Efficacy of a Smartphone Application to Promote Maternal Influenza Vaccination: A Randomized Controlled Trial
Abstract
:1. Introduction
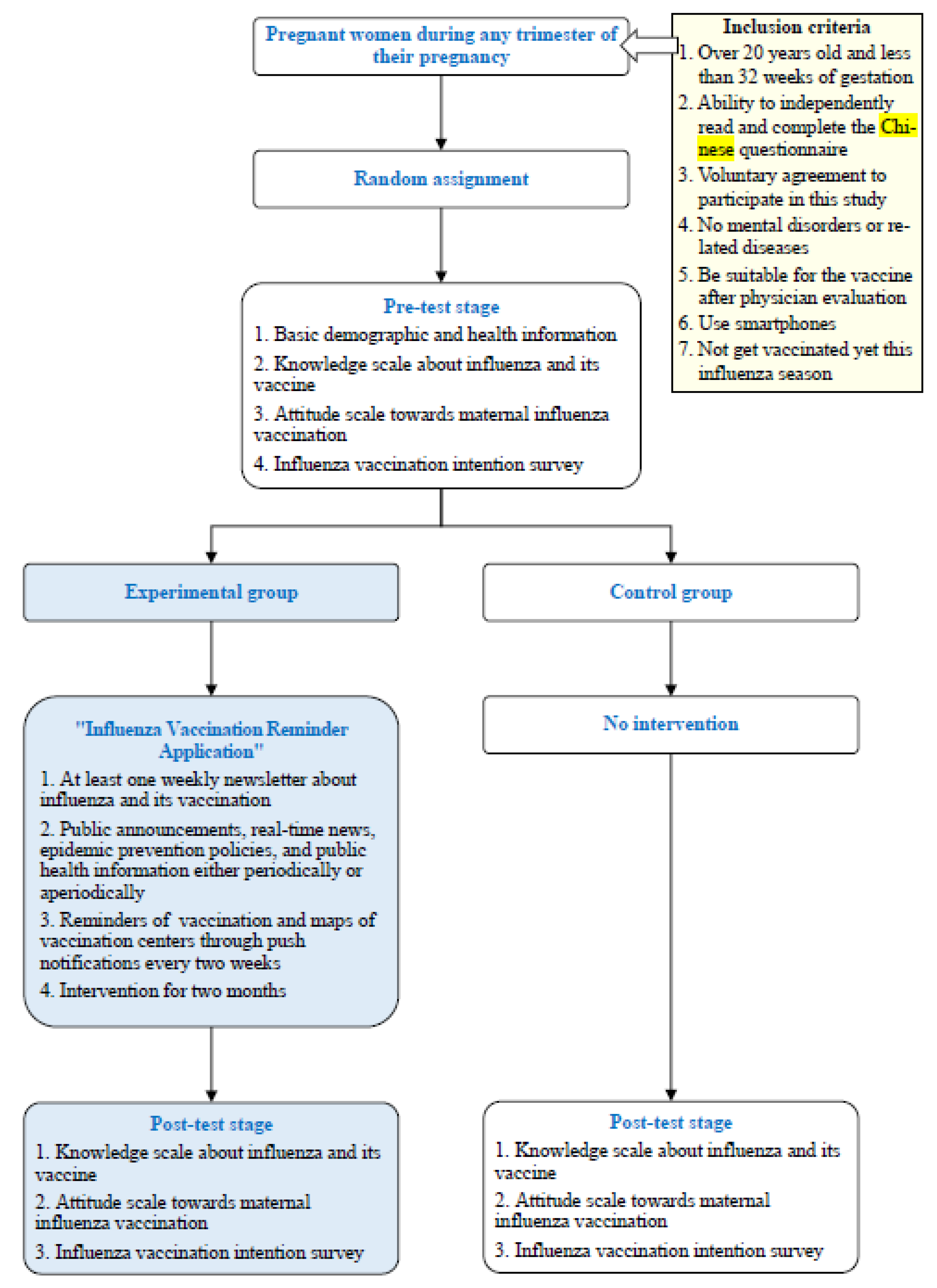
2. Materials and Methods
2.1. Study Design and Population
2.2. Study Procedure
- (1)
- Pre-test stage
- (2)
- Post-test stage
2.3. Development of Influenza Vaccination Reminder Application
- (1)
- Reminder function (Home page)
- (2)
- Knowledge base function
- (3)
- Calendar function
- (4)
- Vaccination feedback function
2.4. Measurement of Characteristics and Outcomes
- (1)
- Knowledge scale about influenza and its vaccine
- (2)
- Attitude scale towards maternal influenza vaccination
- (3)
- Influenza vaccination intention survey
2.5. Statistical Analysis
3. Results
3.1. Sociodemographic Characteristics
3.2. Knowledge about Influenza and Its Vaccine
3.3. Attitudes towards Maternal Influenza Vaccination
3.4. Influenza Vaccination Intention
3.5. Effect of the Intervention on Positive Change in Vaccination Intention
4. Discussion
5. Conclusions
6. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mertz, D.; Kim, T.H.; Johnstone, J.; Lam, P.-P.; Science, M.; Kuster, S.P.; Fadel, S.A.; Tran, D.; Fernandez, E.; Bhatnagar, N.; et al. Populations at risk for severe or complicated influenza illness: Systematic review and meta-analysis. BMJ 2013, 347, f5061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, M.C.; Madhi, S.A. Influenza vaccination during pregnancy for prevention of influenza confirmed illness in the infants: A systematic review and meta-analysis. Hum. Vaccines Immunother. 2018, 14, 758–766. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poehling, K.A.; Szilagyi, P.G.; Staat, M.A.; Snively, B.M.; Payne, D.C.; Bridges, C.B.; Chu, S.Y.; Light, L.S.; Prill, M.M.; Finelli, L.; et al. Impact of maternal immunization on influenza hospitalizations in infants. Am. J. Obstet. Gynecol. 2011, 204, S141–S148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, M.C.; Madhi, S.A. Prevention of influenza-related illness in young infants by maternal vaccination during pregnancy. F1000Research 2018, 7, 122. [Google Scholar] [CrossRef]
- Zaman, K.; Roy, E.; Arifeen, S.E.; Rahman, M.; Raqib, R.; Wilson, E.; Omer, S.B.; Shahid, N.S.; Breiman, R.F.; Steinhoff, M.C. Effectiveness of maternal influenza immunization in mothers and infants. N. Engl. J. Med. 2008, 359, 1555–1564. [Google Scholar] [CrossRef] [Green Version]
- Ding, H.; Black, C.L.; Ball, S.; Donahue, S.; Fink, R.V.; Williams, W.W.; Kennedy, E.D.; Bridges, C.B.; Lu, P.J.; Kahn, K.E.; et al. Influenza vaccination coverage among pregnant women—United States, 2014–2015 influenza season. Morb. Mortal. Wkly. Rep. 2015, 64, 1000–1005. [Google Scholar] [CrossRef]
- Henninger, M.; Naleway, A.; Crane, B.; Donahue, J.; Irving, S. Predictors of Seasonal Influenza Vaccination during Pregnancy. Obstet. Gynecol. 2013, 121, 741–749. [Google Scholar] [CrossRef] [PubMed]
- WHO. Vaccines against influenza WHO position paper—November 2012. Wkly. Epidemiol. Rec. 2012, 87, 461–476. [Google Scholar]
- Blanchard-Rohner, G.; Eberhardt, C. Review of maternal immunisation during pregnancy: Focus on pertussis and influenza. Swiss Med. Wkly. 2017, 147, w14526. [Google Scholar] [CrossRef]
- Sheffield, J.S.; Greer, L.G.; Rogers, V.L.; Roberts, S.W.; Lytle, H.; McIntire, D.D.; Wendel, G.D. Effect of influenza vaccination in the first trimester of pregnancy. Obstet. Gynecol. 2012, 120, 532–537. [Google Scholar] [CrossRef]
- Ahluwalia, I.B.; Jamieson, D.J.; Rasmussen, S.A.; D’Angelo, D.; Goodman, D.; Kim, H. Correlates of seasonal influenza vaccine coverage among pregnant women in Georgia and Rhode Island. Obstet. Gynecol. 2010, 116, 949–955. [Google Scholar] [CrossRef]
- Dlugacz, Y.; Fleischer, A.; Carney, M.T.; Copperman, N.; Ahmed, I.; Ross, Z.; Buchman, T.; Fried, A.M.; Cabello, C.; De Geronimo, M.; et al. 2009 H1N1 vaccination by pregnant women during the 2009-10 H1N1 influenza pandemic. Am. J. Obstet. Gynecol. 2012, 206, 339.e1–339.e8. [Google Scholar] [CrossRef]
- Yudin, M.H.; Mistry, N.; De Souza, L.R.; Besel, K.; Patel, V.; Blanco Mejia, S.; Bernick, R.; Ryan, V.; Urquia, M.; Beigi, R.H.; et al. Text messages for influenza vaccination among pregnant women: A randomized controlled trial. Vaccine 2017, 35, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Shearer, M.P.; Chih, Y.C.; Hsu, Y.C.; Lin, Y.C.; Nuzzo, J.B. Taiwan’s Annual Seasonal Influenza Mass Vaccination Program-Lessons for Pandemic Planning. Am. J. Public Health 2018, 108, S188–S193. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.W.; Tsai, S.M.; Lin, P.C.; Chou, F.H. Willingness to receive influenza vaccination during pregnancy and associated factors among pregnant women in Taiwan. Public Health Nurs. 2019, 36, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Free, C.; Phillips, G.; Galli, L.; Watson, L.; Felix, L.; Edwards, P.; Patel, V.; Haines, A. The effectiveness of mobile-health technology-based health behaviour change or disease management interventions for health care consumers: A systematic review. PLoS Med. 2013, 10, e1001362. [Google Scholar] [CrossRef] [Green Version]
- Regan, A.K.; Bloomfield, L.; Peters, I.; Effler, P.V. Randomized controlled trial of text message reminders for increasing influenza vaccination. Ann. Fam. Med. 2017, 15, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Dudley, M.Z.; Limaye, R.J.; Salmon, D.A.; Omer, S.B.; O’Leary, S.T.; Ellingson, M.K.; Spina, C.I.; Brewer, S.E.; Bednarczyk, R.A.; Malik, F.; et al. Latent Class Analysis of Maternal Vaccine Attitudes and Beliefs. Health Educ. Behav. 2020, 47, 765–781. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, Y.; Liang, H.; Chen, Y. Seasonal Influenza Vaccine Acceptance among Pregnant Women in Zhejiang Province, China: Evidence Based on Health Belief Model. Int. J. Environ. Res. Public Health 2017, 14, 1551. [Google Scholar] [CrossRef] [Green Version]
- Maltezou, H.C.; Pelopidas Koutroumanis, P.; Kritikopoulou, C.; Theodoridou, K.; Katerelos, P.; Tsiaousi, I.; Rodolakis, A.; Loutradis, D. Knowledge about influenza and adherence to the recommendations for influenza vaccination of pregnant women after an educational intervention in Greece. Hum. Vaccines Immunother. 2019, 15, 1070–1074. [Google Scholar] [CrossRef] [PubMed]
- Rosenstock, I.M.; Strecher, V.J.; Becker, M.H. Social learning theory and the health belief model. Health Educ. Q. 1988, 15, 175–183. [Google Scholar] [CrossRef]
- Looijmans-Van Den Akker, I.; Van Delden, J.; Verheij, T.J.; Van Essen, G.; Van der Sande, M.; Hulscher, M.; Hak, E. Which determinants should be targeted to increase influenza vaccination uptake among health care workers in nursing homes? Vaccine 2009, 27, 4724–4730. [Google Scholar] [CrossRef] [PubMed]
- Gorman, J.R.; Brewer, N.T.; Wang, J.B.; Chambers, C.D. Theory-based predictors of influenza vaccination among pregnant women. Vaccine 2012, 31, 213–218. [Google Scholar] [CrossRef]
- Bödeker, B.; Walter, D.; Reiter, S.; Wichmann, O. Cross-sectional study on factors associated with influenza vaccine uptake and pertussis vaccination status among pregnant women in Germany. Vaccine 2014, 32, 4131–4139. [Google Scholar] [CrossRef]
- Eppes, C.; Wu, A.; You, W.; Cameron, K.A.; Garcia, P.; Grobman, W. Barriers to influenza vaccination among pregnant women. Vaccine 2013, 31, 2874–2878. [Google Scholar] [CrossRef]
- Kang, H.S.; De Gagne, J.C.; Kim, J.H. Attitudes, Intentions, and Barriers Toward Influenza Vaccination Among Pregnant Korean Women. Health Care Women Int. 2015, 36, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, F.; Napolitano, P.; Angelillo, I.F. Seasonal influenza vaccination in pregnant women: Knowledge, attitudes, and behaviors in Italy. BMC Infect. Dis. 2017, 17, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamberlain, A.T.; Seib, K.; Ault, K.A.; Orenstein, W.A.; Frew, P.M.; Malik, F.; Cortés, M.; Cota, P.; Whitney, E.A.S.; Flowers, L.C.; et al. Factors associated with intention to receive influenza and tetanus, diphtheria, and acellular pertussis (Tdap) vaccines during pregnancy: A focus on vaccine hesitancy and perceptions of disease severity and vaccine safety. PLoS Curr. Outbreaks 2015, 7. [Google Scholar] [CrossRef]
- Vojtek, I.; Dieussaert, I.; Doherty, T.M.; Franck, V.; Hanssens, L.; Miller, J.; Bekkat-Berkani, R.; Kandeil, W.; Prado-Cohrs, D.; Vyse, A. Maternal immunization: Where are we now and how to move forward? Ann. Med. 2018, 50, 193–208. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Smith, P.; Yee, L.M. Mobile Phone-Based Behavioral Interventions in Pregnancy to Promote Maternal and Fetal Health in High-Income Countries: Systematic Review. JMIR Mhealth Uhealth 2020, 8, e15111. [Google Scholar] [CrossRef]
- Healy, C.M.; Rench, M.A.; Montesinos, D.P.; Ng, N.; Swaim, L.S. Knowledge and attitiudes of pregnant women and their providers towards recommendations for immunization during pregnancy. Vaccine 2015, 33, 5445–5451. [Google Scholar] [CrossRef] [PubMed]
- Marshall, H.; McMillan, M.; Andrews, R.; Macartney, K.; Edwards, K. Vaccines in pregnancy: The dual benefit for pregnant women and infants. Hum. Vaccines Immunother. 2016, 12, 848–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisset, K.A.; Paterson, P. Strategies for increasing uptake of vaccination in pregnancy in high-income countries: A systematic review. Vaccine 2018, 36, 2751–2759. [Google Scholar] [CrossRef] [PubMed]
- Ellingson, M.; Chamberlain, A.T. Beyond the verbal: Pregnant women’s preferences for receiving influenza and Tdap vaccine information from their obstetric care providers. Hum. Vaccines Immunother. 2018, 14, 767–771. [Google Scholar] [CrossRef] [Green Version]
- Forsyth, K.; Plotkin, S.; Tan, T.; Wirsing von König, C.H. Strategies to decrease pertussis transmission to infants. Pediatrics 2015, 135, e1475–e1482. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Blanco, N.; Tuells, J.; Vila-Candel, R.; Nolasco, A. Adherence and Concordance of Influenza and Pertussis Vaccination Coverage in Pregnant Women in Spain. Int. J. Environ. Res. Public Health 2019, 16, 543. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.H.; Lee, J.H.; Kim, B.C.; Jung, S.U.; Park, Y.B.; Lee, C.S. Adverse reaction of influenza A (H1N1) 2009 virus vaccination in pregnant women and its effect on newborns. Vaccine 2010, 28, 7455–7456. [Google Scholar] [CrossRef]
- Cutrona, S.L.; Golden, J.G.; Goff, S.L.; Ogarek, J.; Barton, B.; Fisher, L.; Preusse, P.; Sundaresan, D.; Garber, L.; Mazor, K.M. Improving Rates of Outpatient Influenza Vaccination Through EHR Portal Messages and Interactive Automated Calls: A Randomized Controlled Trial. J. Gen. Intern. Med. 2018, 33, 659–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moniz, M.H.; Hasley, S.; Meyn, L.A.; Beigi, R.H. Improving Influenza Vaccination Rates in Pregnancy Through Text Messaging: A Randomized Controlled Trial. Obstet. Gynecol. 2013, 121, 734–740. [Google Scholar] [CrossRef]
- Iribarren, S.J.; Cato, K.; Falzon, L.; Stone, P.W. What is the economic evidence for mHealth? A systematic review of economic evaluations of mHealth solutions. PLoS ONE 2017, 12, e0170581. [Google Scholar] [CrossRef]
| Sociodemographic Variables | All (n = 243) | Experimental Group (n = 126) | Control Group (n = 117) | ||
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | χ2 a | p | |
| Age group | 0.35 | 0.56 | |||
| <34 years | 150 (61.73) | 80 (63.49) | 70 (59.83) | ||
| ≥34 years | 93 (38.27) | 46 (36.51) | 47 (40.17) | ||
| Trimester of pregnancy | 6.04 | 0.05 | |||
| 1st trimester | 34 (13.99) | 20 (15.87) | 14 (11.97) | ||
| 2nd trimester | 81 (33.33) | 33 (26.19) | 48 (41.03) | ||
| 3rd trimester | 128 (52.68) | 73 (57.94) | 55 (47.00) | ||
| Marital status | 0.17 | 0.68 | |||
| Unmarried | 15 (6.17) | 7 (5.56) | 8 (6.84) | ||
| Married | 228 (93.83) | 119 (94.44) | 109 (93.16) | ||
| Highest education | 2.87 | 0.09 | |||
| ≤High school | 55 (22.63) | 23 (18.25) | 32 (27.35) | ||
| ≥Junior college | 188 (77.37) | 103 (81.75) | 85 (72.65) | ||
| Employment status | 0.03 | 0.86 | |||
| Unemployed | 51 (20.99) | 27 (21.43) | 24 (20.51) | ||
| Employed | 192 (79.01) | 99 (78.57) | 93 (79.49) | ||
| History of illness | 3.25 | 0.07 | |||
| No | 215 (88.48) | 107 (84.92) | 108 (92.31) | ||
| Yes | 28 (11.52) | 19 (15.08) | 9 (7.69) | ||
| Gestational complication | 0.21 | 0.65 | |||
| No | 231 (95.06) | 119 (94.44) | 112 (95.73) | ||
| Yes | 12 (4.94) | 7 (5.56) | 5 (4.27) | ||
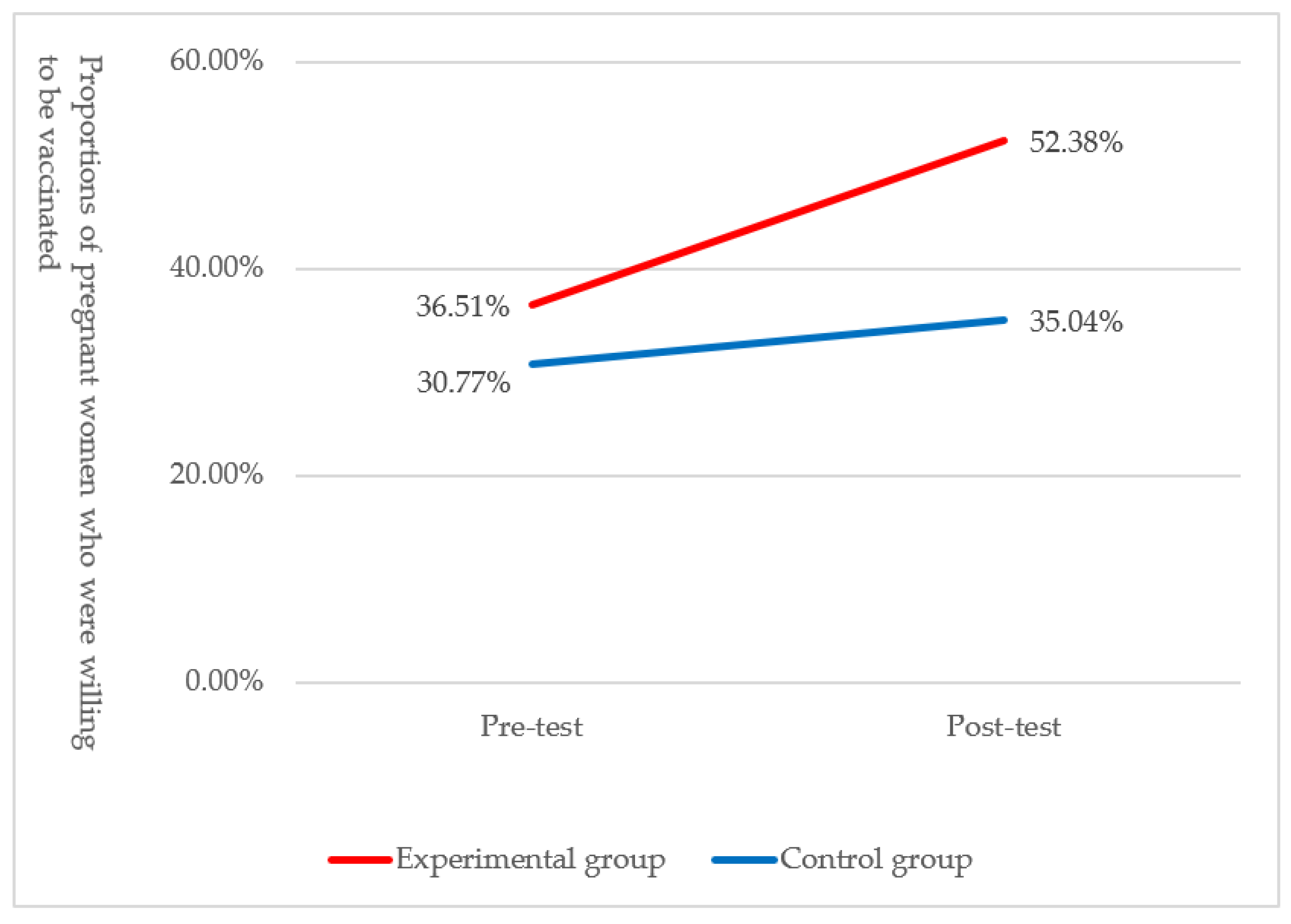
| Post-Test | Experimental Group (n = 126) | Control Group (n = 117) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Test | With Intention (%) a | Without Intention (%) a | Total (%) a | pb | With Intention (%) c | Without Intention (%) c | Total (%) c | pb | |
| With | 44 | 2 | 46 | <0.01 | 32 | 4 | 36 | 0.27 | |
| intention | (34.92) | (1.59) | (36.51) | (27.35) | (3.42) | (30.77) | |||
| Without | 22 | 58 | 80 | 9 | 72 | 81 | |||
| intention | (17.46) | (46.03) | (63.49) | (7.69) | (61.54) | (69.23) | |||
| Total | 66 | 60 | 126 | 41 | 76 | 117 | |||
| (52.38) | (47.62) | (35.04) | (64.96) | ||||||
| Experimental Group (n = 126) | Control Group (n = 117) | pa | Odds Ratio b | |
|---|---|---|---|---|
| Pre-test, n (% c) | 46 (36.51) | 36 (30.77) | 0.34 | |
| Post-test, n (% c) | 66 (52.38) | 41 (35.04) | 0.01 | |
| pd | <0.01 | 0.27 | 1.58 |
| Predictive Variables | n | Unadjusted Odds Ratio | Adjusted Odds Ratio | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p | aOR | 95% CI | p | ||
| Group | ---- | ---- | |||||
| Control group | 117 | (Ref) | (Ref) | ||||
| Experimental group | 126 | 2.54 | 1.12~5.77 | 0.03 | 2.41 | 1.04~5.55 | 0.03 |
| Pre-test total knowledge score | 0.32 | 0.43 | |||||
| ≥15 | 113 | (Ref) | (Ref) | ||||
| 8~14 | 112 | 0.50 | 0.06~4.06 | 0.51 | 0.45 | 0.05~3.90 | 0.47 |
| ≤7 | 18 | 1.61 | 0.74~3.53 | 0.23 | 1.44 | 0.64~3.22 | 0.38 |
| Pre-test total attitude score | 0.10 | 0.11 | |||||
| ≥120 | 17 | (Ref) | (Ref) | ||||
| 88~119 | 181 | 4.57 | 0.54~38.82 | 0.16 | 4.28 | 0.48~38.11 | 0.19 |
| ≤87 | 45 | 1.99 | 0.25~15.80 | 0.52 | 1.78 | 0.22~14.62 | 0.59 |
| Omnibus test | χ2 | df | p | ||||
| 11.63 | 5 | 0.04 | |||||
| Hosmer & Lemeshow goodness-of-fit test | χ2 | df | p | ||||
| 3.97 | 7 | 0.78 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-W.; Tsai, S.-M.; Lin, P.-C.; Chou, F.-H. Efficacy of a Smartphone Application to Promote Maternal Influenza Vaccination: A Randomized Controlled Trial. Vaccines 2022, 10, 369. https://doi.org/10.3390/vaccines10030369
Chang Y-W, Tsai S-M, Lin P-C, Chou F-H. Efficacy of a Smartphone Application to Promote Maternal Influenza Vaccination: A Randomized Controlled Trial. Vaccines. 2022; 10(3):369. https://doi.org/10.3390/vaccines10030369
Chicago/Turabian StyleChang, Ya-Wen, Shiow-Meei Tsai, Pao-Chen Lin, and Fan-Hao Chou. 2022. "Efficacy of a Smartphone Application to Promote Maternal Influenza Vaccination: A Randomized Controlled Trial" Vaccines 10, no. 3: 369. https://doi.org/10.3390/vaccines10030369
APA StyleChang, Y.-W., Tsai, S.-M., Lin, P.-C., & Chou, F.-H. (2022). Efficacy of a Smartphone Application to Promote Maternal Influenza Vaccination: A Randomized Controlled Trial. Vaccines, 10(3), 369. https://doi.org/10.3390/vaccines10030369





