Economic Value of Vaccines to Address the COVID-19 Pandemic in Hong Kong: A Cost-Effectiveness Analysis
Abstract
:1. Background
1.1. Interventions against the COVID-19 Pandemic in Hong Kong
1.2. The Vaccination Program of Hong Kong
1.3. A COVID-19 Vaccination Program May Be Expensive. Are the Benefits Worth the Costs?
2. Methods
2.1. Study Design
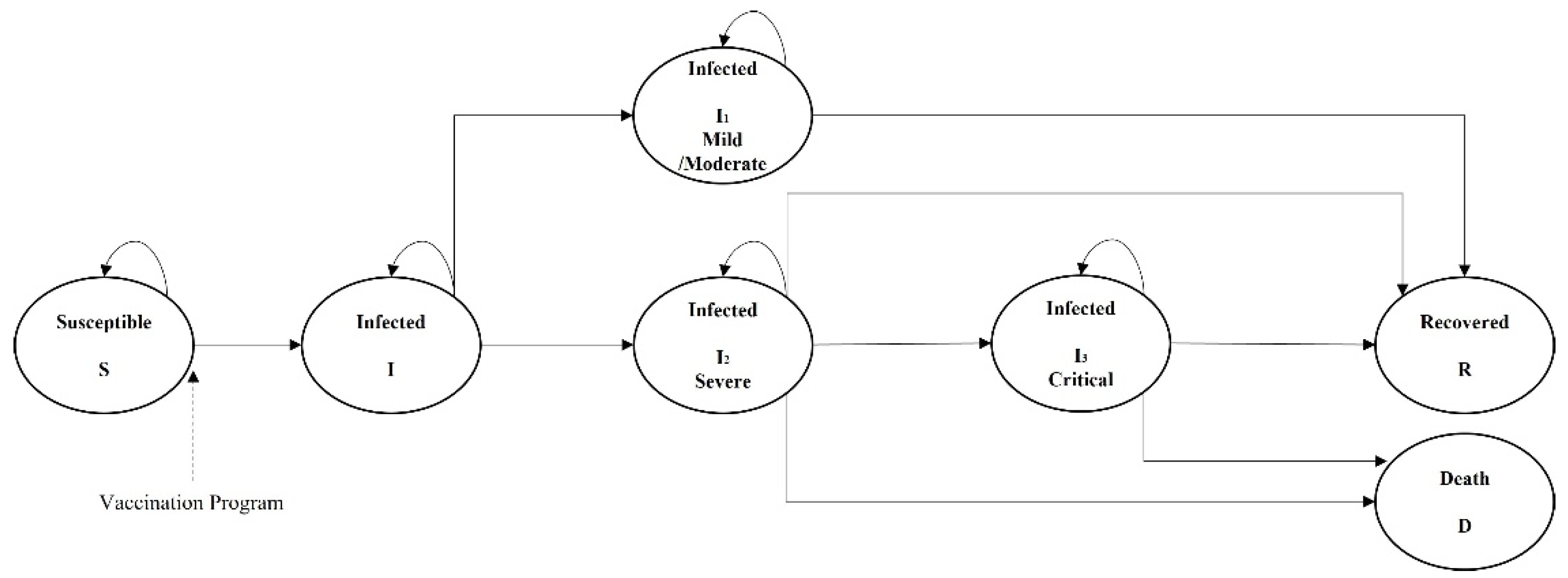
2.2. Model Structure
2.3. Model Parameters
2.3.1. Transition Probabilities
2.3.2. Vaccine Efficacy
2.3.3. Cost
2.3.4. Health Utilities
| Parameter | Parameter | Based Value | References |
|---|---|---|---|
| Transition Parameters | |||
| No vaccination Program | S to I | 0.12% | [2] |
| I to I1 | 95.75% | [36] | |
| I to I2 | 4.25% | ||
| I2 to I3 | 64.00% | ||
| I1 to R | 100% | ||
| I2/I3 to R | 62.35% | ||
| I2/I3 to D | 37.65% | ||
| Effect modification of Vaccine | |||
| Probability of vaccinated | 70.0% | [22] | |
| Age 3–11 | 1.8% | ||
| Age 12–19 | 66.9% | ||
| Age 20–59 | 86.2% | ||
| Age over 60 | 61.6% | ||
| Sinovac | Probability of vaccinated by Sinovac | 38.7% | [22] |
| Primary Efficacy for mild case | 83.70% [57.99–93.67%] | [41] | |
| Primary Efficacy for severe case | 100% [56.4–100%] | ||
| Primary Efficacy for death | 100% [56.4–100%] | Assumed | |
| Biontech | Probability of vaccinated by Biontech | 61.3% | [22] |
| Primary Efficacy for mild case | 91.3% [89.0–93.2%] | [38] | |
| Primary Efficacy for severe case | 95.3% [71.0–100%] | ||
| Primary Efficacy for death | 95.3% [71.0–100%] | Assumed | |
| Cost and Utility Parameters | |||
| Health care cost | Cost of Sinovac (per dose) | HKD 369 | [46] |
| Cost of Biontech (per dose) | HKD 369 | ||
| Operation cost (per dose) | HKD 196 | ||
| Cost of general ward/day | HKD 5100 | [48] | |
| Cost of ICU/day | HKD 24400 | ||
| Cost of Reverse transcription polymerase chain reaction (RT-PCR) Test | HKD 240 | [49] | |
| Productivity Loss | Loss of salary per person per day | HKD 600 | [44] |
| Utility Loss | Health utility loss of S Susceptible | 0.081 | [47] |
| Health utility loss of I1 Mild/moderate | 0.50 | [50] | |
| Health utility loss of I2 | 0.75 | [51,52] | |
| Health utility loss of I3 | 0.95 | [24] | |
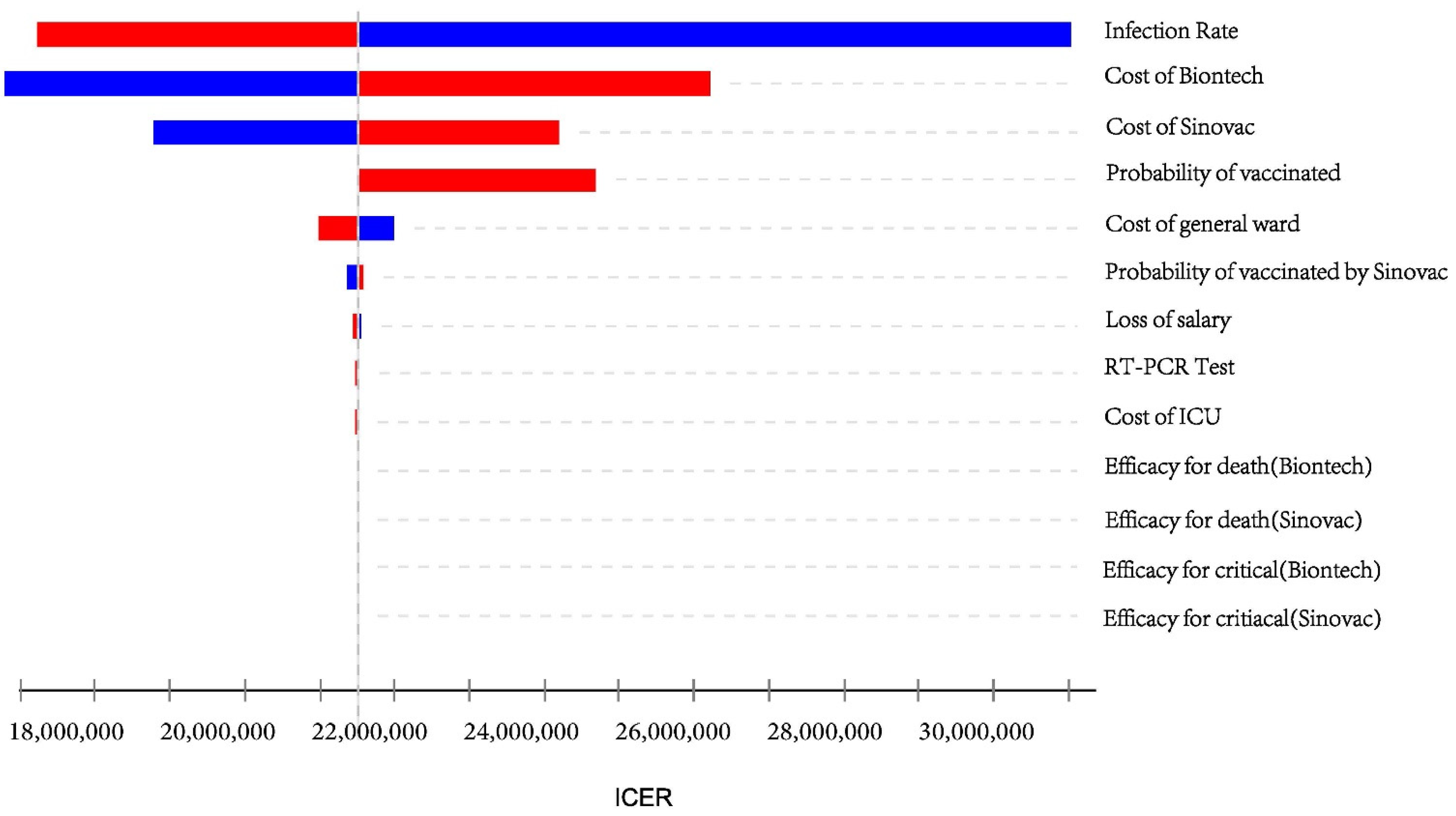
2.4. Sensitivity Analysis
3. Results
3.1. General Results
3.2. Sensitivity Analysis
3.3. Scenario Analysis
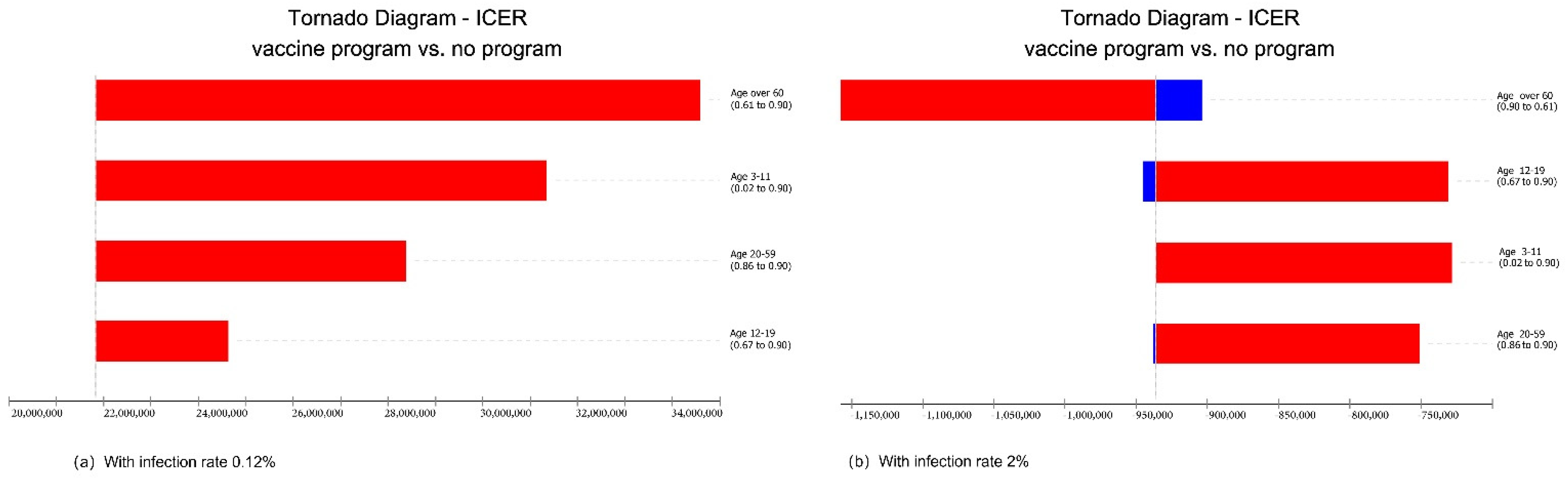
3.3.1. Changing Infection Rate
3.3.2. Changing Protection Rate
3.3.3. Changing Vaccination Rate
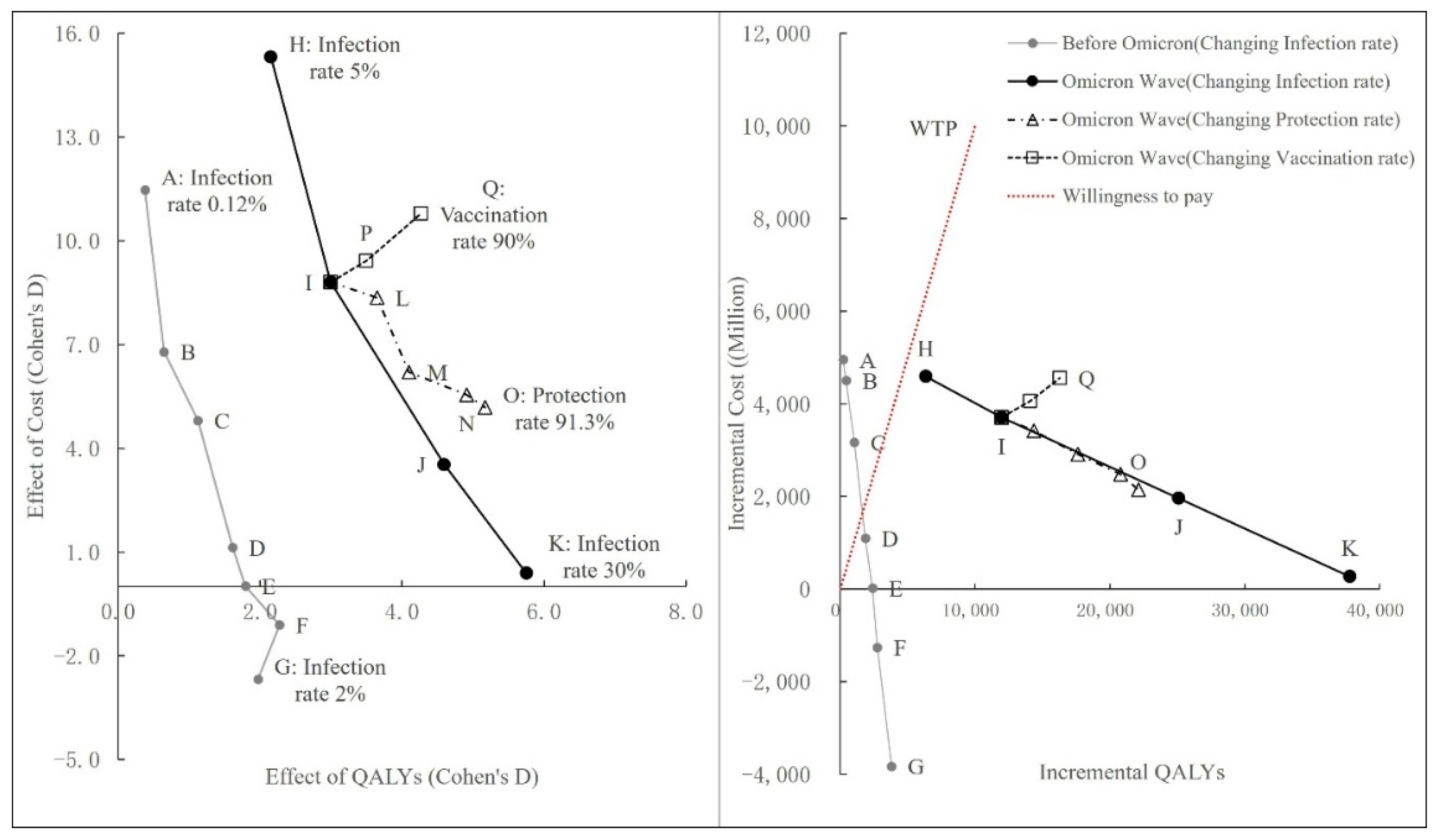
3.3.4. Summary of Scenario Analysis
4. Discussion
4.1. Main Findings
4.2. Limitations
4.3. Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. 2021. Available online: https://covid19.who.int/table (accessed on 3 March 2022).
- Coronavirus Disease (COVID-19) in HK. 2021. Available online: https://www.coronavirus.gov.hk/sim/index.html (accessed on 16 March 2022).
- Nicola, M.; Alsafi, Z.; Sohrabi, C.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, M.; Agha, R. The socio-economic implications of the coronavirus pandemic (COVID-19): A review. Int. J. Surg. 2020, 78, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Flaxman, S.; Mishra, S.; Gandy, A.; Unwin, H.J.T.; Mellan, T.A.; Coupland, H.; Whittaker, C.; Zhu, H.; Berah, T.; Eaton, J.W.; et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature 2020, 584, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.R.; Cook, A.R.; Park, M.; Sun, Y.; Sun, H.; Lim, J.T.; Tam, C.; Dickens, B.L. Interventions to mitigate early spread of SARS-CoV-2 in Singapore: A modelling study. Lancet Infect Dis. 2020, 20, 678–688. [Google Scholar] [CrossRef] [Green Version]
- Ten Years and Beyond; Centre for Health Protection: Hong Kong, China, 2014.
- Wan, K.M.; Ka-Ki Ho, L.; Wong, N.W.M.; Chiu, A. Fighting COVID-19 in Hong Kong: The effects of community and social mobilization. World Dev. 2020, 134, 105055. [Google Scholar] [CrossRef] [PubMed]
- Chow, L. Care homes and COVID-19 in Hong Kong: How the lessons from SARS were used to good effect. Age Ageing 2021, 50, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Control Points for Passengers. 2020. Available online: https://www.coronavirus.gov.hk/sim/control-points.html (accessed on 30 November 2021).
- Prevention and Control of Disease (Prohibition on Group Gathering) Regulation. 2020. Available online: https://www.elegislation.gov.hk/hk/cap599G!en?INDEX_CS=N (accessed on 30 November 2021).
- Prevention and Control of Disease (Wearing of Mask) Regulation. 2020. Available online: https://www.elegislation.gov.hk/hk/cap599I (accessed on 30 November 2021).
- Prevention and Control of Disease (Compulsory Testing for Certain Persons) Regulation. 2020. Available online: https://www.elegislation.gov.hk/hk/cap599J!en@2021-06-05T00:00:00?INDEX_CS=N (accessed on 30 November 2021).
- Designated Hotels for Quarantine. 2020. Available online: https://www.coronavirus.gov.hk/eng/designated-hotel.html (accessed on 30 November 2021).
- Zhang, R.; Liang, Z.; Pang, M.; Yang, X.; Wu, J.; Fang, Y.; Ji, H.; Qi, X. Mobility Trends and Effects on the COVID-19 Epidemic—Hong Kong, China. China CDC Wkly. 2021, 3, 159–161. [Google Scholar] [CrossRef]
- Lau, J.Y.C.; Lee, S.S. Legal provisions for enforcing social distancing to guard against COVID-19: The case of Hong Kong. J. Law Biosci. 2021, 8, lsab006. [Google Scholar] [CrossRef]
- Bonaccorsi, G.; Pierri, F.; Cinelli, M.; Flori, A.; Galeazzi, A.; Porcelli, F.; Schmidt, A.L.; Valensise, C.M.; Scala, A.; Quattrociocchi, W.; et al. Economic and social consequences of human mobility restrictions under COVID-19. Proc. Natl. Acad. Sci. USA 2020, 117, 15530–15535. [Google Scholar] [CrossRef]
- Jin, H.; Wang, H.; Li, X.; Zheng, W.; Ye, S.; Zhang, S.; Zhou, J.; Pennington, M. Economic burden of COVID-19, China, January-March, 2020: A cost-of-illness study. Bull. World Health Organ. 2021, 99, 112–124. [Google Scholar] [CrossRef]
- COVID-19 Vaccines. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines (accessed on 30 November 2021).
- Vaccine Pass. Available online: https://www.coronavirus.gov.hk/sim/vaccine-pass.html (accessed on 19 March 2022).
- Home Quarantine. Available online: https://www.coronavirus.gov.hk/sim/home-quarantine.html (accessed on 19 March 2022).
- Government to Relax Social Distancing Measures under “Vaccine Bubble”. 2021. Available online: https://www.info.gov.hk/gia/general/202104/28/P2021042800868.htm?fontSize=1 (accessed on 30 November 2021).
- Hong Kong Vaccination Dashboard. Available online: https://www.covidvaccine.gov.hk/en/dashboard (accessed on 1 March 2022).
- HKFP. Available online: https://hongkongfp.com/2022/02/26/factwire-sinovac-limits-hong-kongs-protection-against-infection-from-omicron-analysis-suggests/ (accessed on 15 March 2022).
- Marco-Franco, J.E.; Pita-Barros, P.; González-de-Julián, S.; Sabat, I.; Vivas-Consuelo, D. Simplified Mathematical Modelling of Uncertainty: Cost-Effectiveness of COVID-19 Vaccines in Spain. Mathematics 2021, 9, 566. [Google Scholar] [CrossRef]
- Mauskopf, J.; Standaert, B.; Connolly, M.P.; Culyer, A.J.; Garrison, L.P.; Hutubessy, R.; Jit, M.; Pitman, R.; Revill, P.; Severens, J.L. Economic Analysis of Vaccination Programs: An ISPOR Good Practices for Outcomes Research Task Force Report. Value Health 2018, 21, 1133–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, N.; Jit, M.; Cox, S.; Yoong, J.; Hutubessy, R.C.W. Capturing Budget Impact Considerations Within Economic Evaluations: A Systematic Review of Economic Evaluations of Rotavirus Vaccine in Low- and Middle-Income Countries and a Proposed Assessment Framework. Pharmacoeconomics 2018, 36, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chitiga-Mabugu, M.; Henseler, M.; Mabugu, R.; Maisonnave, H. Economic and Distributional Impact of COVID-19: Evidence from Macro-Micro Modelling of the South African Economy. S. Afr. J. Econ. 2021, 89, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.M.C.; Plotkin, S.A. Impact of Vaccines; Health, Economic and Social Perspectives. Front. Microbiol. 2020, 11, 1526. [Google Scholar] [CrossRef] [PubMed]
- Janssens, W.; Pradhan, M.; de Groot, R.; Sidze, E.; Donfouet, H.P.P.; Abajobir, A. The short-term economic effects of COVID-19 on low-income households in rural Kenya: An analysis using weekly financial household data. World Dev. 2021, 138, 105280. [Google Scholar] [CrossRef]
- Sanders, G.D.; Neumann, P.J.; Basu, A.; Brock, D.W.; Feeny, D.; Krahn, M.; Kuntz, K.M.; Meltzer, D.O.; Owens, D.K.; Prosser, L.A.; et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 2016, 316, 1093–1103. [Google Scholar] [CrossRef]
- Marseille, E.; Larson, B.; Kazi, D.S.; Kahn, J.G.; Rosen, S. Thresholds for the cost-effectiveness of interventions: Alternative approaches. Bull. World Health Organ. 2015, 93, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Gross Domestic Product (GDP), Implicit Price Deflator of GDP and per Capita GDP. Available online: https://www.censtatd.gov.hk/en/web_table.html?id=31 (accessed on 30 November 2021).
- Interim Clinical Guidance for Management of Patients with Confirmed Coronavirus Disease (COVID-19). Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html (accessed on 30 November 2021).
- Those Confirmed to have been Vaccinated will be Hospitalized for 3 Days Less. Available online: https://www.wenweipo.com/a/202110/27/AP6178addde4b0c0f6bc91fc10.html (accessed on 30 November 2021).
- Rees, E.M.; Nightingale, E.S.; Jafari, Y.; Waterlow, N.R.; Clifford, S.; BPearson, C.A.; Jombart, T.; Procter, S.R.; Knight, G.M. COVID-19 length of hospital stay: A systematic review and data synthesis. BMC Med. 2020, 18, 270. [Google Scholar] [CrossRef]
- HK01. Available online: https://www.hk01.com/%E7%A4%BE%E6%9C%83%E6%96%B0%E8%81%9E/516409/%E6%96%B0%E5%86%A0%E8%82%BA%E7%82%8E-%E6%B8%AF%E6%82%A3%E8%80%85%E6%AD%BB%E4%BA%A1%E7%8E%871-6-%E9%81%A0%E4%BD%8E%E6%96%BC%E6%B5%B7%E5%A4%96-%E7%94%B7%E5%A5%B3%E5%85%AD%E5%9B%9B%E6%AF%94 (accessed on 30 November 2021).
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines 2021, 6, 28. [Google Scholar] [CrossRef]
- Pfizer and BioNTech Concluse Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints. Available online: https://www.pfizer.com/news/press-release/pressrelease-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine (accessed on 30 November 2021).
- Palacios, R.; Patiño, E.G.; de Oliveira Piorelli, R.; Conde, M.T.R.P.; Batista, A.P.; Zeng, G.; Xin, Q.; Kallas, E.G.; Flores, J.; Ockenhouse, C.F.; et al. Double-Blind, Randomized, Placebo-Controlled Phase III Clinical Trial to Evaluate the Efficacy and Safety of treating Healthcare Professionals with the Adsorbed COVID-19 (Inactivated) Vaccine Manufactured by Sinovac—PROFISCOV: A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 853. [Google Scholar]
- McMenamin, M.E.; Cowling, B.J. CoronaVac efficacy data from Turkey. Lancet 2021, 398, 1873–1874. [Google Scholar] [CrossRef]
- Palacios, R.; Batista, A.P.; Albuquerque, C.S.N.; Patiño, E.G.; Santos, J.D.P.; Tilli Reis Pessoa Conde, M.; Piorelli, R.D.O.; Pereira Júnior, L.C.; Raboni, S.M.; Ramos, F. Efficacy and Safety of a COVID-19 Inactivated Vaccine in Healthcare Professionals in Brazil: The PROFISCOV Study. SSRN. 2021. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3822780 (accessed on 30 November 2021).
- Tartof, S.Y.; Slezak, J.M.; Fischer, H.; Hong, V.; Ackerson, B.K.; Ranasinghe, O.N.; Frankland, T.B.; Ogun, O.A.; Zamparo, J.M.; Gray, S.; et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: A retrospective cohort study. Lancet 2021, 398, 1407–1416. [Google Scholar] [CrossRef]
- Cohn, B.A.; Cirillo, P.M.; Murphy, C.C.; Krigbaum, N.Y.; Wallace, A.W. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science 2021, 375, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Wages and Labor Income. Available online: https://www.censtatd.gov.hk/tc/scode210.html (accessed on 30 November 2021).
- Molinari, N.A.M.; Ortega-Sanchez, I.R.; Messonnier, M.L.; Thompson, W.W.; Wortley, P.M.; Weintraub, E.; Bridges, C.B. The annual impact of seasonal influenza in the US: Measuring disease burden and costs. Vaccine 2007, 25, 5086–5096. [Google Scholar] [CrossRef]
- The 2021–2022 Budget. Available online: https://www.budget.gov.hk/2021/eng/budget04.html (accessed on 30 November 2021).
- Wong, E.L.; Cheung, A.W.; Wong, A.Y.; Xu, R.H.; Ramos-Goñi, J.M.; Rivero-Arias, O. Normative Profile of Health-Related Quality of Life for Hong Kong General Population Using Preference-Based Instrument EQ-5D-5L. Value Health 2019, 22, 916–924. [Google Scholar] [CrossRef]
- Fees and Charges. Available online: https://www.ha.org.hk/visitor/ha_visitor_index.asp?Content_ID=10045&Lang=CHIB5&Dimension=100&Parent_ID=10044 (accessed on 30 November 2021).
- The Fee Is Capped at 240 Yuan. Available online: https://wealth.hket.com/article/2811053/%E3%80%90%E6%A0%B8%E9%85%B8%E6%AA%A2%E6%B8%AC%E3%80%91%E9%86%AB%E7%99%82%E6%AA%A2%E6%B8%AC%E6%A9%9F%E6%A7%8B%E6%96%B0%E5%86%A0%E7%97%85%E6%AF%92%E6%B8%AC%E8%A9%A6%E6%94%B6%E8%B2%BB%E5%8F%8A%E9%9C%80%E6%99%82%E6%AF%94%E8%BC%83%EF%BC%88%E8%B3%87%E6%96%99%E6%9B%B4%E6%96%B0%E8%87%B32021%E5%B9%B412%E6%9C%886%E6%97%A5%EF%BC%89 (accessed on 30 November 2021).
- Kohli, M.; Maschio, M.; Becker, D.; Weinstein, M.C. The potential public health and economic value of a hypothetical COVID-19 vaccine in the United States: Use of cost-effectiveness modeling to inform vaccination prioritization. Vaccine 2021, 39, 1157–1164. [Google Scholar] [CrossRef]
- Khan, K.; Muennig, P.; Gardam, M.; Zivin, J.G. Managing febrile respiratory illnesses during a hypothetical SARS outbreak. Emerg. Infect. Dis. 2005, 11, 191–200. [Google Scholar] [CrossRef]
- You, J.H.; Ming, W.K.; Chan, P.K. Cost-effectiveness of quadrivalent influenza vaccine in Hong Kong—A decision analysis. Hum. Vaccin Immunother. 2015, 11, 564–571. [Google Scholar] [CrossRef] [Green Version]
- Briggs, A.H. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics 2000, 17, 479–500. [Google Scholar] [CrossRef]
- Padula, W.V.; Malaviya, S.; Reid, N.M.; Cohen, B.G.; Chingcuanco, F.; Ballreich, J.; Tierce, J.; Alexander, G.C. Economic value of vaccines to address the COVID-19 pandemic: A U.S. cost-effectiveness and budget impact analysis. J. Med. Econ. 2021, 24, 1060–1069. [Google Scholar] [CrossRef]
- Debrabant, K.; Grønbæk, L.; Kronborg, C. The Cost-Effectiveness of a COVID-19 Vaccine in a Danish Context. Clin. Drug Investig. 2021, 41, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Sandmann, F.G.; Davies, N.G.; Vassall, A.; Edmunds, W.J.; Jit, M. The potential health and economic value of SARS-CoV-2 vaccination alongside physical distancing in the UK: A transmission model-based future scenario analysis and economic evaluation. Lancet Infect. Dis. 2021, 21, 962–974. [Google Scholar] [CrossRef]
- Zhang, J.; Dean, J.; Yin, Y.; Wang, D.; Sun, Y.; Zhao, Z.; Wang, J. Determinants of COVID-19 Vaccine Acceptance and Hesitancy: A Health Care Student-Based Online Survey in Northwest China. Front. Public Health 2022, 9, 777565. [Google Scholar] [CrossRef] [PubMed]
- Solís Arce, J.S.; Warren, S.S.; Meriggi, N.F.; Scacco, A.; McMurry, N.; Voors, M.; Syunyaev, G.; Malik, A.A.; Aboutajdine, S.; Adeojo, O.; et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 2021, 27, 1385–1394. [Google Scholar] [CrossRef]
- Gallè, F.; Sabella, E.A.; Roma, P.; Da Molin, G.; Diella, G.; Montagna, M.T.; Ferracuti, S.; Liguori, G.; Orsi, G.B.; Napoli, C. Acceptance of COVID-19 Vaccination in the Elderly: A Cross-Sectional Study in Southern Italy. Vaccines 2021, 9, 1222. [Google Scholar] [CrossRef]
- Population Clock. Available online: https://www.census.gov/popclock/ (accessed on 10 March 2022).
- Statistics and Research Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 10 March 2022).
- Statistics on Locally Confirmed Cases and Deaths of COVID-19. Available online: https://sites.google.com/cdc.gov.tw/2019ncov/taiwan (accessed on 10 March 2022).
- Demographic Data. Available online: https://www.ris.gov.tw/app/portal/346 (accessed on 25 December 2021).
- Statistics and Research Coronavirus (COVID-19) Vaccinations. 2021. Available online: https://ourworldindata.org/covid-vaccinations?country=TWN (accessed on 10 March 2022).
- Wang, Y.; Dong, C.; Hu, Y.; Li, C.; Ren, Q.; Zhang, X.; Shi, H.; Zhou, M. Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study. Radiology 2020, 296, E55–E64. [Google Scholar] [CrossRef] [Green Version]
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.W.; Pollak, T.A.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M.; et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry 2020, 7, 875–882. [Google Scholar] [CrossRef]
- Schoch-Spana, M.; Brunson, E.K.; Long, R.; Ruth, A.; Ravi, S.J.; Trotochaud, M.; Borio, L.; Brewer, J.; Buccina, J.; Connell, N.; et al. The public’s role in COVID-19 vaccination: Human-centered recommendations to enhance pandemic vaccine awareness, access, and acceptance in the United States. Vaccine 2021, 39, 6004–6012. [Google Scholar] [CrossRef]
| Scenario | Cost (HKD Billion) | Outcome | ICER | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Infection Rate | Comparators | Cases | Death | QALYs | HKD/Case | HKD/Death | HKD/QALYs | ||
| 0.12% (Before Omicron) | No Vaccine | 0.84 | 9630 | 269 | 7,393,955 | ||||
| Vaccination Program | 5.80 | 3704 | 67 | 7,394,177 | 836,364 | 24,533,333 | 22,339,700 | ||
| 10.0% (Omicron Wave) | Lower Protect | No Vaccine | 85.1 | 953,215 | 25,053 | 7,358,898 | |||
| Vaccination Program | 73.8 | 831,656 | 8620 | 7,372,388 | −31,468 | −687,705 | −837,699 | ||
| Home quarantine | No Vaccine | 5.1 | 954,158 | 23,975 | 7,359,845 | ||||
| Vaccination Program | 7.4 | 355,385 | 8688 | 7,381,766 | −6388 | 146,696 | 102,303 | ||
| Home quarantine and Lower Protect | No Vaccine | 5.1 | 948,703 | 24,043 | 7,360,067 | ||||
| Vaccination Program | 8.8 | 827,682 | 9092 | 7,372,033 | −31,608 | 248,198 | 310,094 | ||
| Scenario | Cost (HKD Billion) | Outcome | ICER | Cohen’s D | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Basic Infection Rate | Comparators | Cases | Death | QALYs | HKD/Case | HKD/Death | HKD/QALYs | Cost | QALYs | ||
| A | 0.12% | No Vaccine | 0.8 | 9630 | 269 | 7,393,955 | |||||
| Vaccination Program | 5.8 | 3704 | 67 | 7,394,177 | 836,364 | 24,533,333 | 22,339,700 | 11.5 | 0.4 | ||
| B | 025% | No Vaccine | 1.6 | 15,422 | 539 | 7,393,519 | |||||
| Vaccination Program | 6.2 | 5657 | 202 | 7,393,962 | 461,379 | 13,380,000 | 10,153,029 | 6.8 | 0.7 | ||
| C | 0.5% | No Vaccine | 3.6 | 40,273 | 1145 | 7,392,690 | |||||
| Vaccination Program | 6.8 | 14,681 | 404 | 7,393,740 | 123,684 | 4,272,727 | 3,013,910 | 4.8 | 1.1 | ||
| D | 1% | No Vaccine | 6.9 | 77,717 | 2424 | 7,391,470 | |||||
| Vaccination Program | 8.0 | 29,093 | 875 | 7,393,334 | 22,576 | 708,696 | 588,990 | 1.1 | 1.6 | ||
| E | 1.2% | No Vaccine | 8.6 | 97,315 | 2559 | 7,390,538 | |||||
| Vaccination Program | 8.7 | 35,626 | 943 | 7,392,934 | 17,795 | 12,500 | 8431 | 0.0 | 1.8 | ||
| F | 1.5% | No Vaccine | 10.6 | 118,529 | 2829 | 7,390,072 | |||||
| Vaccination Program | 9.3 | 43,910 | 808 | 7,392,831 | −16,877 | −623,333 | −456,513 | −1.1 | 2.3 | ||
| G | 2% | No Vaccine | 14.5 | 160,216 | 4175 | 7,388,097 | |||||
| Vaccination Program | 10.6 | 59,669 | 1549 | 7,391,899 | −38,044 | −1,456,410 | −1,006,250 | −2.7 | 2.0 | ||
| H* | 5% | No Vaccine | 2.6 | 475,867 | 12,392 | 7,376,796 | |||||
| Vaccination Program | 7.2 | 415,323 | 4512 | 7,383,127 | −63,181 | 582,906 | 725,490 | 15.3 | 2.2 | ||
| I* | 10% | No Vaccine | 5.1 | 948,703 | 24,043 | 7,360,067 | |||||
| Vaccination Program | 8.8 | 827,682 | 9092 | 7,372,033 | −31,608 | 248,198 | 310,094 | 8.8 | 3.0 | ||
| J* | 20% | No Vaccine | 10.2 | 1,885,755 | 49,499 | 7,325,343 | |||||
| Vaccination Program | 12.2 | 1,646,812 | 17,443 | 7,350,423 | −16,009 | 61,345 | 78,411 | 3.5 | 4.6 | ||
| K* | 30% | No Vaccine | 15.1 | 2,795,734 | 73,138 | 7,291,951 | |||||
| Vaccination Program | 15.3 | 2,448,094 | 24,985 | 7,329,714 | −11,003 | 5734 | 7312 | 0.4 | 5.8 | ||
| Scenario | Cost (HKD Billion) | Outcome | ICER | Cohen’s D | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Efficacy for Mild | Comparators | Cases | Death | QALYs | HKD/Case | HKD/Death | HKD/QALYs | Cost | QALYs | ||
| I | 16.5% | No Vaccine | 5.1 | 948,703 | 24,043 | 7,360,067 | |||||
| Vaccination Program | 8.8 | 827,682 | 9092 | 7,372,033 | −31,608 | 248,198 | 310,094 | 8.8 | 3.0 | ||
| L | 30.0% | No Vaccine | 5.2 | 941,834 | 23,504 | 7,360,363 | |||||
| Vaccination Program | 8.5 | 723,229 | 8553 | 7,374,733 | −17,498 | 228,378 | 237,605 | 8.4 | 3.7 | ||
| M | 45.6% | No Vaccine | 5.2 | 953,821 | 24,716 | 7,359,623 | |||||
| Vaccination Program | 8.1 | 601,871 | 8082 | 7,377,233 | −10,869 | 174,899 | 165,213 | 6.2 | 4.1 | ||
| N | 70.0% | No Vaccine | 5.2 | 947,558 | 25,996 | 7,358,802 | |||||
| Vaccination Program | 7.7 | 462,600 | 9092 | 7,379,585 | −7888 | 146,614 | 119,251 | 5.6 | 4.9 | ||
| O | 91.3% | No Vaccine | 5.1 | 951,060 | 24,851 | 7,359,934 | |||||
| Vaccination Program | 7.3 | 33,4036 | 9024 | 7,382,040 | −6200 | 135,319 | 96,877 | 5.2 | 5.2 | ||
| Scenario | Cost (HKD Billion) | Outcome | ICER | Cohen’s D | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccination Rate | Comparators | Cases | Death | QALYs | HKD/Case | HKD/Death | HKD/QALYs | Cost | QALYs | ||
| I | 72% | No Vaccine | 5.1 | 948,703 | 24,043 | 7,360,067 | |||||
| Vaccination Program | 8.8 | 827,682 | 9092 | 7,372,033 | −31,608 | 248,198 | 310,094 | 8.8 | 3.0 | ||
| P | 80% | No Vaccine | 5.2 | 950,791 | 25,053 | 7,359,512 | |||||
| Vaccination Program | 9.5 | 815,223 | 7004 | 7,373,594 | −28,217 | 225,000 | 288,384 | 9.4 | 3.5 | ||
| Q | 90% | No Vaccine | 5.2 | 947,356 | 25,120 | 7,359,453 | |||||
| Vaccination Program | 9.7 | 798,589 | 4512 | 7,375,754 | −25,713 | 220,915 | 279,290 | 10.8 | 4.3 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, X.; Li, J.; Huang, B.; Tam, T.; Hong, Y.; Chong, K.-C.; Huo, Z. Economic Value of Vaccines to Address the COVID-19 Pandemic in Hong Kong: A Cost-Effectiveness Analysis. Vaccines 2022, 10, 495. https://doi.org/10.3390/vaccines10040495
Xiong X, Li J, Huang B, Tam T, Hong Y, Chong K-C, Huo Z. Economic Value of Vaccines to Address the COVID-19 Pandemic in Hong Kong: A Cost-Effectiveness Analysis. Vaccines. 2022; 10(4):495. https://doi.org/10.3390/vaccines10040495
Chicago/Turabian StyleXiong, Xuechen, Jing Li, Bo Huang, Tony Tam, Yingyi Hong, Ka-Chun Chong, and Zhaohua Huo. 2022. "Economic Value of Vaccines to Address the COVID-19 Pandemic in Hong Kong: A Cost-Effectiveness Analysis" Vaccines 10, no. 4: 495. https://doi.org/10.3390/vaccines10040495
APA StyleXiong, X., Li, J., Huang, B., Tam, T., Hong, Y., Chong, K.-C., & Huo, Z. (2022). Economic Value of Vaccines to Address the COVID-19 Pandemic in Hong Kong: A Cost-Effectiveness Analysis. Vaccines, 10(4), 495. https://doi.org/10.3390/vaccines10040495








