elwood, D.; Zhu, X.; Yan, J.; Gary, E.N.; Walker, S.N.; Schultheis, K.; Purwar, M.; et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat. Commun. 2020, 11, 2601. [Google Scholar] [CrossRef]
- Knezevic, I.; Liu, M.A.; Peden, K.; Zhou, T.; Kang, H.N. Development of mRNA Vaccines: Scientific and Regulatory Issues. Vaccines 2021, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA Vaccines: Immunological Mechanism and Beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.A.C.; Kester, K.E.; Casimiro, D.; Gurunathan, S.; DeRosa, F. The promise of mRNA vaccines: A biotech and industrial perspective. NPJ Vaccines 2020, 5, 11. [Google Scholar] [CrossRef]
- Stone, C.A., Jr.; Rukasin, C.R.F.; Beachkofsky, T.M.; Phillips, E.J. Immune-mediated adverse reactions to vaccines. Br. J. Clin. Pharmacol. 2019, 85, 2694–2706. [Google Scholar] [CrossRef]
- Blumenthal, K.G.; Robinson, L.B.; Camargo, C.A., Jr.; Shenoy, E.S.; Banerji, A.; Landman, A.B.; Wickner, P. Acute Allergic Reactions to mRNA COVID-19 Vaccines. JAMA 2021, 325, 1562–1565. [Google Scholar] [CrossRef]
- Tirado, S.M.; Yoon, K.J. Antibody-dependent enhancement of virus infection and disease. Viral Immunol. 2003, 16, 69–86. [Google Scholar] [CrossRef]
- Garber, K. Coronavirus vaccine developers wary of errant antibodies. Nat. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Sadoff, J.; Davis, K.; Douoguih, M. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination—Response from the Manufacturer. N. Engl. J. Med. 2021, 384, 1965–1966. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.; Nair, N. Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Pfizer-BioNTech COVID-19 Vaccine. JAMA 2021, 325, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hu, Y.; Xu, M.; Chen, Z.; Yang, W.; Jiang, Z.; Li, M.; Jin, H.; Cui, G.; Chen, P.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021, 21, 803–812. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Han, B.; Song, Y.; Li, C.; Yang, W.; Ma, Q.; Jiang, Z.; Li, M.; Lian, X.; Jiao, W.; Wang, L.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: A double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021, 21, 1645–1653. [Google Scholar] [CrossRef]
- Tanriover, M.D.; Doğanay, H.L.; Akova, M.; Güner, H.R.; Azap, A.; Akhan, S.; Köse, Ş.; Erdinç, F.; Akalın, E.H.; Tabak, Ö.F.; et al. Efficacy and safety of an inactivated whole-virion SARS-CoV-2 vaccine (CoronaVac): Interim results of a double-blind, randomised, placebo-controlled, phase 3 trial in Turkey. Lancet 2021, 398, 213–222. [Google Scholar] [CrossRef]
- Fadlyana, E.; Rusmil, K.; Tarigan, R.; Rahmadi, A.R.; Prodjosoewojo, S.; Sofiatin, Y.; Khrisna, C.V.; Sari, R.M.; Setyaningsih, L.; Surachman, F.; et al. A phase III, observer-blind, randomized, placebo-controlled study of the efficacy, safety, and immunogenicity of SARS-CoV-2 inactivated vaccine in healthy adults aged 18–59 years: An interim analysis in Indonesia. Vaccine 2021, 39, 6520–6528. [Google Scholar] [CrossRef]
- Jara, A.; Undurraga, E.A.; González, C.; Paredes, F.; Fontecilla, T.; Jara, G.; Pizarro, A.; Acevedo, J.; Leo, K.; Leon, F.; et al. Effectiveness of an Inactivated SARS-CoV-2 Vaccine in Chile. N. Engl. J. Med. 2021, 385, 875–884. [Google Scholar] [CrossRef]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Zhu, F.C.; Li, Y.H.; Guan, X.H.; Hou, L.H.; Wang, W.J.; Li, J.X.; Wu, S.P.; Wang, B.S.; Wang, Z.; Wang, L.; et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. Lancet 2020, 395, 1845–1854. [Google Scholar] [CrossRef]
- Zhu, F.; Jin, P.; Zhu, T.; Wang, W.; Ye, H.; Pan, H.; Hou, L.; Li, J.; Wang, X.; Wu, S.; et al. Safety and immunogenicity of a recombinant adenovirus type-5-vectored COVID-19 vaccine with a homologous prime-boost regimen in healthy participants aged 6 years and above: A randomised, double-blind, placebo-controlled, phase 2b trial. Clin. Infect Dis. 2021, ciab845. [Google Scholar] [CrossRef] [PubMed]
- Halperin, S.A.; Ye, L.; MacKinnon-Cameron, D.; Smith, B.; Cahn, P.E.; Ruiz-Palacios, G.M.; Ikram, A.; Lanas, F.; Lourdes Guerrero, M.; Muñoz Navarro, S.R.; et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: An international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet 2021, 399, 237–248. [Google Scholar] [PubMed]
- Walsh, E.E.; Frenck, R.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. RNA-Based COVID-19 Vaccine BNT162b2 Selected for a Pivotal Efficacy Study. medRxiv 2020. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Kim, H.W.; Jenista, E.R.; Wendell, D.C.; Azevedo, C.F.; Campbell, M.J.; Darty, S.N.; Parker, M.A.; Kim, R.J. Patients With Acute Myocarditis Following mRNA COVID-19 Vaccination. JAMA Cardiol. 2021, 6, 1196–1201. [Google Scholar] [CrossRef]
- Mansour, J.; Short, R.G.; Bhalla, S.; Woodard, P.K.; Verma, A.; Robinson, X.; Raptis, D.A. Acute myocarditis after a second dose of the mRNA COVID-19 vaccine: A report of two cases. Clin. Imaging 2021, 78, 247–249. [Google Scholar] [CrossRef]
- Fronza, M.; Thavendiranathan, P.; Chan, V.; Karur, G.R.; Udell, J.A.; Wald, R.M.; Hong, R.; Hanneman, K. Myocardial Injury Pattern at MRI in COVID-19 Vaccine-associated Myocarditis. Radiology 2022, 212559. [Google Scholar] [CrossRef]
- Ghincea, A.; Ryu, C.; Herzog, E.L. An Acute Exacerbation of Idiopathic Pulmonary Fibrosis After BNT162b2 mRNA COVID-19 Vaccination: A Case Report. Chest 2022, 161, e71–e73. [Google Scholar] [CrossRef]
- Mumm, T.; Elbashir, M. A Copd Exacerbation That Occurred after the Mrna COVID-19 Vaccine. Chest 2021, 160, A1764. [Google Scholar] [CrossRef]
- Chen, R.T.; Hibbs, B. Vaccine safety: Current and future challenges. Pediatr. Ann. 1998, 27, 445–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, S.; Huang, J.; Zhang, Z.; Wu, J.; Zhang, J.; Hu, H.; Zhu, T.; Zhang, J.; Luo, L.; Fan, P.; et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: Preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect Dis. 2021, 21, 1654–1664. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Stone, C.A., Jr.; Jakubovic, B.; Phillips, E.J.; Sussman, G.; Park, J.; Hoang, U.; Kirshner, S.L.; Levin, R.; Kozlowski, S. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J. Allergy Clin. Immunol. Pract. 2021, 9, 1731–1733.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, J.; Perlman, S. T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J. Virol. 2010, 84, 9318–9325. [Google Scholar] [CrossRef] [Green Version]
- Channappanavar, R.; Fett, C.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J. Virol. 2014, 88, 11034–11044. [Google Scholar] [CrossRef] [Green Version]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Zhu, F.C.; Hou, L.H.; Li, J.X.; Wu, S.P.; Liu, P.; Zhang, G.R.; Hu, Y.M.; Meng, F.Y.; Xu, J.J.; Tang, R.; et al. Safety and immunogenicity of a novel recombinant adenovirus type-5 vector-based Ebola vaccine in healthy adults in China: Preliminary report of a randomised, double-blind, placebo-controlled, phase 1 trial. Lancet 2015, 385, 2272–2279. [Google Scholar] [CrossRef]
- Buchbinder, S.P.; Mehrotra, D.V.; Duerr, A.; Fitzgerald, D.W.; Mogg, R.; Li, D.; Gilbert, P.B.; Lama, J.R.; Marmor, M.; Del Rio, C.; et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008, 372, 1881–1893. [Google Scholar] [CrossRef] [Green Version]
- Haas, E.J.; Angulo, F.J.; McLaughlin, J.M.; Anis, E.; Singer, S.R.; Khan, F.; Brooks, N.; Smaja, M.; Mircus, G.; Pan, K.; et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: An observational study using national surveillance data. Lancet 2021, 397, 1819–1829. [Google Scholar] [CrossRef]
- Mittelman, M.; Magen, O.; Barda, N.; Dagan, N.; Oster, H.S.; Leader, A.; Balicer, R. Effectiveness of the BNT162b2mRNA COVID-19 vaccine in patients with hematological neoplasms in a nationwide mass vaccination setting. Blood 2022, 139, 1439–1451. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Tauzin, A.; Nayrac, M.; Benlarbi, M.; Gong, S.Y.; Gasser, R.; Beaudoin-Bussières, G.; Brassard, N.; Laumaea, A.; Vézina, D.; Prévost, J.; et al. A single dose of the SARS-CoV-2 vaccine BNT162b2 elicits Fc-mediated antibody effector functions and T cell responses. Cell Host Microbe. 2021, 29, 1137–1150.e6. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.Y.; McMahan, K.; Yu, J.; Tostanoski, L.H.; Aguayo, R.; Ansel, J.; Chandrashekar, A.; Patel, S.; Apraku Bondzie, E.; Sellers, D.; et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA 2021, 325, 2370–2380. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Assessing the Programmatic Suitability of Vaccine Candidates for WHO Prequalification. Available online: https://apps.who.int/iris/bitstream/handle/10665/148168/WHO_IVB_14.10_eng.pdf;jsessionid=2B8144B8E176C5D64BCD778FA63C3110?sequence=1 (accessed on 10 January 2022).
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of infection, hospitalisation, and death up to 9 months after a second dose of COVID-19 vaccine: A retrospective, total population cohort study in Sweden. Lancet 2022, 399, 814–823. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 2119451. [Google Scholar] [CrossRef]
- Widge, A.T.; Rouphael, N.G.; Jackson, L.A.; Anderson, E.J.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Durability of Responses after SARS-CoV-2 mRNA-1273 Vaccination. N. Engl. J. Med. 2021, 384, 80–82. [Google Scholar] [CrossRef]
- Zhang, J.; He, Q.; An, C.; Mao, Q.; Gao, F.; Bian, L.; Wu, X.; Wang, Q.; Liu, P.; Song, L.; et al. Boosting with heterologous vaccines effectively improves protective immune responses of the inactivated SARS-CoV-2 vaccine. Emerg. Microbes Infect. 2021, 10, 1598–1608. [Google Scholar] [CrossRef]


| Vaccine Name | Technology | Developer/Company | Expiration Date | Immunization Protocol | Approved |
|---|---|---|---|---|---|
| CoronaVac | Inactivated vaccine | Sinovac Biotech Ltd. (Beijing, China) | 2–8 °C for 24 months | 2 doses (600SU/0.5 mL/dose), 2–4 weeks apart | WHO 2021.6.1 |
| BBIBP-CorV | Inactivated vaccine | Sinopharm Beijing Institute of Biotechnology (Beijing, China) | 2–8 °C for 24 months | 2 doses (6.5U/0.5 mL/dose), 3–4 weeks apart | WHO 2021.5.7 |
| Convidecia | Adenovirus vector vaccine | Cansino Biologics (Tianjin, China) | 2–8 °C for 12 months | 1 dose (5 × 1010 virus particles/0.5 mL) | China 2021.2. 25 |
| AZD1222 | Adenovirus vector vaccine | AstraZeneca (Cambridge, UK), Oxford University (Oxford, UK) | 2–8 °C for 6 months | 2 dose (5 × 1010 virus particles/0.5 mL), 4–12 weeks apart | WHO 2021.3.1 |
| Ad26.COV2.S | Adenovirus vector vaccine | Johnson & Johnson (New Brunswick, NJ, USA) | 2–8 °C for 3 months | 1 dose (5 × 1010 virus particles/0.5 mL) | WHO 2021.3.17 |
| Sputnik V | Adenovirus vector vaccine | Gamaleya Research Institute (Moscow, Russia) | −18 °C/2–8 °C | 2 dose (1011 viral particles /0.5 mL/dose), 2–3 weeks apart | Multiple countries without WHO |
| BNT162b2 | mRNA vaccine | Pfizer (New York, NY, USA)/BioNTech (Mainz, Germany) | Ultralow-temperature freezer for 6 months/−70 ± 10 °C for 10 days/2–8 °C for 5 days | 2 doses (30 μg/0.3 mL/dose), 3 weeks apart | WHO 2021.1.14 |
| mRNA-1273 | mRNA vaccine | Moderna (Cambridge, MA, USA) | Between −25 °C and −15 °C for supply/2–8 °C for 30 days | 2 doses (100 μg/0.5 mL/dose), 28 days apart | WHO 2021.2.3 |
| NVX-CoV2373 | Recombinant vaccine | Novavax and the Serum Institute of India (Pune, India) | 2–8 °C for 9 months | 2 doses (55 μg/0.5 mL/dose), 3–4 weeks apart | WHO 2021.12.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Ye, Q. Safety and Efficacy of the Common Vaccines against COVID-19. Vaccines 2022, 10, 513. https://doi.org/10.3390/vaccines10040513
Liu Y, Ye Q. Safety and Efficacy of the Common Vaccines against COVID-19. Vaccines. 2022; 10(4):513. https://doi.org/10.3390/vaccines10040513
Chicago/Turabian StyleLiu, Ying, and Qing Ye. 2022. "Safety and Efficacy of the Common Vaccines against COVID-19" Vaccines 10, no. 4: 513. https://doi.org/10.3390/vaccines10040513
APA StyleLiu, Y., & Ye, Q. (2022). Safety and Efficacy of the Common Vaccines against COVID-19. Vaccines, 10(4), 513. https://doi.org/10.3390/vaccines10040513





