Immunogenicity and Safety of Childhood Combination Vaccines: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
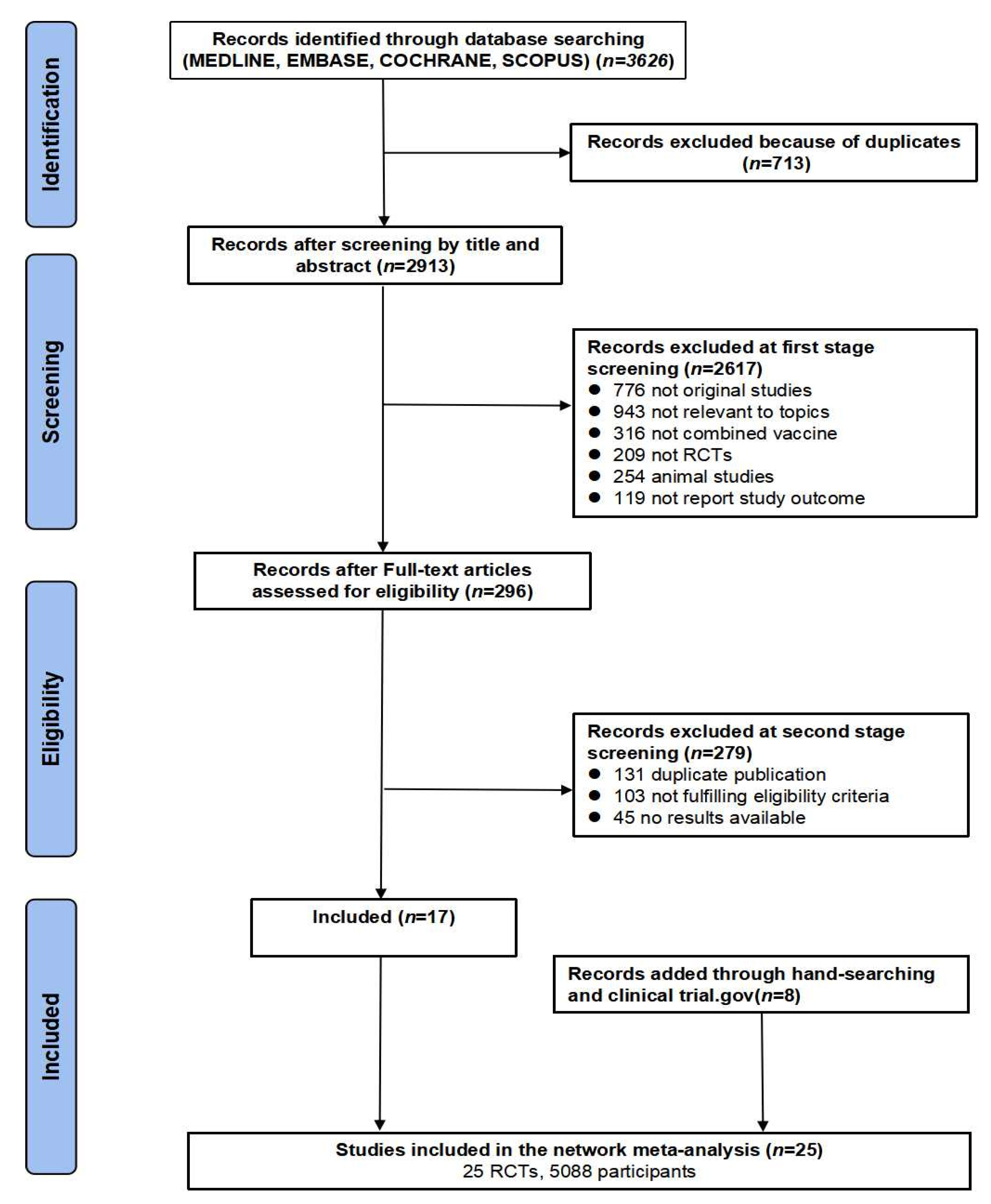
2.1. Literature Search
2.2. Selection Criteria
2.3. Data Extraction and Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Pooled Immunogenicity of Combined Vaccine
3.3. Pooled Acceptability of Combined Vaccines
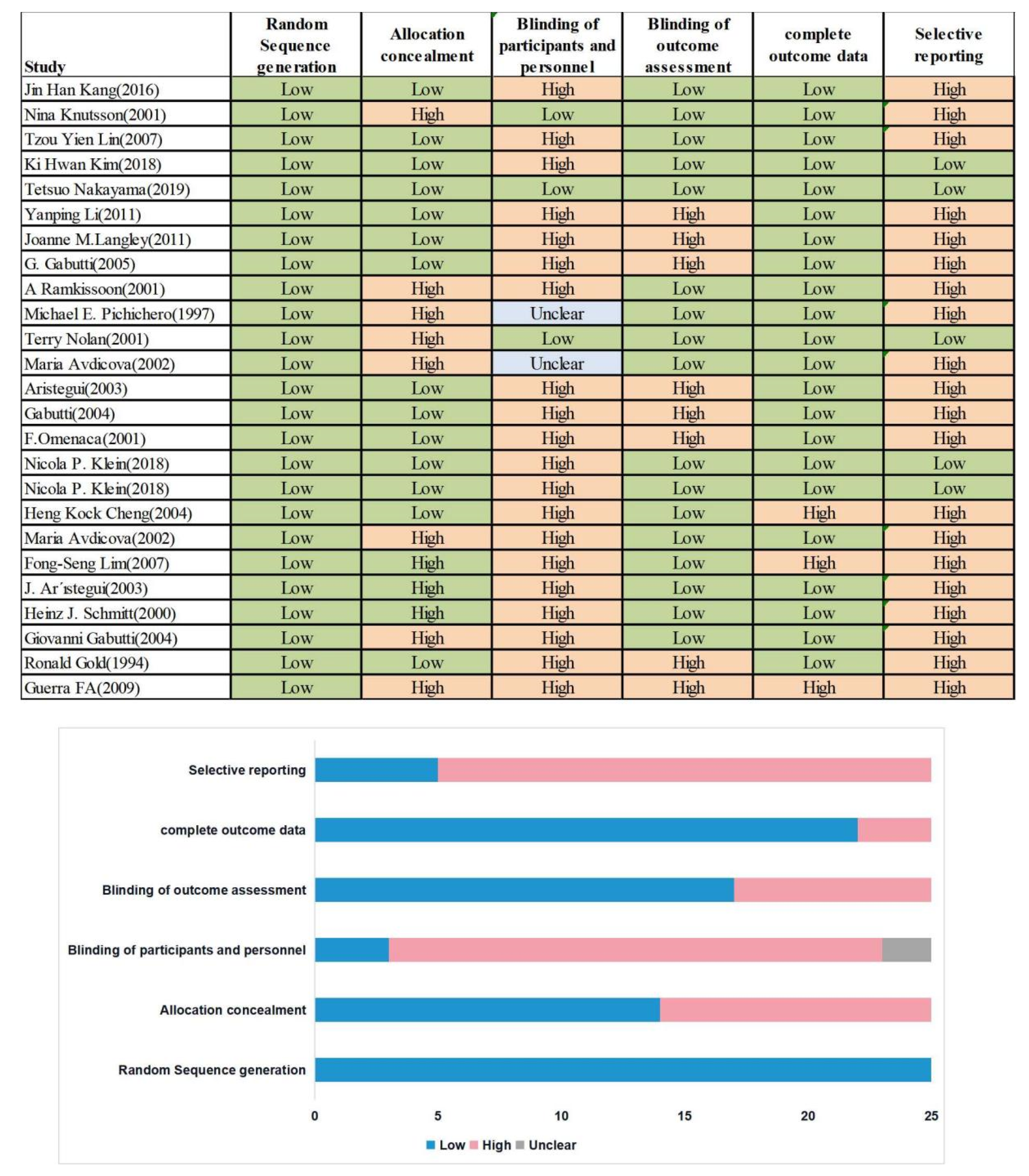
3.4. Risk of Bias and Quality Assessment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meng, Q.; Sun, Y.; Cong, H.; Hu, H.; Xu, F.J. An overview of chitosan and its application in infectious diseases. Drug Deliv. Transl. Res. 2021, 11, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Ehreth, J. The global value of vaccination. Vaccine 2003, 21, 596–600. [Google Scholar] [CrossRef]
- Patel, V.; Saxena, S.; Lund, C.; Thornicroft, G.; Baingana, F.; Bolton, P.; Chisholm, D.; Collins, P.Y.; Cooper, J.L.; Eaton, J.; et al. The Lancet Commission on global mental health and sustainable development. Lancet 2018, 392, 1553–1598. [Google Scholar] [CrossRef]
- World Health Organization. Vaccine Introduction Guidelines. 2018. Available online: http://www.who.int/immunization/hpv/plan/vaccine:introduction_guidelines_who_2018.pdf.2018 (accessed on 5 February 2022).
- World Health Organization. Global Immunization Vision and Strategy, 2006–2015. 2005. Available online: http://whqlibdoc.who.int/hq/2005/WHO_IVB_05.05.pdf.2005. (accessed on 5 February 2022).
- Galazka, A.M.; Robertson, S.E. Immunization against diphtheria with special emphasis on immunization of adults. Vaccine 1996, 14, 845–857. [Google Scholar] [CrossRef]
- Hanifi, S.M.A.; Fisker, A.B.; Welaga, P.; Rieckmann, A.; Jensen, A.G.; Benn, C.S.; Aaby, P. Diphtheria-Tetanus-Pertussis (DTP) Vaccine Is Associated With Increased female-Male Mortality. Studies of DTP administered before and after measles vaccine. J. Infect. Dis. 2021, 223, 1984–1991. [Google Scholar] [CrossRef]
- Tafreshi, S.H. Efficacy, safety, and formulation issues of the combined vaccines. Expert Rev. Vaccines 2020, 19, 949–958. [Google Scholar] [CrossRef]
- Chitkara, A.J.; Parikh, R.; Mihalyi, A.; Kolhapure, S. Hexavalent Vaccines in India: Current Status. Indian Pediatr. 2019, 56, 939–950. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Combination Vaccines. Available online: https://www.cdc.gov/vaccines/parents/why-vaccinate/combination-vaccines.html (accessed on 5 February 2022).
- Maman, K.; Zöllner, Y.; Greco, D.; Duru, G.; Sendyona, S.; Remy, V. The value of childhood combination vaccines: From beliefs to evidence. Hum. Vaccines Immunother. 2015, 11, 2132–2141. [Google Scholar] [CrossRef]
- Johns, T.L.; Hutter, G.E. New combination vaccines: DTaP-IPV (Kinrix) and DTaP-IPV/Hib (Pentacel). Ann. Pharmacother. 2010, 44, 515–523. [Google Scholar] [CrossRef]
- Smith, L.E.; Amlôt, R.; Weinman, J.; Yiend, J.; Rubin, G.J. A systematic review of factors affecting vaccine uptake in young children. Vaccine 2017, 35, 6059–6069. [Google Scholar] [CrossRef]
- Karafillakis, E.; Larson, H.J. The benefit of the doubt or doubts over benefits? A systematic literature review of perceived risks of vaccines in European populations. Vaccine 2017, 35, 4840–4850. [Google Scholar] [CrossRef] [PubMed]
- Baldo, V.; Bonanni, P.; Castro, M.; Gabutti, G.; Franco, E.; Marchetti, F.; Prato, R.; Vitale, F. Combined hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated poliovirus-Haemophilus influenzae type B vaccine; Infanrix™ hexa: Twelve years of experience in Italy. Hum. Vaccines Immunother. 2014, 10, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Vidor, E.; Tsuzuki, D.; Nishina, S.; Sasaki, T.; Ishii, Y.; Mizukami, H.; Tsuge, H. Immunogenicity and safety of a DTaP-IPV/Hib pentavalent vaccine given as primary and booster vaccinations in healthy infants and toddlers in Japan. J. Infect. Chemother. 2020, 26, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Avdicova, M.; Crasta, P.D.; Hardt, K.; Kovac, M. Lasting immune memory against hepatitis B following challenge 10–11 years after primary vaccination with either three doses of hexavalent DTPa-HBV-IPV/Hib or monovalent hepatitis B vaccine at 3, 5 and 11–12 months of age. Vaccine 2015, 33, 2727–2733. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Lee, H.J.; Kim, K.H.; Oh, S.H.; Cha, S.H.; Lee, J.; Kim, N.H.; Eun, B.W.; Kim, C.H.; Hong, Y.J.; et al. The Immunogenicity and Safety of a Combined DTaP-IPV//Hib Vaccine Compared with Individual DTaP-IPV and Hib (PRP~T) Vaccines: A Randomized Clinical Trial in South Korean Infants. J. Korean Med. Sci. 2016, 31, 1383–1391. [Google Scholar] [CrossRef]
- Bar-On, E.S.; Goldberg, E.; Fraser, A.; Vidal, L.; Hellmann, S.; Leibovici, L. Combined DTP-HBV-HIB vaccine versus separately administered DTP-HBV and HIB vaccines for primary prevention of diphtheria, tetanus, pertussis, hepatitis B and Haemophilus influenzae B (HIB). Cochrane Database Syst. Rev. 2009, Cd005530. [Google Scholar] [CrossRef]
- World Health Organization. Vaccine-Preventable Diseases and Vaccines; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Farrington, P.; Pugh, S.; Colville, A.; Flower, A.; Nash, J.; Morgan-Capner, P.; Rush, M.; Miller, E. A new method for active surveillance of adverse events from diphtheria/tetanus/pertussis and measles/mumps/rubella vaccines. Lancet 1995, 345, 567–569. [Google Scholar] [CrossRef]
- Sun, Y.; Christensen, J.; Hviid, A.; Li, J.; Vedsted, P.; Olsen, J.; Vestergaard, M. Risk of Febrile Seizures and Epilepsy After Vaccination With Diphtheria, Tetanus, Acellular Pertussis, Inactivated Poliovirus, and Haemophilus Influenzae Type b. Jama-J. Am. Med. Assoc. 2012, 307, 823–831. [Google Scholar] [CrossRef]
- Traversa, G.; Spila-Alegiani, S.; Bianchi, C.; Ciofi degli Atti, M.; Frova, L.; Massari, M.; Raschetti, R.; Salmaso, S.; Scalia Tomba, G. Sudden unexpected deaths and vaccinations during the first two years of life in Italy: A case series study. PLoS ONE 2011, 6, e16363. [Google Scholar] [CrossRef]
- Puliyel, J.; Sathyamala, C. Infanrix hexa and sudden death: A review of the periodic safety update reports submitted to the European Medicines Agency. Indian J. Med. Ethics 2018, 3, 43–47. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Rappuoli, R. A crisis of public confidence in vaccines. Sci. Transl. Med. 2010, 2, 61mr1. [Google Scholar] [CrossRef] [PubMed]
- Le, C.T. Combination vaccines: Choices or chaos? A practitioner’s perspective. Clin. Infect. Dis. 2001, 33 (Suppl. S4), S367–S371. [Google Scholar] [CrossRef] [PubMed]
- Konini, A.; Kang, M.; Moghadas, S.M. Simulating Immune Interference on the Effect of a Bivalent Glycoconjugate Vaccine against Haemophilus influenzae Serotypes “a” and “b”. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 5486869. [Google Scholar] [CrossRef]
- Halperin, S.A.; King, J.; Law, B.; Mills, E.; Willems, P. Safety and immunogenicity of Haemophilus influenzae-tetanus toxoid conjugate vaccine given separately or in combination with a three-component acellular pertussis vaccine combined with diphtheria and tetanus toxoids and inactivated poliovirus vaccine for the first four doses. Clin. Infect. Dis. 1999, 28, 995–1001. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Li, R.C.; Ye, Q.; Li, C.; Liu, Y.P.; Ma, X.; Li, Y.; Zhao, H.; Chen, X.; Assudani, D.; et al. Safety, immunogenicity and persistence of immune response to the combined diphtheria, tetanus, acellular pertussis, poliovirus and Haemophilus influenzae type b conjugate vaccine (DTPa-IPV/Hib) administered in Chinese infants. Hum. Vaccines Immunother. 2017, 13, 588–598. [Google Scholar] [CrossRef]
- Phua, K.B.; Quak, S.H.; Lim, F.S.; Goh, P.; Teoh, Y.L.; Datta, S.K.; Han, H.H.; Bock, H.L. Immunogenicity, reactogenicity and safety of a diphtheria-tetanus-acellular pertussis-inactivated polio and Haemophilus influenzae type b vaccine in a placebo-controlled rotavirus vaccine study. Ann. Acad. Med. Singap. 2008, 37, 546–553. [Google Scholar]
- Scheifele, D.W.; Ferguson, M.; Predy, G.; Dawar, M.; Assudani, D.; Kuriyakose, S.; Van Der Meeren, O.; Han, H.H. Immunogenicity and safety of 3-dose primary vaccination with combined DTPa-HBV-IPV/Hib vaccine in Canadian Aboriginal and non-Aboriginal infants. Vaccine 2015, 33, 1897–1900. [Google Scholar] [CrossRef][Green Version]
- Van Der Meeren, O.; Kuriyakose, S.; Kolhe, D.; Hardt, K. Immunogenicity of Infanrix™ hexa administered at 3, 5 and 11 months of age. Vaccine 2012, 30, 2710–2714. [Google Scholar] [CrossRef]
- Gabutti, G.; Zepp, F.; Schuerman, L.; Dentico, P.; Bamfi, F.; Soncini, R.; Habermehl, P.; Knuf, M.; Crovari, P. Evaluation of the immunogenicity and reactogenicity of a DTPa-HBV-IPV Combination vaccine co-administered with a Hib conjugate vaccine either as a single injection of a hexavalent combination or as two separate injections at 3, 5 and 11 months of age. Scand. J. Infect. Dis. 2004, 36, 585–592. [Google Scholar] [CrossRef]
- Tejedor, J.C.; Moro, M.; Ruiz-Contreras, J.; Castro, J.; Gómez-Campderá, J.A.; Navarro, M.L.; Merino, J.M.; Martín-Ancel, A.; Roca, J.; García-del-Río, M.; et al. Immunogenicity and reactogenicity of primary immunization with a hexavalent diphtheria-tetanus-acellular pertussis-hepatitis B-inactivated polio-Haemophilus influenzae type B vaccine coadministered with two doses of a meningococcal C-tetanus toxoid conjugate vaccine. Pediatr. Infect. Dis. J. 2006, 25, 713–720. [Google Scholar] [CrossRef]
- Insel, R.A. Potential alterations in immunogenicity by combining or simultaneously administering vaccine components. Ann. N. Y. Acad. Sci. 1995, 754, 35–47. [Google Scholar] [CrossRef]
- Del Giudice, G.; Rappuoli, R.; Didierlaurent, A.M. Correlates of adjuvanticity: A review on adjuvants in licensed vaccines. Semin. Immunol. 2018, 39, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Zepp, F.; Schmitt, H.J.; Kaufhold, A.; Schuind, A.; Knuf, M.; Habermehl, P.; Meyer, C.; Bogaerts, H.; Slaoui, M.; Clemens, R. Evidence for induction of polysaccharide specific B-cell-memory in the 1st year of life: Plain Haemophilus influenzae type b-PRP (Hib) boosters children primed with a tetanus-conjugate Hib-DTPa-HBV combined vaccine. Eur. J. Pediatr. 1997, 156, 18–24. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Pertussis vaccines: WHO position paper–September 2015. Wkly. Epidemiol. Rec. 2015, 90, 433–458. [Google Scholar]
- Zhang, L.; Prietsch, S.O.; Axelsson, I.; Halperin, S.A. Acellular vaccines for preventing whooping cough in children. Cochrane Database Syst. Rev. 2014, Cd001478. [Google Scholar] [CrossRef]
- Klein, N.P.; Abu-Elyazeed, R.; Cheuvart, B.; Janssens, W.; Mesaros, N. Immunogenicity and safety following primary and booster vaccination with a hexavalent diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated poliovirus and Haemophilus influenzae type b vaccine: A randomized trial in the United States. Hum. Vaccines Immunother. 2019, 15, 809–821. [Google Scholar] [CrossRef]
- Van Der Meeren, O.; Bleckmann, G.; Crasta, P.D. Immune memory to hepatitis B persists in children aged 7-8 years, who were vaccinated in infancy with 4 doses of hexavalent DTPa-HBV-IPV/Hib (Infanrix™ hexa) vaccine. Hum. Vaccines Immunother. 2014, 10, 1682–1687. [Google Scholar] [CrossRef]
- Zepp, F.; Knuf, M.; Heininger, U.; Jahn, K.; Collard, A.; Habermehl, P.; Schuerman, L.; Sänger, R. Safety, reactogenicity and immunogenicity of a combined hexavalent tetanus, diphtheria, acellular pertussis, hepatitis B, inactivated poliovirus vaccine and Haemophilus influenzae type b conjugate vaccine, for primary immunization of infants. Vaccine 2004, 22, 2226–2233. [Google Scholar] [CrossRef]
- Ellenberg, S.S. Evaluating the safety of combination vaccines. Clin. Infect. Dis. 2001, 33 (Suppl. S4), S319–S322. [Google Scholar] [CrossRef]
- McGirr, A.; Fisman, D.N. Duration of pertussis immunity after DTaP immunization: A meta-analysis. Pediatrics 2015, 135, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Ortega-Sanchez, I.R.; Guris, D.; Shefer, A.; Lieu, T.; Seward, J.F. An economic analysis of the universal varicella vaccination program in the United States. J. Infect. Dis. 2008, 197, S156–S164. [Google Scholar] [CrossRef] [PubMed]
- Valentim, J.; Sartori, A.M.C.; de Soarez, P.C.; Amaku, M.; Azevedo, R.S.; Novaes, H.M.D. Cost-effectiveness analysis of universal childhood vaccination against varicella in Brazil. Vaccine 2008, 26, 6281–6291. [Google Scholar] [CrossRef] [PubMed]
- Aljunid, S.M.; Al Bashir, L.; Ismail, A.B.; Aizuddin, A.N.; Rashid, S.; Nur, A.M. Economic impact of switching from partially combined vaccine “Pentaxim® and hepatitis B” to fully combined vaccine “Hexaxim®” in the Malaysian National Immunization Program. BMC Health Serv. Res. 2022, 22, 34. [Google Scholar] [CrossRef]
- Li, H.; Tan, Y.; Zeng, H.; Zengl, F.; Xu, X.; Liao, Y.; Zhu, Q.; Zhang, M.; Chen, X.; Kang, M.; et al. Co-Administration of Multiple Childhood Vaccines–Guangdong Province, 2019. China CDC Wkly. 2020, 2, 13–17. [Google Scholar] [CrossRef]
| ID | First Author | Year | Country | Study Design | Age Range | Vaccine Comparisons | Company Funding |
|---|---|---|---|---|---|---|---|
| 1 | Jin Han Kang | 2016 | Korea | Open-label, randomized, and controlled trial | 1.8–2.3 months | DTaP–IPV–Hib vs. DTaP–IPV+Hib | Yes (Sanofi PasteurSA, Lyon, France) |
| 2 | Nina Knutsson | 2001 | Swedish | Randomized, a double-blind placebo-controlled efficacy trial | _ | DTaP–IPV–Hib vs. DTaP–IPV+Hib | Yes (North American Vaccine Inc., Maryland, USA) |
| 3 | Tzou Yien Lin | 2007 | China | Open-label, randomized, controlled trial | 8 weeks | DTaP–IPV–Hib vs. DTaP–IPV+Hib | Yes (Sanofi Pasteur, Lyon, France) |
| 4 | Ki Hwan Kim | 2018 | Korea | A Phase III, open-label, randomized, controlled trial | 42–69 days | DTaP–IPV–Hib vs. DTaP–IPV+Hib | Yes (GlaxoSmithKline Biologicals SA) |
| 5 | Tetsuo Nakayama | 2019 | Japan | A Phase III, modified double-blind, active-controlled, 2-arm, balanced trial | _ | DTaP–IPV–Hib vs. DTaP–IPV+Hib | Yes |
| 6 | Yanping Li | 2011 | China | A Phase III, open-label, randomized, controlled trial | _ | DTaP–IPV–Hib vs. DTaP–IPV+Hib | Yes (GlaxoSmithKline Biologicals SA) |
| 7 | Joanne M.Langley | 2011 | Canada | Randomized controlled trial | _ | DTaP–IPV–Hib vs. DTaP–IPV+Hib | No data |
| 8 | Ronald Gold | 1994 | Canada | Randomized controlled trial | _ | DTaP–IPV–Hib vs. DTaP–IPV+Hib | No data |
| 9 | Guerra FA | 2009 | US | Randomized controlled trial | _ | DTaP–IPV–Hib vs. DTaP–IPV+Hib | _ |
| 10 | G. Gabutti | 2005 | Italy | Open, randomized, multicentre | 12–16 weeks | DTaP–HBV–Hib vs. DTaP–HBV+Hib | Yes (GSK Biologicals, Rixensart, Belgium) |
| 11 | A Ramkissoon | 2001 | South Africa | Open, randomized comparative trial | _ | DTaP–HBV–Hib vs. DTaP–HBV+Hib | No |
| 12 | Michael E. Pichichero | 1997 | UK | A multicenter, prospective, randomized trial | 6–12 weeks | DTaP–HBV–Hib vs. DTaP–HBV+Hib | No |
| 13 | Terry Nolan | 2001 | Melbourne | A randomised double-blind trial | _ | DTaP–HBV–Hib vs. DTaP–HBV+Hib | Yes |
| 14 | Maria Avdicova | 2002 | Slovak | Open-label, randomized, controlled trial | 8–20 weeks | DTaP–HBV–Hib vs. DTaP–HBV+Hib | Yes (GlaxoSmithKline Biologicals, Rixensart, Belgium) |
| 15 | Aristegui | 2003 | Spain | Open randomized, comparative Phase III multicenter trial | _ | DTaP–HBV–Hib vs. DTaP–HBV+Hib | Yes (GSK Biologicals, Rixensart, Belgium) |
| 16 | Gabutti | 2004 | Germany and Italy | Open, Phase III, randomized trial | 12–16 weeks | DTaP–HBV–Hib vs. DTaP–HBV+Hib | Yes (GSK Biologicals, Rixensart, Belgium) |
| 17 | F.Omenaca | 2001 | Greece, Spain, and Switzerland | Open, Phase III, randomized trial | 8–12 weeks | DTaP–HBV–Hib vs. DTaP–HBV+Hib | Yes |
| 18 | Nicola P. Klein | 2018 | US | Open-label, randomized, controlled trial | 6–12 weeks | DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | Yes (GlaxoSmithKline Biologicals S.A) |
| 19 | Nicola P. Klein | 2018 | US | Open-label, randomized, controlled trial | 6–12 weeks | DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+HBV | Yes (GlaxoSmithKline Biologicals S.A) |
| 20 | Heng Kock Cheng | 2004 | Singapore | Open-label, randomized, controlled trial | _ | DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+HBV | _ |
| 21 | Maria Avdicova | 2002 | Slovakia | Open-label, randomized, controlled trial | 8–20 weeks | DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+HBV | Yes (GlaxoSmithKline Biologicals) |
| 22 | Fong-Seng Lim | 2007 | Singapore | Open-label, randomized, controlled trial | 12–16 weeks | DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+HBV | _ |
| 23 | J. Ar’ıstegui | 2003 | Spain | An open, randomized, multi-center, comparative Phase IIIb clinical trial | 8–11 weeks | DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+HBV | Yes (GlaxoSmithKline Biologicals S.A) |
| 24 | Heinz J. Schmitt | 2000 | Germany | An open, randomized, multi-center trial | 8–16 weeks | DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | No |
| 25 | Giovanni Gabutti | 2004 | Germany and Italy | An open, randomized, multi-center trial | _ | DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | Yes (GSK Biologicals, Rixensart, Belgium) |
| Variables | Vaccine Group | No. of Studies | SMD (95% CI)/GMTs | % Weight | z | p-Effect | I2 | p-Heterogeneity |
|---|---|---|---|---|---|---|---|---|
| Anti-diphtheria | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 8 | 0 (−0.08, 0.07) | 48.18 | −0.109 | 0.913 | 27.80% | 0.206 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 7 | −0.08 (−0.23, 0.06) | 27.61 | −1.115 | 0.265 | 41.90% | 0.112 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 2 | 0.03 (−0.13, 0.18) | 11.14 | 0.336 | 0.737 | 0.00% | 0.326 | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 3 | 0.19 (−0.13, 0.51) | 13.06 | 1.186 | 0.236 | 74.50% | 0.020 | |
| Overall | 20 | 0.01 (−0.07, 0.08) | 100 | 0.124 | 0.902 | 53.50% | 0.003 | |
| Anti-tetanus | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 8 | −0.12 (−0.3, 0.05) | 44.79 | −1.396 | 0.163 | 85.5% | <0.001 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 7 | −0.23 (−0.42, −0.05) | 30.45 | −2.487 | 0.013 | 63.7% | 0.011 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 2 | −0.2 (−0.41, 0.02) | 10.90 | −1.790 | 0.073 | 49.3% | 0.160 | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 3 | −0.02 (−0.37, 0.34) | 13.86 | −0.082 | 0.935 | 79.5% | 0.008 | |
| Overall | 20 | −0.15 (−0.26, −0.04) | 100.00 | −2.737 | 0.006 | 77.7% | <0.001 | |
| Anti-hepatitis B | ||||||||
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 8 | −0.21 (−0.44, 0.02) | 60.55 | −1.817 | 0.069 | 82.2% | <0.001 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 2 | −0.02 (−0.4, 0.36) | 16.62 | −0.091 | 0.928 | 83.7% | 0.013 | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 3 | 0.15 (−0.33, 0.63) | 22.83 | 0.619 | 0.536 | 88.7% | <0.001 | |
| Overall | 13 | −0.09 (−0.31, 0.12) | 100.00 | −0.870 | 0.385 | 88.9% | <0.001 | |
| Anti-pertussis | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 8 | 0.27 (−0.16, 0.69) | 43.57 | 1.240 | 0.215 | 97.5% | <0.001 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 6 | 0.07 (−0.22, 0.35) | 30.13 | 0.444 | 0.657 | 80.1% | <0.001 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 2 | −0.15 (−0.3, 0.01) | 10.86 | −1.889 | 0.059 | 0.0% | 0.999 | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 3 | 0.6 (0.45, 0.75) | 15.45 | 7.962 | 0.000 | 0.0% | 0.450 | |
| Overall | 19 | 0.21 (−0.03, 0.44) | 100.00 | 1.735 | 0.083 | 95.2% | <0.001 | |
| Anti-FHA | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 6 | 0.28 (−0.2, 0.76) | 38.86 | 1.153 | 0.249 | 96.7% | <0.001 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 5 | −0.08 (−0.3, 0.15) | 29.83 | −0.640 | 0.522 | 65.3% | 0.021 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 2 | −0.13 (−0.3, 0.04) | 12.98 | −1.464 | 0.143 | 22.3% | 0.257 | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 3 | 0.4 (0.01, 0.78) | 18.33 | 2.034 | 0.042 | 82.4% | 0.003 | |
| Overall | 16 | 0.14 (−0.09, 0.38) | 100.00 | 1.183 | 0.237 | 93.5% | <0.001 | |
| Anti-PRN | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 3 | −0.13 (−0.27, 0) | 24.66 | −1.984 | 0.047 | 0.0% | 0.565 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 5 | −0.2 (−0.53, 0.13) | 35.85 | −1.200 | 0.230 | 83.4% | <0.001 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 2 | 0.08 (−0.19, 0.35) | 16.92 | 0.566 | 0.571 | 67.7% | 0.078 | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 3 | 0.08 (−0.5, 0.66) | 22.58 | 0.281 | 0.778 | 92.4% | <0.001 | |
| Overall | 13 | −0.07 (−0.25, 0.1) | 100.00 | −0.806 | 0.420 | 83.6% | <0.001 | |
| Anti-PRP | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 6 | −0.83 (−1.44, −0.22) | 34.06 | −2.680 | 0.007 | 97.6% | <0.001 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 7 | −0.37 (−0.73, −0.01) | 37.92 | −2.029 | 0.043 | 91.2% | <0.001 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 2 | −0.61 (−1.03, −0.19) | 11.50 | −2.828 | 0.005 | 86.2% | 0.007 | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 3 | −0.24 (−0.69, 0.22) | 16.52 | −1.003 | 0.316 | 87.8% | <0.001 | |
| Overall | 18 | −0.53 (−0.79, −0.27) | 100.00 | −4.005 | 0.000 | 94.9% | <0.001 | |
| Polio serotype 1 | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 7 | 0.06 (−0.19, 0.3) | 49.24 | 0.474 | 0.636 | 89.6% | <0.001 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 4 | −0.04 (−0.18, 0.1) | 25.25 | −0.539 | 0.590 | 14.2% | 0.321 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 2 | 0.24 (−0.39, 0.87) | 14.04 | 0.747 | 0.455 | 94.0% | <0.001 | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 2 | −0.09 (−0.3, 0.12) | 11.46 | −0.805 | 0.421 | 0.0% | 0.634 | |
| Overall | 15 | 0.04 (−0.12, 0.19) | 100.00 | 0.444 | 0.657 | 84.4% | <0.001 | |
| Polio serotype 2 | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 7 | 0.06 (−0.16, 0.27) | 49.01 | 0.521 | 0.602 | 85.9% | <0.001 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 4 | −0.02 (−0.24, 0.19) | 25.40 | −0.205 | 0.838 | 59.9% | 0.058 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 2 | 0.4 (−0.41, 1.21) | 13.95 | 0.961 | 0.336 | 96.3% | <0.001 | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 2 | −0.12 (−0.33, 0.09) | 11.64 | −1.108 | 0.268 | 0.0% | 0.701 | |
| Overall | 15 | 0.06 (−0.11, 0.22) | 100.00 | 0.692 | 0.489 | 86.4% | <0.001 | |
| Polio serotype 3 | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 7 | 0.03 (−0.22, 0.28) | 48.69 | 0.253 | 0.801 | 89.7% | <0.001 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 4 | 0.04 (−0.2, 0.27) | 25.58 | 0.330 | 0.742 | 66.8% | 0.029 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 2 | 0.21 (−0.77, 1.2) | 13.87 | 0.422 | 0.673 | 97.5% | <0.001 | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 2 | −0.08 (−0.54, 0.38) | 11.86 | −0.328 | 0.743 | 68.6% | 0.074 | |
| Overall | 15 | 0.05 (−0.13, 0.23) | 100.00 | 0.543 | 0.587 | 88.6% | 0.000 | |
| Variables | Vaccine Group | No. of Studies | RR (95% CI) | % Weight | z | p-Effect | I2 | p-Heterogeneity |
|---|---|---|---|---|---|---|---|---|
| Redness | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 5 | 1.04 (0.87, 1.24) | 28.24 | 0.398 | 0.691 | 67.00% | 0.017 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 7 | 0.92 (0.81, 1.06) | 50.85 | −1.129 | 0.259 | 88.70% | 0.000 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 2 | 0.80 (0.63, 1.03) | 13.73 | −1.742 | 0.082 | 75.20% | 0.045 | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 1 | 1.17 (1.01, 1.36) | 7.18 | 2.040 | 0.041 | - | - | |
| Overall | 15 | 0.95 (0.86, 1.05) | 100.00 | −0.982 | 0.326 | 86.80% | 0.000 | |
| Pain | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 4 | 0.94 (0.86, 1.02) | 24.35 | −1.512 | 0.131 | 0.00% | 0.574 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 7 | 1.04 (0.94, 1.15) | 49.9 | 0.673 | 0.501 | 81.10% | 0.000 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 2 | 0.80 (0.69, 0.91) | 12.58 | −3.239 | 0.001 | 9.70% | 0.293 | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 2 | 0.76 (0.34, 1.73) | 13.17 | −0.656 | 0.512 | 97.60% | 0.000 | |
| Overall | 15 | 0.94 (0.85, 1.04) | 100.00 | −1.151 | 0.25 | 87.60% | 0.000 | |
| Swelling | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 5 | 1.07 (0.86, 1.33) | 28.6 | 0.623 | 0.534 | 82.60% | 0.000 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 7 | 0.97 (0.85, 1.11) | 46.68 | −0.439 | 0.66 | 86.50% | 0.000 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 2 | 0.87 (0.78, 0.98) | 12.57 | −2.331 | 0.02 | 0.00% | 0.402 | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 2 | 0.90 (0.52, 1.58) | 12.15 | −0.358 | 0.72 | 93.90% | 0.000 | |
| Overall | 16 | 0.98 (0.89, 1.09) | 100.00 | −0.359 | 0.72 | 86.50% | 0.000 | |
| Diarrhea | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 1 | 0.58 (1.2, 0) | 35.49 | −0.961 | 0.336 | - | - | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 2 | 0.95 (1.43, 0) | 64.51 | 1.436 | 0.151 | 0.00% | 0.321 | |
| Overall | 3 | 0.87 (1.26, 0) | 100.00 | 0.499 | 0.618 | 47.90% | 0.147 | |
| Fever | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 7 | 1.00 (0.92, 1.09) | 37.79 | 0.051 | 0.959 | 17.60% | 0.296 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 6 | 0.99 (0.79, 1.24) | 38.74 | −0.104 | 0.917 | 95.20% | 0.000 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 2 | 1.13 (1.02, 1.26) | 11.76 | 2.308 | 0.021 | 0.00% | 0.397 | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 2 | 1.26 (1.08, 1.47) | 11.71 | 2.985 | 0.003 | 20.30% | 0.263 | |
| Overall | 17 | 1.03 (0.93, 1.15) | 100.00 | 0.575 | 0.565 | 87.70% | 0.000 | |
| Irritability | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 6 | 0.92 (0.85, 1) | 36.15 | −1.945 | 0.052 | 13.80% | 0.326 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 6 | 0.95 (0.83, 1.1) | 46.28 | −0.671 | 0.502 | 89.40% | 0.000 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 1 | 1.10 (0.85, 1.42) | 4.95 | 0.716 | 0.474 | - | - | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 2 | 1.18 (1.04, 1.33) | 12.62 | 2.587 | 0.010 | 0.00% | 0.674 | |
| Overall | 15 | 0.97 (0.9, 1.06) | 100.00 | −0.692 | 0.489 | 78.60% | 0.000 | |
| Loss of appetite | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 6 | 1.01 (0.93, 1.1) | 30.75 | 0.247 | 0.805 | 0.00% | 0.682 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 4 | 1.03 (0.98, 1.09) | 51.51 | 1.092 | 0.275 | 24.40% | 0.265 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 1 | 0.99 (0.86, 1.15) | 10.66 | −0.093 | 0.926 | - | - | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 1 | 1.11 (0.94, 1.3) | 7.07 | 1.256 | 0.209 | - | - | |
| Overall | 12 | 1.03 (0.98, 1.07) | 100.00 | 1.188 | 0.235 | 0.00% | 0.671 | |
| Restlessness | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 3 | 0.96 (0.84, 1.1) | 16.24 | −0.579 | 0.562 | 68.40% | 0.042 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 3 | 0.96 (0.91, 1.01) | 64.16 | −1.475 | 0.14 | 8.90% | 0.334 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 1 | 0.96 (0.85, 1.08) | 19.59 | −0.675 | 0.500 | - | - | |
| Overall | 7 | 0.96 (0.92, 1.01) | 100.00 | −1.687 | 0.092 | 29.60% | 0.202 | |
| Sleepiness | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 2 | 0.97 (0.87, 1.08) | 29.33 | −0.528 | 0.597 | 61.60% | 0.106 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 1 | 0.99 (0.94, 1.04) | 64.69 | −0.402 | 0.688 | - | - | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 1 | 1.02 (0.8, 1.3) | 5.98 | 0.127 | 0.899 | - | - | |
| Overall | 4 | 0.99 (0.94, 1.04) | 100.00 | −0.585 | 0.559 | 0.00% | 0.438 | |
| Unusual drowsiness | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 5 | 0.95 (0.85, 1.06) | 31.28 | −0.912 | 0.362 | 36.90% | 0.175 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 3 | 0.94 (0.85, 1.04) | 34.07 | −1.16 | 0.246 | 57.60% | 0.095 | |
| DTaP–HBV–IPV–Hib vs. DTaP–HBV–IPV+Hib | 1 | 1.02 (0.9, 1.16) | 21.47 | 0.339 | 0.734 | - | - | |
| DTaP–HBV–IPV–Hib vs. DTaP–IPV–Hib+HBV | 1 | 0.97 (0.82, 1.14) | 13.18 | −0.412 | 0.681 | - | - | |
| Overall | 10 | 0.96 (0.91, 1.02) | 100.00 | −1.187 | 0.235 | 24.10% | 0.222 | |
| Vomiting | ||||||||
| DTaP–IPV–Hib vs. DTaP–IPV+Hib | 4 | 1.05 (0.86, 1.28) | 52.58 | 0.483 | 0.629 | 56.90% | 0.073 | |
| DTaP–HBV–Hib vs. DTaP–HBV+Hib | 3 | 1.05 (0.91, 1.2) | 47.42 | 0.653 | 0.514 | 30.90% | 0.235 | |
| Overall | 7 | 1.07 (0.95, 1.21) | 100.00 | 1.046 | 0.295 | 59.90% | 0.020 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Cao, B.; Wang, C.; Han, B.; Sun, T.; Miao, Y.; Lu, Q.; Cui, F. Immunogenicity and Safety of Childhood Combination Vaccines: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 472. https://doi.org/10.3390/vaccines10030472
Liu B, Cao B, Wang C, Han B, Sun T, Miao Y, Lu Q, Cui F. Immunogenicity and Safety of Childhood Combination Vaccines: A Systematic Review and Meta-Analysis. Vaccines. 2022; 10(3):472. https://doi.org/10.3390/vaccines10030472
Chicago/Turabian StyleLiu, Bei, Bing Cao, Chao Wang, Bingfeng Han, Tao Sun, Yudong Miao, Qingbin Lu, and Fuqiang Cui. 2022. "Immunogenicity and Safety of Childhood Combination Vaccines: A Systematic Review and Meta-Analysis" Vaccines 10, no. 3: 472. https://doi.org/10.3390/vaccines10030472
APA StyleLiu, B., Cao, B., Wang, C., Han, B., Sun, T., Miao, Y., Lu, Q., & Cui, F. (2022). Immunogenicity and Safety of Childhood Combination Vaccines: A Systematic Review and Meta-Analysis. Vaccines, 10(3), 472. https://doi.org/10.3390/vaccines10030472






