COVID-19 in Patients with Inflammatory Bowel Disease: The Israeli Experience
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Participants
2.3. Outcomes and Definitions
2.4. Research Ethics and Patient Consent
2.5. Statistical Analysis
3. Results
3.1. IBD Patient Characteristics
3.2. COVID-19 Characteristics in IBD Patients
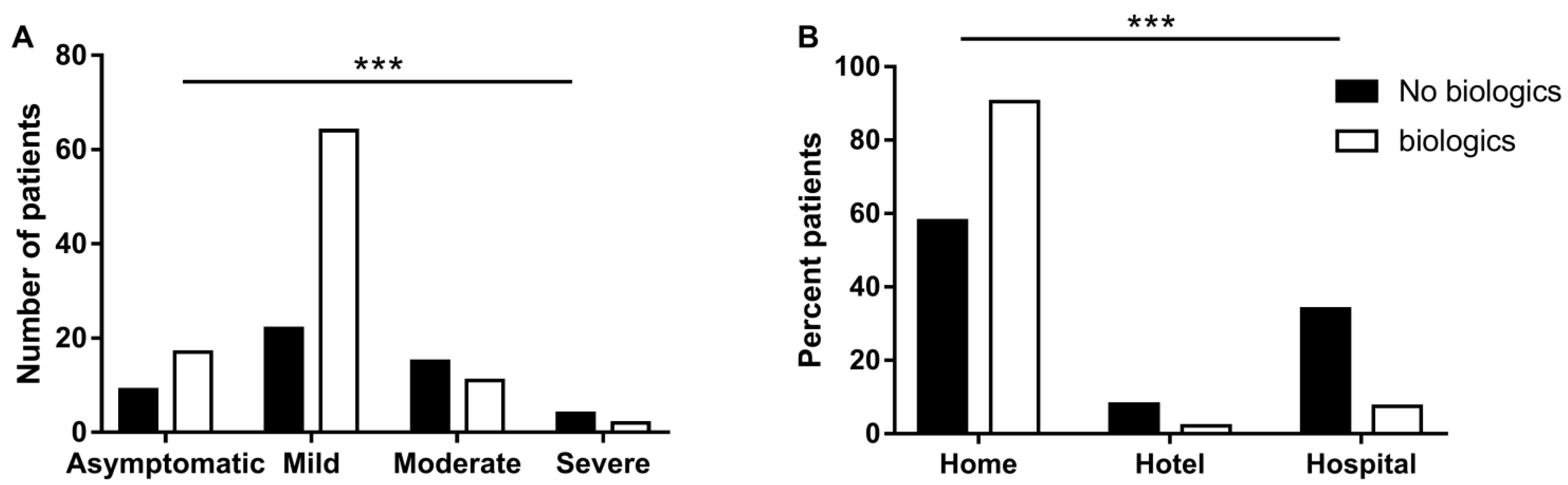
3.3. Biological Treatments for IBD May Be Associated with Favorable Course of COVID-19
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirchgesner, J.; Lemaitre, M.; Carrat, F.; Zureik, M.; Carbonnel, F.; Dray-Spira, R. Risk of Serious and Opportunistic Infections Associated with Treatment of Inflammatory Bowel Diseases. Gastroenterology 2018, 155, 337–346.e10. [Google Scholar] [CrossRef] [Green Version]
- Beaugerie, L.; Kirchgesner, J. Balancing Benefit vs. Risk of Immunosuppressive Therapy for Individual Patients with Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2019, 17, 370–379. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Harmer, D.; Gilbert, M.; Borman, R.; Clark, K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002, 532, 107–110. [Google Scholar] [CrossRef] [Green Version]
- Allocca, M.; Fiorino, G.; Zallot, C.; Furfaro, F.; Gilardi, D.; Radice, S.; Danese, S.; Peyrin-Biroulet, L. Incidence and Patterns of COVID-19 Among Inflammatory Bowel Disease Patients From the Nancy and Milan Cohorts. Clin. Gastroenterol. Hepatol. 2020, 18, 2134–2135. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839. [Google Scholar] [CrossRef]
- Burke, K.E.; Kochar, B.; Allegretti, J.R.; Winter, R.W.; Lochhead, P.; Khalili, H.; Colizzo, F.P.; Hamilton, M.J.; Chan, W.W.; Ananthakrishnan, A.N. Immunosuppressive Therapy and Risk of COVID-19 Infection in Patients with Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2021, 27, 155–161. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Axelrad, J.; Halfvarson, J.; Khalili, H.; Larsson, E.; Lochhead, P.; Roelstraete, B.; Simon, T.G.; Söderling, J.; Olén, O. Inflammatory bowel disease and risk of severe COVID-19: A nationwide population-based cohort study in Sweden. United Eur. Gastroenterol. J. 2021, 9, 177–192. [Google Scholar] [CrossRef]
- Rubin, D.T.; Abreu, M.T.; Rai, V.; Siegel, C.A.; Ahuja, V.; Allez, M.; Ananthakrishnan, A.N.; Bernstein, C.N.; Braun, J.G.; Chowers, Y.; et al. Management of Patients with Crohn’s Disease and Ulcerative Colitis During the Coronavirus Disease-2019 Pandemic: Results of an International Meeting. Gastroenterology 2020, 159, 6–13.e6. [Google Scholar] [CrossRef]
- Kennedy, N.A.; Jones, G.-R.; Lamb, C.A.; Appleby, R.; Arnott, I.; Beattie, R.M.; Bloom, S.; Brooks, A.J.; Cooney, R.; Dart, R.J.; et al. British Society of Gastroenterology guidance for management of inflammatory bowel disease during the COVID-19 pandemic. Gut 2020, 69, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Satsangi, J.; Silverberg, M.S.; Vermeire, S.; Colombel, J.F. The Montreal classification of inflammatory bowel disease: Controversies, consensus, and implications. Gut 2006, 55, 749–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 COVID-19 Treatment Guidelines; The National Institutes of Health: Bethesda, MD, USA, 2019.
- Aziz, M.; Fatima, R.; Haghbin, H.; Lee-Smith, W.; Nawras, A. The Incidence and Outcomes of COVID-19 in IBD Patients: A Rapid Review and Meta-analysis. Inflamm. Bowel Dis. 2020, 26, e132–e133. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Ardizzone, S. Are Patients with Inflammatory Bowel Disease at Increased Risk for Covid-19 Infection? J. Crohn’s Colitis 2020, 14, 1334–1336. [Google Scholar] [CrossRef]
- Singh, S.; Khan, A.; Chowdhry, M.; Bilal, M.; Kochhar, G.S.; Clarke, K. Risk of Severe Coronavirus Disease 2019 in Patients with Inflammatory Bowel Disease in the United States: A Multicenter Research Network Study. Gastroenterology 2020, 159, 1575–1578.e4. [Google Scholar] [CrossRef]
- Singh, A.K.; Jena, A.; Kumar-M, P.; Sharma, V.; Sebastian, S. Risk and outcomes of coronavirus disease in patients with inflammatory bowel disease: A systematic review and meta-analysis. UEG J. 2020, 9, 159–176. [Google Scholar] [CrossRef]
- An, P.; Ji, M.; Ren, H.; Su, J.; Ding, N.S.; Kang, J.; Yin, A.; Zhou, Q.; Shen, L.; Zhao, L.; et al. Prevention of COVID-19 in patients with inflammatory bowel disease in Wuhan, China. Lancet Gastroenterol. Hepatol. 2020, 5, 525–527. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Feagan, B.G.; Lichtenstein, G.R.; Mayer, L.F.; Schreiber, S.; Colombel, J.F.; Rachmilewitz, D.; Wolf, D.C.; Olson, A.; Bao, W.; et al. Maintenance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet 2002, 359, 1541–1549. [Google Scholar] [CrossRef]
- Colombel, J.F.; Sandborn, W.J.; Reinisch, W.; Mantzaris, G.J.; Kornbluth, A.; Rachmilewitz, D.; Lichtiger, S.; D’Haens, G.; Diamond, R.H.; Broussard, D.L.; et al. Diamond Infliximab, azathioprine, or combination therapy for Crohn’s disease. N. Engl. J. Med. 2010, 362, 1383–1395. [Google Scholar] [CrossRef] [Green Version]
- Din, S.; Cochrane, C.J.; Noble, C.L.; Satsangi, J.; Arnott, I.D. Combination therapy of infliximab and azathioprine reduces disease progression in Crohn’s disease. Inflamm. Bowel Dis. 2008, 14, 143–145. [Google Scholar] [CrossRef]
- Gianfrancesco, M.; Hyrich, K.L.; Al-Adely, S.; Carmona, L.; Danila, M.I.; Gossec, L.; Izadi, Z.; Jacobsohn, L.; Katz, P.; Lawson-Tovey, S.; et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: Data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann. Rheum. Dis. 2020, 79, 859–866. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Royce, S.G.; Tikellis, C.; Shallue, C.; Batu, D.; Velkoska, E.; Burrell, L.M.; Patel, S.K.; Beswick, L.; Jackson, A.; et al. Imbalance of the renin-angiotensin system may contribute to inflammation and fibrosis in IBD: A novel therapeutic target? Gut 2020, 69, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Burrell, L.M.; Velkoska, E.; Griggs, K.; Angus, P.W.; Gibson, P.R.; Lubel, J.S. Upregulation of circulating components of the alternative renin-angiotensin system in inflammatory bowel disease: A pilot study. J. Renin-Angiotensin-Aldosterone Syst. 2014, 16, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Batlle, D.; Wysocki, J.; Satchell, K. Soluble angiotensin-converting enzyme 2: A potential approach for coronavirus infection therapy? Clin. Sci. 2020, 134, 543–545. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.K.; Gracias, D.; Croft, M. TNF activity and T cells. Cytokine 2018, 101, 14–18. [Google Scholar] [CrossRef]
- Burgueño, J.F.; Abreu, M.T. Reply to the Letter to the Editor: The Incidence and Outcomes of COVID-19 in Patients with IBD: A Rapid Review and Meta-Analysis. Inflamm. Bowel Dis. 2020, 26, e127. [Google Scholar] [CrossRef]
- Mahase, E. Covid-19: Anti-TNF drug adalimumab to be trialled for patients in the community. BMJ 2020, 371, m3847. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Ghosh, S.; Panes, J.; Vranic, I.; Su, C.; Rousell, S.; Niezychowski, W. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N. Engl. J. Med. 2012, 367, 616–624. [Google Scholar] [CrossRef] [Green Version]
- Weisshof, R.; Golan, M.A.; Sossenheimer, P.H.; El Jurdi, K.; Ollech, J.E.; Pekow, J.; Cohen, R.D.; Sakuraba, A.; Dalal, S.; Rubin, D.T. Real-World Experience with Tofacitinib in IBD at a Tertiary Center. Am. J. Dig. Dis. 2019, 64, 1945–1951. [Google Scholar] [CrossRef]
- Brenner, E.J.; Ungaro, R.C.; Gearry, R.B.; Kaplan, G.G.; Kissous-Hunt, M.; Lewis, J.D.; Ng, S.C.; Rahier, J.-F.; Reinisch, W.; Ruemmele, F.M.; et al. Corticosteroids, But Not TNF Antagonists, Are Associated with Adverse COVID-19 Outcomes in Patients with Inflammatory Bowel Diseases: Results From an International Registry. Gastroenterology 2020, 159, 481–491.e3. [Google Scholar] [CrossRef]
- Vardavas, C.I.; Nikitara, K. COVID-19 and smoking: A systematic review of the evidence. Tob. Induc. Dis. 2020, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Ruiz, C.A.; Lopez-Padilla, D.; Alonso-Arroyo, A.; Aleixandre-Benavent, R.; Solano-Reina, S.; de Granda-Orive, J.I. COVID-19 and Smoking: A Systematic Review and Meta-Analysis of the Evidence. Arch. Bronconeumol. 2020, 57, 21–34. [Google Scholar] [PubMed]
| CD (n = 104) | UC (n = 40) | p | |||
|---|---|---|---|---|---|
| Gender (% Male) | 46 (44.2%) | 18 (45%) | 0.934 | ||
| Montreal classification | |||||
| B1 | 69 (66.3%) | E1 | 4 (10%) | ||
| B2 | 23 (22.1%) | E2 | 12 (30%) | ||
| B3 | 12 (11.5%) | E3 | 20 (50%) | ||
| perianal disease | 21 (20.2%) | colectomy | 4 (10%) | ||
| IBD disease severity | |||||
| Remission/Asymptomatic | 17 (16.3%) | 12 (30%) | p < 0.001 | ||
| S1—Mild | 51 (49%) | 6 (15%) | |||
| S2—Moderate | 25 (24%) | 10 (25%) | |||
| S3—Severe | 11 (10.6%) | 8 (20%) | |||
| Treatment | Total (n) | Mild/Asymptomatic | Moderate/Severe | p |
|---|---|---|---|---|
| Anti-TNF | 52 | 50 | 2 | p = 0.002 |
| Vedolizumab | 23 | 22 | 1 | p = 0.084 |
| Ustekinumab | 12 | 10 | 2 | 1 |
| Other * | 7 | |||
| Total biologics | 94 | 87 | 7 | <0.001 |
| Biologics only | 76 | 71 | 5 | 0.001 |
| Biologics + steroids | 9 | 7 | 2 | 0.644 |
| Biologics + IM | 10 | 10 | 0 | 0.143 |
| IM monotherapy | 12 | 11 | 1 | 0.418 |
| Steroids only | 8 | 7 | 1 | 0.745 |
| IM monotherapy + steroids | 2 | 2 | 0 | 0.524 |
| 5-ASA | 29 | 25 | 4 | 0.642 |
| none | 18 | 7 | 11 | <0.001 |
| IBD | CD (n = 104) | UC (n = 40) | p Value |
|---|---|---|---|
| Asymptomatic | 25 (24%) | 5 (12.5%) | 0.08 |
| Mild | 64 (61.5%) | 26 (65%) | |
| Moderate | 8 (7.7%) | 8 (20%) | |
| Severe | 7 (6.7%) | 1 (2.5%) | |
| Setting | |||
| Home | 84 (80.8%) | 30 (75%) | 0.747 |
| Hotel | 4 (3.8%) | 2 (5%) | |
| Hospital | 16 (15.4%) | 8 (20%) | |
| Symptoms for COVID-19 | |||
| Fever | 49 (47.1%) | 16 (40%) | 0.442 |
| Cough | 43 (41.3%) | 15 (37.5%) | 0.673 |
| Shortness of breath | 9 (8.7%) | 10 (25%) | 0.009 |
| Fatigue | 9 (8.7%) | 3 (7.5%) | 0.822 |
| Headache | 9 (8.7%) | 5 (12.5%) | 0.485 |
| Dysgeusia | 13 (12.5%) | 5 (12.5%) | 1 |
| Throat ache | 4 (3.8%) | 3 (7.5%) | 0.361 |
| GI Pain | 19 (18.3%) | 8 (20%) | 0.3 |
| Vomiting | 5 (4.8%) | 2 (5%) | 0.693 |
| Nausea | 2 (1.9%) | 1 (2.5%) | 0.65 |
| Diarrhea * | 11 (10.5%) | 6 (15%) | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lichtenstein, L.; Koslowsky, B.; Ben Ya’acov, A.; Avni-Biron, I.; Ovadia, B.; Ben-Bassat, O.; Naftali, T.; Kopylov, U.; Haberman, Y.; Eran, H.B.; et al. COVID-19 in Patients with Inflammatory Bowel Disease: The Israeli Experience. Vaccines 2022, 10, 376. https://doi.org/10.3390/vaccines10030376
Lichtenstein L, Koslowsky B, Ben Ya’acov A, Avni-Biron I, Ovadia B, Ben-Bassat O, Naftali T, Kopylov U, Haberman Y, Eran HB, et al. COVID-19 in Patients with Inflammatory Bowel Disease: The Israeli Experience. Vaccines. 2022; 10(3):376. https://doi.org/10.3390/vaccines10030376
Chicago/Turabian StyleLichtenstein, Lev, Benjamin Koslowsky, Ami Ben Ya’acov, Irit Avni-Biron, Baruch Ovadia, Ofer Ben-Bassat, Timna Naftali, Uri Kopylov, Yael Haberman, Hagar Banai Eran, and et al. 2022. "COVID-19 in Patients with Inflammatory Bowel Disease: The Israeli Experience" Vaccines 10, no. 3: 376. https://doi.org/10.3390/vaccines10030376
APA StyleLichtenstein, L., Koslowsky, B., Ben Ya’acov, A., Avni-Biron, I., Ovadia, B., Ben-Bassat, O., Naftali, T., Kopylov, U., Haberman, Y., Eran, H. B., Eliakim, R., Lahat-Zok, A., Hirsch, A., Zittan, E., Maharshak, N., Waterman, M., Israeli, E., Goren, I., Ollech, J. E., ... Bar-Gil Shitrit, A. (2022). COVID-19 in Patients with Inflammatory Bowel Disease: The Israeli Experience. Vaccines, 10(3), 376. https://doi.org/10.3390/vaccines10030376








