Vaccines Against COVID-19: A Review
Abstract
:1. Introduction
2. Methodology
3. COVID-19 Vaccines Development
| Type of Vaccine | Development | Invention Year | Target Desease |
|---|---|---|---|
| Attenuated pathogen | Through physicochemical treatments, the pathogen loses features that allow an effective infection. Due to the intact antigens on the membrane surface, they can be recognized by the immune system. | 1798 | Smallpox |
| Dead/inactivated pathogen | Through physicochemical treatments, the bacterial pathogen is killed and viral pathogen is inactivated. It cannot infect, but the antigens must remain on the membrane to be recognized by the immune system. | 1896 | Typhoid |
| Toxoids | The bacterial toxins are attenuated with chemical agents such as formaldehyde or the effects of heat, preserving their high immunogenicity. | 1923 | Diphtheria |
| Protein subunits | They contain only harmless proteins of the microorganism. They are made by recombinant expression in cell models such as bacteria or fungi, or obtained by lysis of the pathogen, but the proteins that join to the host’s receptors are preserved to protect their three-dimensional conformation. | 1970 | Anthrax |
| Viral particles | The structural proteins of the pathogen are assembled using a matrix (which can be a lipid bilayer) which allows simulating the pathogen’s spatial conformation without genetic content. They have high immunogenicity since most of the pathogen’s proteins are present. | 1986 | Hepatitis B |
| Viral Vectors | These are genetically modified viruses, which have already been well characterized. The genetic content is eliminated, except for those genes that give a cell the ability to infect. The removed genetic material is replaced by that that is of interest (DNA or mRNA *) and incorporated into the virus for protection and transport. Once the vector comes into contact with human cells, it instructs them to produce a protein exclusive for the microorganism. Thus, the body begins to manufacture components of the immune system. Most of these viral vectors cannot replicate. | 2019 | Ebola |
| Nucleic acids | They can be DNA or mRNA. In both cases, the genetic material is protected by a nanoparticle, mainly lipids, since it becomes permeable to the phospholipid bilayer of the cell membrane. DNA travels through the cytosol until incorporated into the nucleus, where it is transcribed into mRNA and later translated into a chain of amino acids. Something similar happens with the mRNA, but it does not enter the nucleus, instead passsing directly to the ribosomes to synthesize the chain of amino acids. Finally, this genetic material allows the production of pathogenic proteins which will be expressed at the membrane surface level, thus achieving the creation of antigens through our cells, which will stimulate the immune system. | 2020 | SARS-CoV-2 |
| Platform | Advantages | Disadvantages |
|---|---|---|
| Attenuated pathogen | Produces humoral and cellular response with a single dose. | Safety problems in immunosuppressed people. Strains are difficult to obtain. |
| Dead/inactivated pathogen | Safe due to the nature of its composition. Very easy to transport and store. | Large amounts of the pathogen. Possible effects on the immunogenicity of the antigen |
| Protein subunits | Safe during production and for immunosuppressed people. | Decrease in APC * capacity due to particle size. Limited production due to product scalability. |
| Polysaccharides | Alternative against bacterias with abundant polysaccharide antigens. | There is only IgM production. Low memory immunity. Low efficiency in children. |
| Viral particles | Combines the efficacy of live and subunit vaccines. High scalability production. | Particle assembly is a complex process. |
| Viral Vectors | It can induce a humoral and cellular response. Safe. | Pre-existing immunity is used against the vector. It needs low temperatures to store. |
| Nucleic acid | Scalability. Rapid design and development. Very secure. Induces humoral and cellular responses. | Its storage and handling are delicate. |
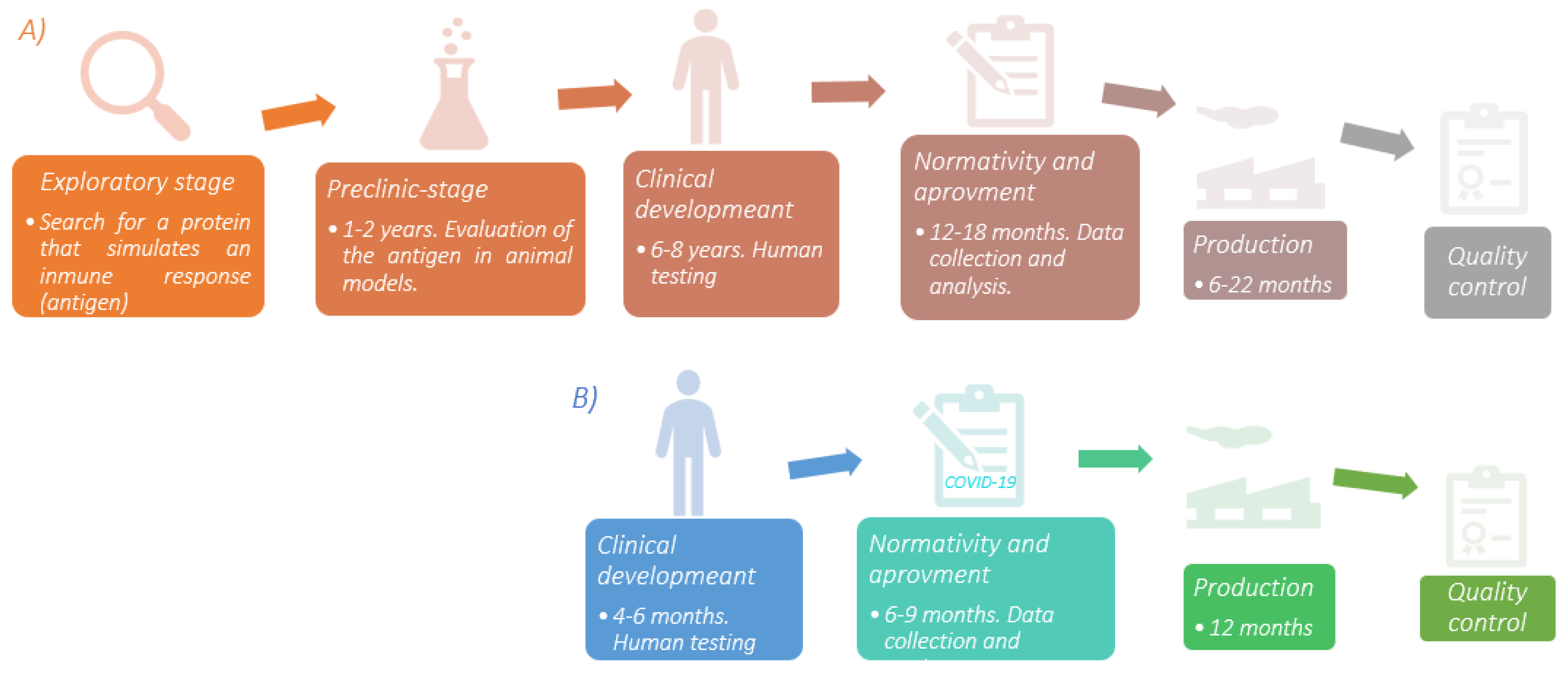
Adjuvants
4. COVID-19 Vaccines
4.1. Dose Immunization
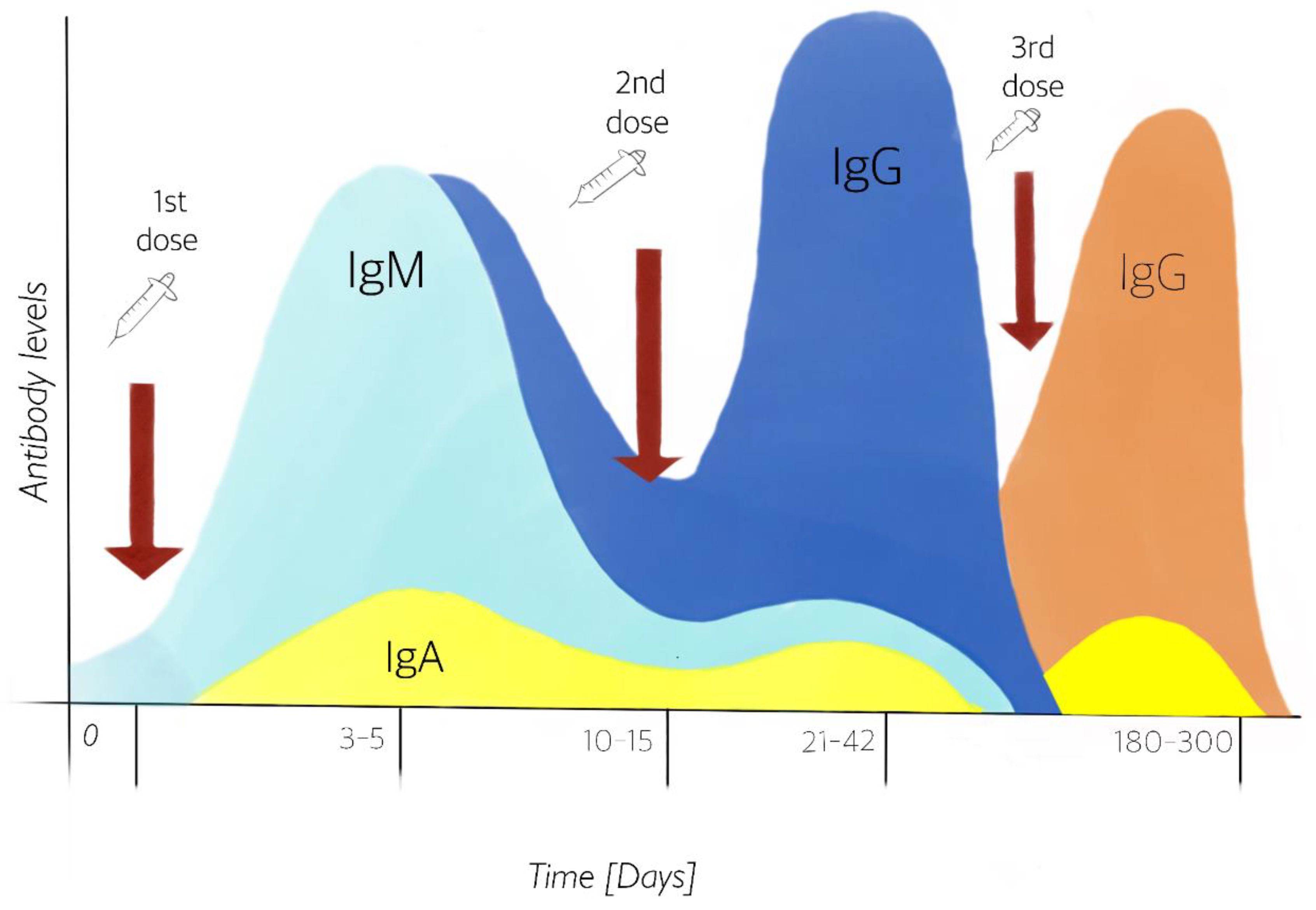
4.2. Heterologous Vaccines
5. Humoral and Cellular Immunity Generated by Vaccines
5.1. Pregnant Women and Vaccination against COVID-19
5.2. Population with Medical Conditions and COVID-19 Vaccination
5.3. Hybrid Immunity
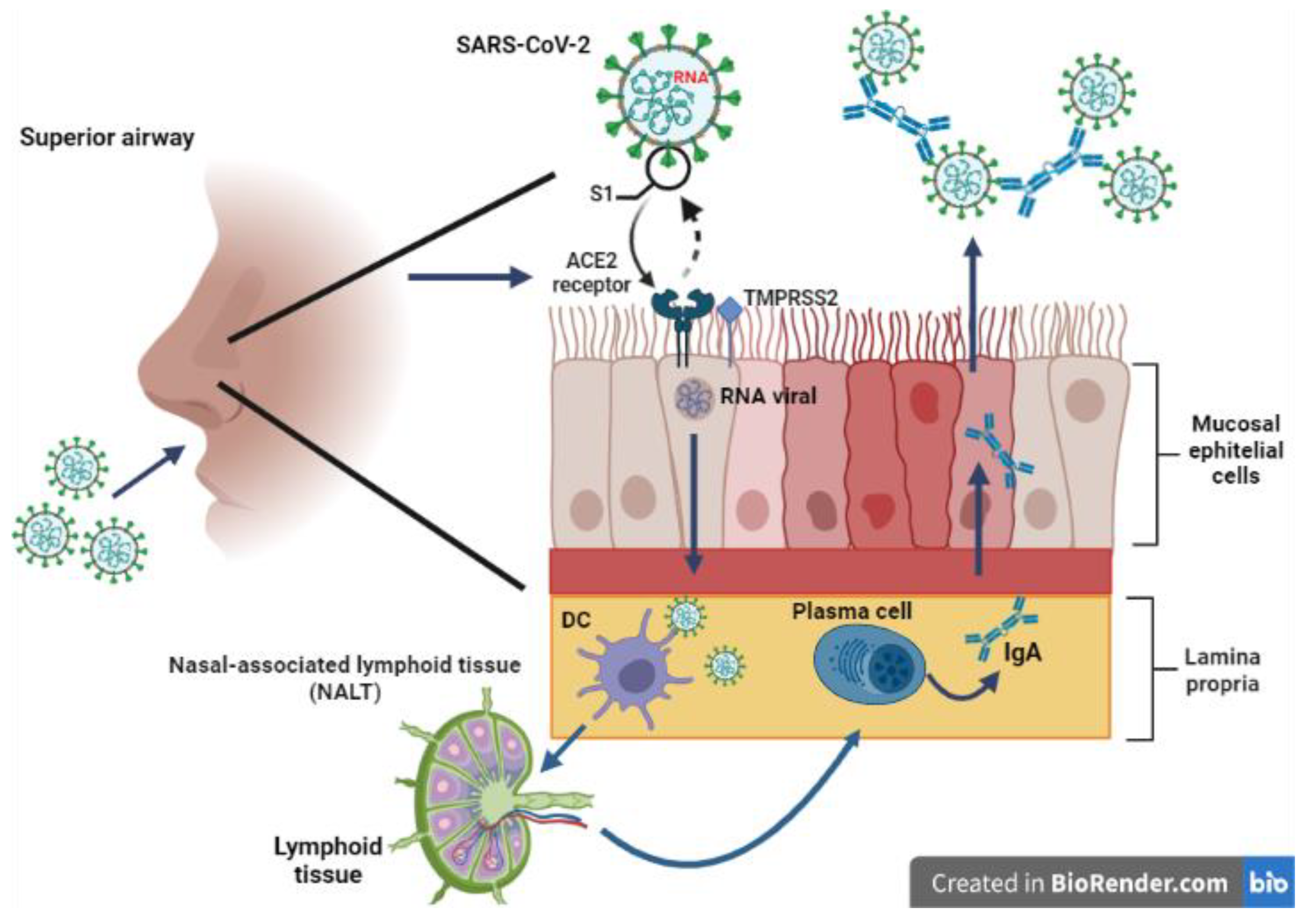
6. Immunization Alternatives
7. Mexico and Vaccination against COVID-19
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Los 7 Tipos de Coronavirus Que Infectan Humanos. Available online: https://www.nationalgeographic.com.es/ciencia/siete-tipos-coronavirus-que-infectan-humanos_15353 (accessed on 31 March 2021).
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Yang, C.; Xu, X.; Feng, X.; Liu, W.; Wen, S. Structural and functional properties os SARS-CoV-2 spike protein: Potential antivurs drug developmeant for COVID-19. Acta Pharmacol. Sin. 2020, 41, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Patrucco, F.; Gavelli, F.; Shi, R.; De Vita, N.; Pavot, A.; Castello, L.M.; Ravanini, P.; Balbo, P.E. Corona-virus disease 2019 outbreak. Panminerva Med. 2020, 62, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Forni, G.; Mantovani, A. COVID-19 vaccines: Where we stand and challenges ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.A.; Sacco, O.; Mancino, E.; Cristiani, L.; Midulla, F. Differences and similarities between SARS-CoV and SARS-CoV-2: Spike receptor-binding domain recognition and host cell infection with support of cellular serine proteases. Infection 2020, 48, 665–669. [Google Scholar] [CrossRef]
- Karikò, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Roldão, A.; Mellado, M.C.; Castilho, L.R.; Carrondo, M.J.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 vaccines strategies: A comprehensive review of phase 3 candidates. NPJ Vaccines 2021, 6, 28. [Google Scholar] [CrossRef]
- Urbiztondo, L.; Borràs, E.; Mirada, G. Vacunas contra el coronavirus. Vacunas 2020, 21, 69–72. [Google Scholar] [CrossRef]
- Acosta Altamirano, G.; Frías De León, M.G.; Reyes Acosta, M.R.; Torres Estrella, C.U.; Reyes Monte, M.R. Respuesta inmunitaria a COVID-19 y vacunas—Cap. 20. In Estrategias del HRAEI ante el reto de COVID-19; Acosta Altamirano, G., Camacho Olivares, G., Carrasco Valdez, M., Robledo Cayetano, M., Eds.; Editorial HRAEI Ixtapaluca: Mexico City, Mexico, 2021; pp. 240–246. ISBN 978-607-99330-1-2. [Google Scholar]
- Hotez, P.J.; Corry, D.B.; Strych, U.; Bottazzi, M.E. COVID-19 vaccines: Neutralizing antibodies and the alum advantage. Nat. Rev. Immunol. 2020, 20, 399–400. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.H.; Beiss, V.; Fiering, S.N.; Steinmetz, N.F. COVID-19 vaccine frontrunners and their nano-technology design. ACS Nano 2020, 14, 12522–12537. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, S.E.; Altenburg, A.F.; Bengtsson, K.L.; Bosman, F.; de Vries, R.D.; Rimmelzwaan, G.F.; Stertman, L. Matrix-MTM adjuvant enhances immunogenicity of both protein- and modified vaccinia virus Ankara-based influenza vaccines in mice. Immunol. Res. 2018, 66, 224–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, J.H.; Patel, N.; Haupt, R.; Zhou, H.; Weston, S.; Hammond, H.; Logue, J.; Portnoff, A.D.; Norton, J.; Guebre-Xabier, M.; et al. SARS-CoV-2 spike glycoprotein vaccine candidate NVX-CoV2373 immunogenicity in baboons and protection in mice. Nat. Commun. 2021, 12, 372. [Google Scholar] [CrossRef]
- Francica, J.R.; Flynn, B.J.; Foulds, K.E.; Noe, A.T.; Werner, A.P.; Moore, I.N.; Gagne, M.; Johnston, T.S.; Tucker, C.; Davis, R.L.; et al. Vaccination with SARS-CoV-2 Spike Protein and AS03 Adjuvant Induces Rapid Anamnestic Antibodies in the Lung and Protects Against Virus Challenge in Nonhuman Primates. bioRxiv 2021. [Google Scholar] [CrossRef]
- Watterson, D.; Wijesundara, D.K.; Modhiran, N.; Mordant, F.L.; Li, Z.; Avumegah, M.S.; McMillan, C.L.; Lackenby, J.; Guilfoyle, K.; van Amerongen, G.; et al. Preclinical development of a molecular clamp-stabilised subunit vaccine for severe acute respiratory syndrome coronavirus 2. Clin. Transl. Immunol. 2021, 10, e1269. [Google Scholar] [CrossRef]
- Bevington, S.L.; Cauchy, P.; Withers, D.R.; Lane, P.J.L.; Cockerill, P.N. T cell receptor and cytokine signaling can function at different stages to establish and maintain transcriptional memory and enable T helper cell differentiation. Front Immunol. 2017, 8, 204. [Google Scholar] [CrossRef] [Green Version]
- CIGB 2020 en Contactos y Sospechosos de Infección por SARS-CoV-2. Available online: https://rpcec.sld.cu/ensayos/RPCEC00000306-Sp (accessed on 18 April 2021).
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four ran-domized controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Kleine-Tebbe, J.; Klimek, L.; Hamelmann, E.; Pfaar, O.; Taube, C.; Wagenmann, M.; Werfel, T.; Worm, M. Severe allergic reactions to the COVID-19 vaccine—Statement and practical consequences. Allergol. Select. 2021, 5, 26–28. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rou-phael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: Novavax vaccine efficacy is 86% against UK variant and 60% against South African variant. BMJ 2021, 372. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Pfizer and BioNTech Initiate a Study as Part of Broad Develop-ment Plan to Evaluate COVID-19 Booster and New Vaccine Variants. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-initiate-study-part-broad-development (accessed on 25 February 2021).
- The First Interim Data Analysis of the Sputnik V Vaccine against COVID-19 Phase III Clinical Trials in the Russian Federation Demonstrated 92% Efficacy. Available online: https://www.businesswire.com/news/home/20201111005675/en/The-First-Interim-Data-Analysis-of-the-Sputnik-V-Vaccine-Against-COVID-19-Phase-III-Clinical-Trials-in-the-Russian-Federation-Demonstrated-92-Efficacy (accessed on 18 May 2021).
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef]
- Wu, S.; Zhong, G.; Zhang, J.; Shuai, L.; Zhang, Z.; Wen, Z.; Wang, B.; Zhao, Z.; Song, X.; Chen, Y.; et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun. 2020, 11, 4081. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Stephenson, K.E.; Le Gars, M.; Sadoff, J.; De Groot, A.M.; Heerwegh, D.; Truyers, C.; Atyeo, C.; Loos, C.; Chandrashekar, A.; McMahan, K.; et al. Immunogenicity of the Ad26.COV2.S Vaccine for COVID-19. JAMA 2021, 325, 1535–1544. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: An interim analysis of a randomised con-trolled phase 3 trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A ran-domized, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 181–192. [Google Scholar] [CrossRef]
- Bharat Biotech and ICMR Announce Interim Results from Phase 3 Trials of COVAXIN®. Demostraste Overall Interim Clinical Efficacy of 78% and 100% Efficacy against Severe COVID-19 Disease. Available online: https://www.bharatbiotech.com/images/press/covaxin-phase3-clinical-trials-interim-results.pdf (accessed on 1 May 2021).
- Ella, R.; Vadrevu, K.M.; Jogdand, H.; Prasad, S.; Reddy, S.; Sarangi, V.; Ganneru, B.; Sapkal, G.; Yadav, P.; Abraham, P.; et al. Safety and immu-nogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: A double-blind, randomised, phase 1 trial. Lancet Infect. Dis. 2021, 21, 637–646. [Google Scholar] [CrossRef]
- Explicación de la Autorización de Uso de Emergencia para las Vacunas. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/explicacion-de-la-autorizacion-de-uso-de-emergencia-para-las-vacunas (accessed on 20 March 2021).
- Cominarty and Pfizer-BioNTech COVID-19 Vaccine. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine (accessed on 6 February 2022).
- Spikevax and Moderna COVID-19 Vaccine. Available online: fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/spikevax-and-moderna-covid-19-vaccine (accessed on 6 February 2022).
- Statement of the WHO Global Advisory Committee on Vaccine Safety (GACVS) COVID-19 Subcom-Mittee on Safety Signals Related to the AstraZeneca COVID-19 Vaccine. Available online: https://www.who.int/news/item/19-03-2021-statement-of-the-who-global-advisory-committee-on-vaccine-safety-(gacvs)-covid-19-subcommittee-on-safety-signals-related-to-the-astrazeneca-covid-19-vaccine (accessed on 20 March 2021).
- Coronavirus Vaccine Tracker. Available online: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html (accessed on 15 April 2021).
- Owen, J.A.; Punt, J.; Stranford, S.A. (Eds.) Kuby Immunology, 7th ed.; W. H. Freeman and Company: New York, NY, USA, 2014; Volume 148, pp. 148–162. [Google Scholar]
- Kaul, D.; Ogra, P.L. Mucosal responses to parenteral and mucosal vaccines. Dev. Biol. Stand. 1998, 95, 141–146. [Google Scholar] [PubMed]
- Johns Hopkins. Booster Shots, Third Dose and Additinoal Doses for COVID-19 Vaccines—What You Need to Know. Available online: https://www.hopkinsmedicine.org/health/conditions-and-diseases/coronavirus/booster-shots-and-third-doses-for-covid19-vaccines-what-you-need-to-know (accessed on 20 February 2022).
- Centers for Disease Control and Prevention (CDC). Effectiveness of a Third Dose of mRNA Vaccines against COVID-19-Associated Emergency Department and Urgent Care Encounters and Hospitalizations among Adults during Periods of Delta and Omicro Variant Predominance—VISION Network, 10 States, August 2021–January 2022. Available online: https://www.cdc.gov/mmwr/volumes/71/wr/mm7104e3.htm?s_cid=mm7104e3_w (accessed on 20 February 2022).
- Mûller, M.; Volzke, J.; Subin, B.; Mûller, S.; Sombetzki, M.; Reisinger, E.C.; Mûller-Hilke, B. Sin-gle-dose SARS-CoV-2 vaccinations with either BNT162b2 or AZD1222 induce disparate Th1 responses and IgA production. BMC Med. 2022, 20, 29. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Mao, Q.; An, C.; Zhang, J.; Gao, F.; Bian, L.; Li, C.; Liang, Z.; Xu, M.; Wang, J. Heterologous prime-boost: Breaking the protective immune response bottleneck of COVID-19 vaccine candidates. Emerg Microbes Infect. 2021, 10, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir. Med. 2021, 9, 1255–1265. [Google Scholar] [CrossRef]
- Eroglu, B.; Nuwarda, R.F.; Ramzan, I.; Kayser, V. A Narrative Review of COVID-19 Vaccines. Vaccines 2022, 10, 62. [Google Scholar] [CrossRef]
- Vacunas e Inmunización: Qué es la Vacunación? Available online: https://www.who.int/es/news-room/q-a-detail/vaccines-and-immunization-what-is-vaccination (accessed on 10 April 2021).
- Clements, J.D.; Freytag, L.C. Parenteral vaccination can be an effective means of inducing protective mucosal responses. Clin. Vaccine Immunol. 2016, 23, 438–441. [Google Scholar] [CrossRef] [Green Version]
- Ketas, T.J.; Chaturbhuj, D.; Cruz-Portillo, V.M.; Francomano, E.; Golden, E.; Chandrasekhar, S.; De-bnath, G.; Diaz-Tapia, R.; Yasmeen, A.; Leconet, W.; et al. Antibody responses to SARS-CoV-2 mRNA vaccines are detectable in saliva. bioRxiv 2021. [Google Scholar] [CrossRef]
- Shimabukuro, T.T.; Kim, S.Y.; Myers, T.R.; Moro, P.L.; Oduyebo, T.; Panagiotakopoulos, L.; Marquez, P.L.; Olson, C.K.; Liu, R.; Chang, K.T.; et al. Preliminary Findings of mRNA COVID-19 Vaccine Safety in Pregnant Persons. N. Engl. J. Med. 2021, 384, 2273–2282. [Google Scholar] [CrossRef]
- COVID-19 Vaccination—Clinical Care. Available online: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-product%2Fclinical-considerations.html (accessed on 12 April 2021).
- Whitaker, J.A.; Valles, K.; Tosh, P.K.; Poland, G.A. Vaccine use in immunocompromised adults. In Vaccinations; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 139–162. [Google Scholar] [CrossRef]
- Yau, K.T.; Chan, C.T.; Abe, K.; Jiang, Y.; Atiquzzaman, M.I.; Mullin, S.; Shadowitz, E.; Liu, L.; Kostadinovic, E.; Sukovic, T.; et al. Differences in Mrna-1273 (Moderna) and BNT162b2 (Pfizer-BioNTech)) SARS-CoV-2 vaccine immu-nogenicity among patients undergoing dialysis. CMAJ 2022, 194, E297–E305. [Google Scholar] [CrossRef]
- Callaway, E. Coronavirus variants get Greek names—But will scientists use them? Nature 2021, 594, 162. [Google Scholar] [CrossRef]
- Madhi, S.A.; Baillie, V.; Cutland, C.L.; Voysey, M.; Koen, A.L.; Fairlie, L.; Padayachee, S.D.; Dheda, K.; Barnabas, S.L.; Bhorat, Q.E.; et al. Efficacy of the ChAdOx1 nCoV-19 COVID-19 vaccine against the B.1.351 variant. N. Engl. J. Med. 2021, 384, 1885–1898. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Chemaitelly, H.; Butt, A.A.; National Study Group for COVID-19 Vaccination. Effectiveness of the BNT162b2 COVID-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021, 385, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Moyo-Gwete, T.; Madzivhandila, M.; Makhado, Z.; Ayres, F.; Mhlanga, D.; Oosthuysen, B.; Lambson, B.E.; Kgagudi, P.; Tegally, H.; Iranzadeh, A.; et al. Cross-Reactive Neutralizing Antibody Responses Elicited by SARS-CoV-2 501Y.V2 (B.1.351). N. Engl. J. Med. 2021, 384, 2161–2163. [Google Scholar] [CrossRef] [PubMed]
- Stamatatos, L.; Czartoski, J.; Wan, Y.H.; Homad, L.J.; Rubin, V.; Glantz, H.; Neradilek, M.; Seydoux, E.; Jennewein, M.F.; MacCamy, A.J.; et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science 2021, 372, 1413–1418. [Google Scholar] [CrossRef]
- Goel, R.R.; Apostolidis, S.A.; Painter, M.M.; Mathew, D.; Pattekar, A.; Kuthuru, O.; Gouma, S.; Hicks, P.; Meng, W.; Rosenfeld, A.M.; et al. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci. Immunol. 2021, 6, eabi6950. [Google Scholar] [CrossRef] [PubMed]
- Urbanowicz, R.A.; Tsoleridis, T.; Jackson, H.J.; Cusin, L.; Duncan, J.D.; Chappell, J.G.; Tarr, A.W.; Nightingale, J.; Norrish, A.R.; Ikram, A.; et al. Two doses of the SARS-CoV-2 BNT162b2 vaccine enhance antibody responses to variants in individuals with prior SARS-CoV-2 infection. Sci. Transl. Med. 2021, 13, eabj0847. [Google Scholar] [CrossRef]
- Crotty, S. Hybrid immunity. Science 2021, 372, 1392–1393. [Google Scholar] [CrossRef]
- Wang, Z.; Muecksch, F.; Schaefer-Babajew, D.; Finkin, S.; Viant, C.; Gaebler, C.; Hoffmann, H.H.; Barnes, C.O.; Cipolla, M.; Ramos, V.; et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature 2021, 595, 426–431. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Pade, C.; Gibbons, J.M.; Butler, D.K.; Otter, A.D.; Menacho, K.; Fontana, M.; Smit, A.; Sackville-West, J.E.; Cutino-Moguel, T.; et al. Prior SARS-CoV-2 infection rescues B and T cell responses to variants after first vaccine dose. Science 2021, 372, 1418–1423. [Google Scholar] [CrossRef]
- Andreano, E.; Paciello, I.; Piccini, G.; Manganaro, N.; Pileri, P.; Hyseni, I.; Leonardi, M.; Pantano, E.; Abbiento, V.; Benincasa, L.; et al. Hybrid immunity improves B cells and antibodies against SARS-CoV-2 variants. Nature 2021, 600, 530–535. [Google Scholar] [CrossRef]
- Andreano, E.; Rappuoli, R. Immunodominant antibody germlines in COVID-19. J. Exp. Med. 2021, 218, e20210281. [Google Scholar] [CrossRef] [PubMed]
- Su, F.; Patel, G.B.; Hu, S.; Chen, W. Induction of mucosal immunity through systemic immunization: Phantom or reality? Hum. Vaccin. Immunother. 2016, 12, 1070–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierra-Martinez, S.; Cruz-Zàrate, D.; Rodriguez-Cornejo, E.R.; Macias-Kauffer, L.R.; Reyes-Montes, M.R.; Acosta-Altamirano, G. Use of a Non-Invasive Method for Detection of nasal and Pharyngeal Neu-tralizing Antibodies in Inmunized Patients against Sars-CoV- 2. Immunol. Res. Ther. J. 2021, 3, 120. [Google Scholar]
- Travis, C.R. As Plain as the Nose on Your Face: The Case for A Nasal (Mucosal) Route of Vaccine Ad-ministration for COVID-19 Disease Prevention. Front. Immunol. 2020, 11, 591897. [Google Scholar] [CrossRef]
- Ejemel, M.; Li, Q.; Hou, S.; Schiller, Z.; Wallace, A.; Amcheslavsky, A.; Yilmaz, N.K.; Toomey, J.; Schneider, R.; Close, B.; et al. IgA MAb blocks SARS-CoV-2 Spike-ACE2 interaction providing mucosal immunity. bioRxiv 2020. [Google Scholar] [CrossRef]
- México Tiene la Mayor Letalidad por COVID-19 Entre las 20 Naciones más Afectadas, Según la Universidad Johns Hopkins. Available online: https://cnnespanol.cnn.com/2021/02/10/mexico-tiene-la-mayor-letalidad-por-covid-19-entre-las-20-naciones-mas-afectadas-segun-la-universidad-johns-hopkins/ (accessed on 6 May 2021).
- Coronavirus (COVID-19) Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations?country=MEX (accessed on 30 January 2022).
- BioNTech Starts Vaccine Production at New Site in Germany. Available online: https://www.dw.com/en/biontech-starts-vaccine-production-at-new-site-in-germany/a-56524305 (accessed on 8 May 2021).
- Secretaria de Relaciones Exteriores: Arriba a México Cargamento con Antígeno de Aztra Zeneca para Producción de Vacunas Contra COVID-19. Available online: https://www.gob.mx/salud/prensa/023-arriba-a-mexico-cargamento-con-antigeno-de-astrazene-ca-para-produccion-de-vacuna-contra-covid-19 (accessed on 20 January 2021).
- Secretaria de Relaciones Exteriores: México Recibe 3 Millones de Dosis de la Vacuna Cansino para su Envasado y Proceso Final. Available online: https://www.gob.mx/sre/prensa/mexico-recibe-3-millones-de-dosis-de-la-vacuna-de-can-sino-para-su-envasado-y-proceso-final?idiom=es (accessed on 11 March 2021).
- Arrecia la Carrera de Mexicanos por el Desarrollo de Vacunas Contra COVID-19. Available online: https://www.conexiones365.com/nota/expo-med/innovacion/vacuna-mexicana-covid19-proyectos (accessed on 1 September 2020).
- Sun, W.; Leist, S.R.; McCroskery, S.; Liu, Y.; Slamanig, S.; Oliva, J.; Amanat, F.; Schäfer, A.; Dinnon, K.H.; García-Sastre, A.; et al. Newcastle disease virus (NDV) expressing the spike protein of SARS-CoV-2 as a live virus vaccine candidate. EBioMedicine 2020, 62, 103132. [Google Scholar] [CrossRef]
- Avimex® Anuncia el Desarrollo de Patria: Vacuna Mexicana contra SARS-CoV-2. Available online: https://avimex.com.mx/noticias-y-eventos/38 (accessed on 16 May 2021).
- Zimmermann, P.; Pittet, L.F.; Finn, A.; Pollard, A.J.; Curtis, N. Should children be vaccinated against COVID-19? Arch. Dis. Child. 2022, 107, e1. [Google Scholar] [CrossRef]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Muñoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 35–46. [Google Scholar] [CrossRef]
- González Farías, A.M.; Martínez Sierra, M.; Sánchez-Conejo, A.R.; González Gozález, T.F.; Juárez García, V.; Acosta-Altamirano, G. Nasal Mask: An alternative to prevent contagion during essential activities. Biomed. J. Sci. Tech. Res. 2021, 34, 27018–27022. [Google Scholar] [CrossRef]
- SARS-CoV-2 Virus Mutations & Variants. Available online: https://www.who.int/docs/default-source/coronaviruse/risk-comms-updates/update47-sars-cov-2-variants.pdf?sfvrsn=f2180835_4 (accessed on 20 May 2021).
- Gupta, T.; Gupta, S.K. Potential adjuvants for the development of a SARS-CoV-2 vaccine based on experimental results from similar coronaviruses. Int. Immunopharmacol. 2020, 86, 106717. [Google Scholar] [CrossRef]
- Liang, Z.; Zhu, H.; Wang, X.; Jing, B.; Li, Z.; Xia, X.; Sun, H.; Yang, Y.; Zhang, W.; Shi, L.; et al. Adjuvants for Coronavirus Vaccines. Front. Immunol. 2020, 11, 589833. [Google Scholar] [CrossRef] [PubMed]
- Chabeda, A.; Yanez, R.J.R.; Lamprecht, R.; Meyers, A.E.; Rybicki, E.P.; Hitzeroth, I.I. Therapeutic vac-cines for high-risk HPV-associated diseases. Papillomavirus Res. 2018, 5, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Nova, E.; Montero, S.; Marcos, A. La estrecha relación entre la nutrición y el sistema inmunitario. In Soporte Nutricional en el Paciente Oncológico; Gloss: Madrid, Spain, 2004; pp. 9–21. [Google Scholar]
- Calder, P.C. Nutrition, immunity and COVID-19. BMJ Nutr. Prev. Health 2020, 3, 74–92. [Google Scholar] [CrossRef] [PubMed]
- Preciado-Ortiz, M.E.; Sánchez-Reyes, K.; Álvarez-Zavala, M.; González-Hernández, L.A.; Ramos-Solano, M.; Andrade-Villanueva, J. Obesidad e infecciones. Rev. Med. 2018, 9, 341–344. [Google Scholar]
- Vacunas Contra el COVID-19 | En Qué Consiste la Pugna por las Patentes (y Cuál Puede ser el Riesgo de Liberarlas). Available online: https://www.bbc.com/mundo/noticias-57016980 (accessed on 21 May 2021).

| Type of Vaccine | |||||||
|---|---|---|---|---|---|---|---|
| Nucleic Acids | Vector Viral | ||||||
| Name | Comirnaty (BNT162b2 mRNA) | mRNA-1273 | CVnCoV | AZD1222 (ChAdOx1) | Ad5-nCov | Sputnik V (Gam-COVID-Vac) | Ad26.COV2.21S (JNJ-78436735 |
| Manufacturing Company | Pfizer/BioNTech | MODERNA | CureVac/Bayer/GSK/No vartis | AstraZeneca/Oxford | CanSino Biological | Gamaleya Research Institute | Janssen Pharmaceutical Companies of Johnson & Johnson (J & J) |
| Handling/ Storage | −70 °C up to 6 months, 2–8 °C up to 5 days, reconstituted up to 6 hrs | −20 °C up to 6 months, 2–8 °C up to 30 days | 2–8 °C up to 3 months | 2–8 °C | 2–8 °C | 1st vial frozen at −18 °C 2nd vial lyophilized at 2–8 °C | 2–8 °C |
| Doses required | Three doses Second dose 21–42 days after the first dose Third dose 6 to 12 months after the second dose | Second dose 28 days after the first one | Second dose 28 days after the first one | Second dose 28 days after the first one | Single dose | Second dose 21 days after the first one | Single dose * |
| Immunization per dose | 100 µg 30 µg (3rd doses) | 30 µg | 12 µg | 0.5 × 1011 Vp | 0.5 × 1011 Vp | 0.5 mL | 0.5 × 1011 Vp |
| % Efficacy in preventing infection | 95% | 94.1% | Phase III data to be published | 82.4% | 65.28% | 92% | 72% in the USA 61% in Latin America |
| Observations | It contains a strand of mRNA that codes for the protein S “Spike” wrapped in a lipid nanoparticle using polyethylene glycol as a stabilizing agent. The third dose is being evaluated in patients 18–55 years and 65–85 years. | It contains a strand of mRNA that codes for the protein S “Spike” wrapped in a lipid nanoparticle. | It contains a strand of mRNA that codes for the protein S “Spike” wrapped in a lipid nanoparticle. Mexico is one of the countries selected for phase III. | Chimpanzee adenovirus containing mRNA encoding protein S “Spike.” | Modified adenovirus serotype Ad5 containing mRNA encoding protein S “Spike.” | The first vial is a modified adenovirus serotype Ad26. The second one is a modified adenovirus serotype Ad5. Both contain double-stranded DNA with the S gene for the “Spike” protein. | Modified adenovirus serotype Ad26 containing double-stranded DNA with the “Spike” protein S gene. |
| Type of Vaccine | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Attenuated Pathogen | Protein Subunities | ||||
| Name | CoronaVac | Covaxin (BBV152 A, B, C) | Not Available | BBIBP-CorV | NVX-CoV2373 | ZF2001 |
| Manufacturing Company/Institution | Sinovac | Bharat Biotech/Indian Council of Medical Research | Sinopharm/Wuhan Institute of Biological Products | Sinopharm/Beijing Institute of Biological Products | NOVAVAX | Anhui Zhifei Longcom Biopharmaceutical Co./Government of Uzbekistan |
| Handling/Storage | 2–8 °C | 2–8 °C | 2–8 °C | 2–8 °C | 2–8 °C | 2–8 °C |
| Doses required | Second dose 14 days after the first one | Second doses 28 days after the first one | Second dose 21 days after the first one | Second dose 21 days after the first one | Second dose 21 days after the first one | 2–3 doses 28 days after the first one |
| Immunization per dose | 3 µg | 3 µg | Unknown | 4 µg | 5 µg SARS-CoV-2 rS + 50 µg of Matrix-M1 adjuvant | 25 µg/0.5 mL |
| % Efficacy in preventing infection | 83.7% in Turkey 50.3% in Brazil | 81% | 72.5% | 79.34% | 96% Original coronavirus 86% variant B.1.1.7 49% variant B.1.351 | Not reported |
| Observations | - | - | - | - | Nanoparticles containing the protein subunit S. | Recombinant origin using CHO cell line to express protein S. |
| Vaccine | Maximum Antibodies | Type of Immunity Reported | Detection Method |
|---|---|---|---|
| RNm-1273 NIAID Moderna | Antibodies have been reported six months after vaccination | CD4 + T H 1 cells (TNF-α> IL-2> IFN-γ), low expression of TH2 cytokines (IL-4 and IL-13) and detectable CD8 + T cell responses | ELISA |
| NT162b1 Pfizer/BioNTech | Antibody rise 14 days after the booster dose | Concurrent production of neutralizing antibodies, activation of CD4 + T lymphocytes biased to TH1 with little response of TH2 (IL-4) and CD8+, virus-specific, and the solid release of immunomodulatory cytokines such as IFNγ. | Flow cytometry, IFNγ ELISpot and cytokine profile |
| CanSino | IgG antibodies at 28 days. Neutralizing antibodies at 8 weeks. | CD4 + and CD8 + T cells produced IFN-γ, TNF-α, and IL-2, with a large proportion of both subsets of T cells being unique IFN-γ producers. Strong IgG1 and IgG2 responses. | ELISA IgG NAb by virus-specific microneutralization |
| ChAdOx1 CoV-19/AZD1222 AstraZeneca | T-cell response from day 7, peaking on day 14 and remaining detectable until day 56. The last analysis detected IgG being at its peak on day 28 and remaining until day 56. | CD4 T + predominantly secreted Th1 cytokines (IFN-γ, IL-2, and TNF-α) rather than Th2 (IL-5 and IL-13). | Detection by IFN-γ ELISPOT assay before and after vaccination and flow cytometry. |
| VX-CoV237 (Novavax) | IgG anti-S: 31/32 days after one dose. Neutralizing antibodies: 21–28 days after the first dose. IgG anti-S: Titers increased 1 to 35-fold within ten days after second dose immunization. | Induced CD4 + and CD8 + T cell response. Matrix-M adjuvant improves the development of Tfh cells and GC B. cells (Vaccine in phase III of clinical trials). | ELISA |
| 26.COV2.S Janssen/Johnson & Johnson | The first dose showed neutralizing antibodies on days 57 and 71. The second dose showed an increase in neutralizing antibody titers at day 57 | Central memory CD27 +/CD45RA−/CD4 + and CD8 + T cell response. Biased TH1 cellular immune response. | LISA, ELISPOT, and IFN-γ assays for cellular immune response. Intracellular cytokine staining for CD4 + and CD8 + T cells. |
| Institution/Company | Financing | Type of Vaccine |
|---|---|---|
| Avimex®, Universidad Nacional Autónoma de México and Instituo Mexicano del Seguro Social. | AMEXCID, CONACyT and SECTEI | Viral vector with nucleic acids. Veterinary platform. |
| Instituto de Biotecnología, Universidad Nacional Autónoma de México | AMEXCID, CONACyT and SECTEI | Viral vector |
| Universidad Autónoma de Querétaro and Instituto Politécnico Nacional | AMEXCID, CONACyT and SECTEI. | Viral vector |
| Universidad Autónoma de Baja California and Tecnológico de Monterrey | AMEXCID, CONACyT and SECTEI | Synthetic nanoparticle |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Estrella, C.U.; Reyes-Montes, M.d.R.; Duarte-Escalante, E.; Sierra Martínez, M.; Frías-De-León, M.G.; Acosta-Altamirano, G. Vaccines Against COVID-19: A Review. Vaccines 2022, 10, 414. https://doi.org/10.3390/vaccines10030414
Torres-Estrella CU, Reyes-Montes MdR, Duarte-Escalante E, Sierra Martínez M, Frías-De-León MG, Acosta-Altamirano G. Vaccines Against COVID-19: A Review. Vaccines. 2022; 10(3):414. https://doi.org/10.3390/vaccines10030414
Chicago/Turabian StyleTorres-Estrella, Carlos U., María del Rocío Reyes-Montes, Esperanza Duarte-Escalante, Mónica Sierra Martínez, María Guadalupe Frías-De-León, and Gustavo Acosta-Altamirano. 2022. "Vaccines Against COVID-19: A Review" Vaccines 10, no. 3: 414. https://doi.org/10.3390/vaccines10030414
APA StyleTorres-Estrella, C. U., Reyes-Montes, M. d. R., Duarte-Escalante, E., Sierra Martínez, M., Frías-De-León, M. G., & Acosta-Altamirano, G. (2022). Vaccines Against COVID-19: A Review. Vaccines, 10(3), 414. https://doi.org/10.3390/vaccines10030414







