Duration of SARS-CoV-2 Immune Responses Up to Six Months Following Homologous or Heterologous Primary Immunization with ChAdOx1 nCoV-19 and BNT162b2 mRNA Vaccines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Humoral Immune Response
2.2. Live-Virus Microneutralization
2.3. T-Cell Responses
2.4. Statistics
3. Results
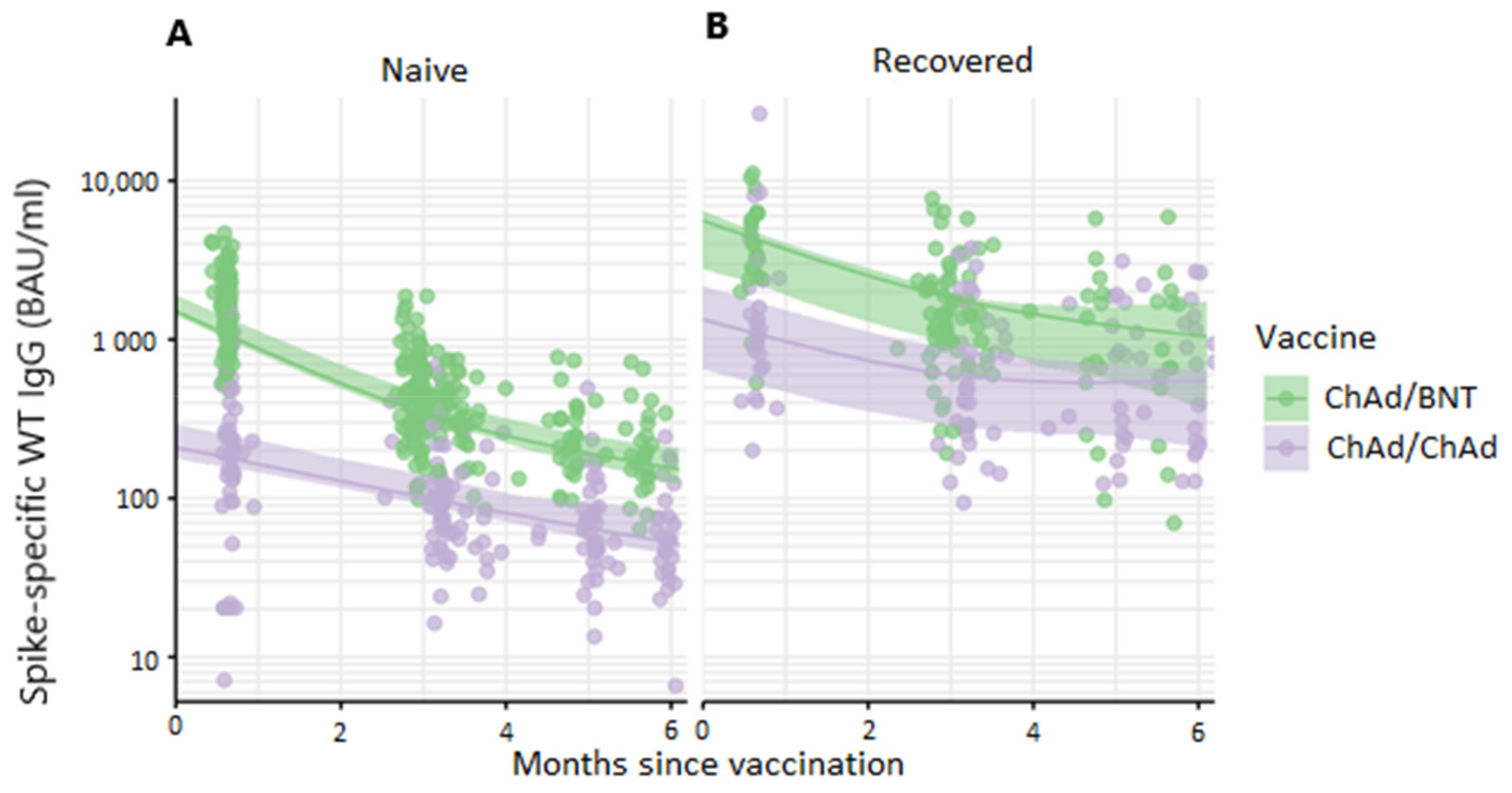
3.1. Antibody Titers Up to Six Months after Primary Immunization with Heterologous ChAd/BNT or Homologous ChAd
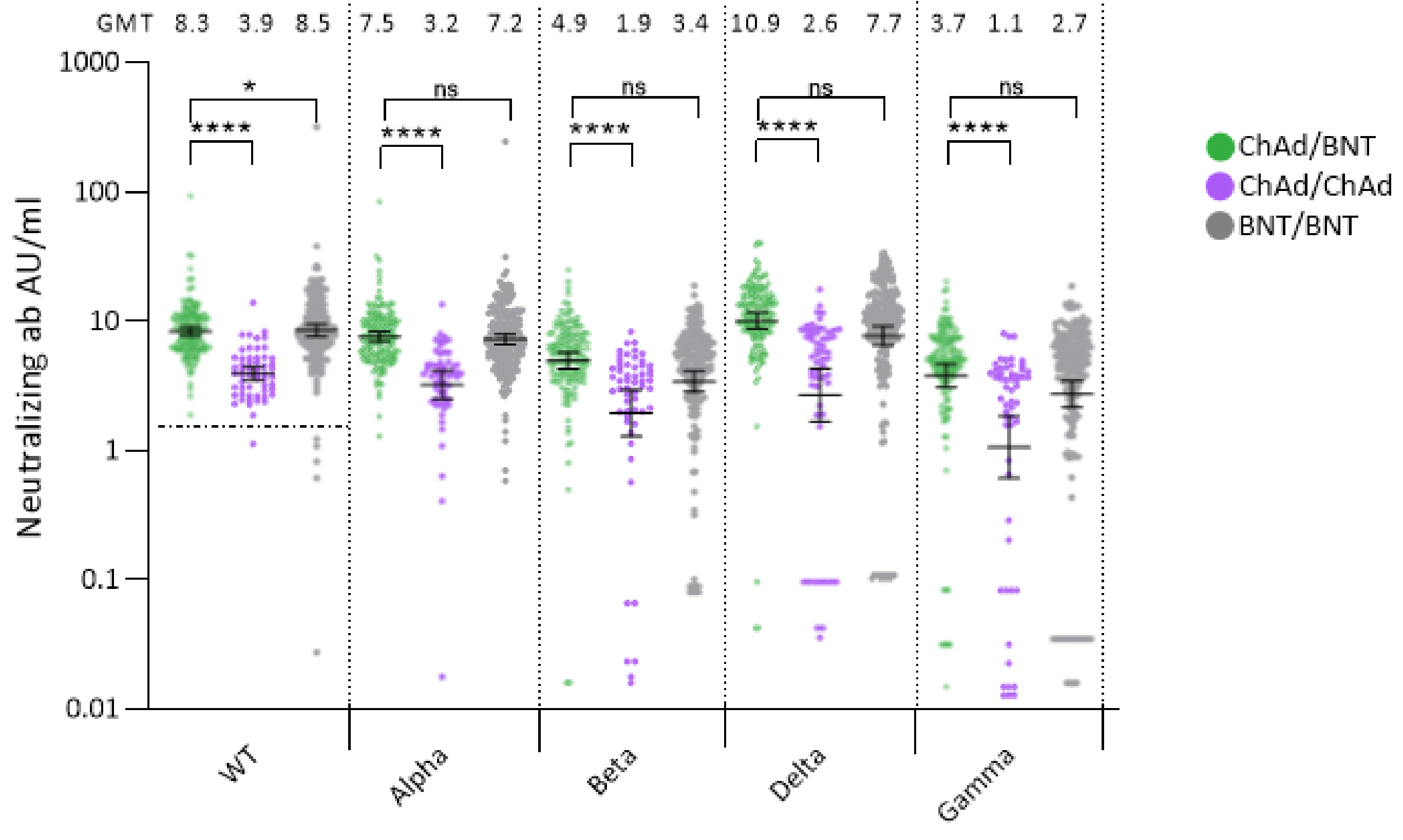
3.2. Neutralizing Antibody Responses
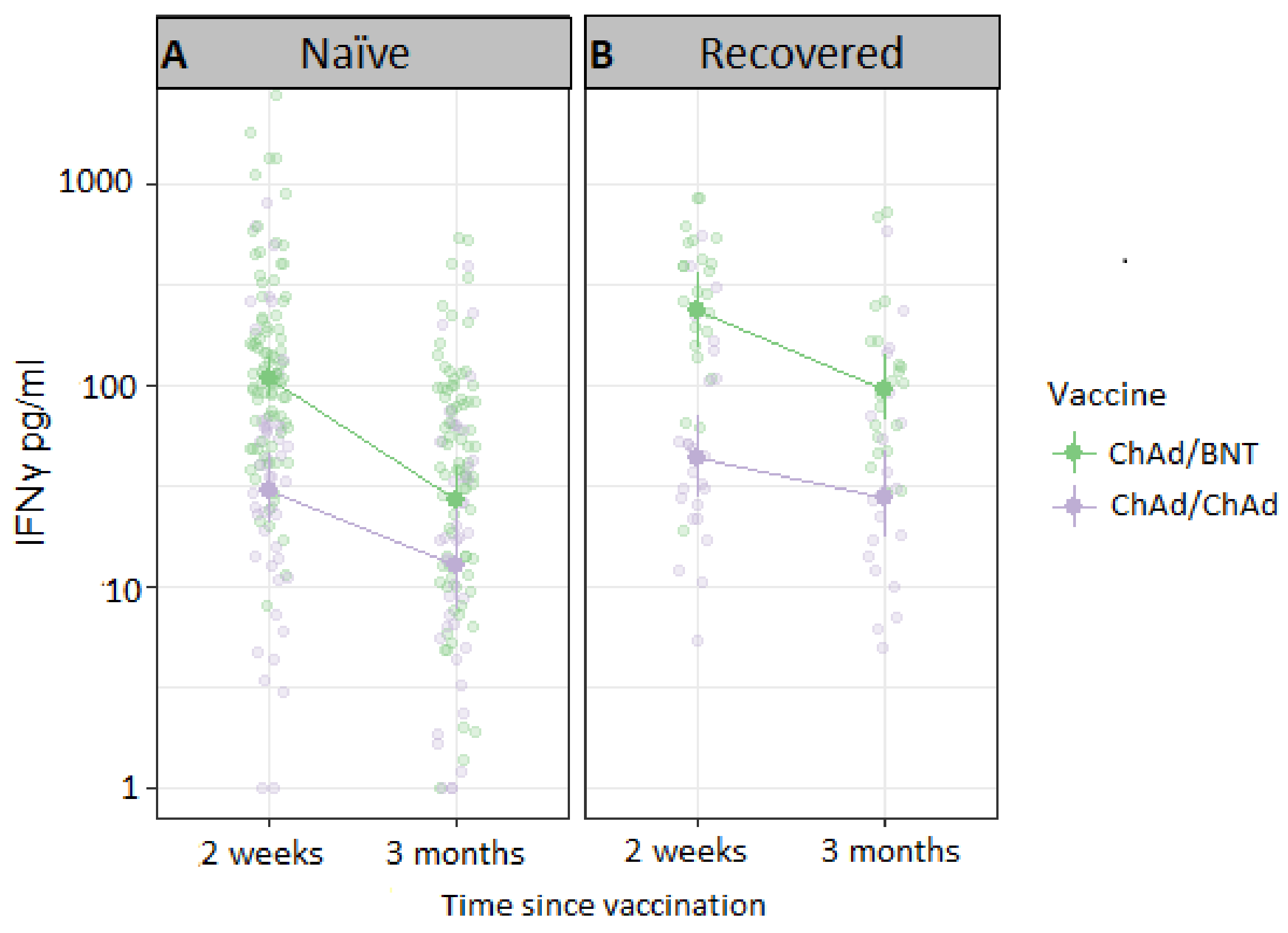
3.3. T-Cell Responses
3.4. Reactogenicity Following Second Vaccine Dose in Heterologous ChAd/BNT and Homologous ChAd/ChAd Primary Vaccination Regimens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shaw, R.H.; Stuart, A.; Greenland, M.; Liu, X.; Nguyen Van-Tam, J.S.; Snape, M.D.; Com-COV Study Group. Heterologous prime-boost COVID-19 vaccination: Initial reactogenicity data. Lancet 2021, 397, 2043–2046. [Google Scholar] [CrossRef]
- Borobia, A.M.; Carcas, A.J.; Pérez-Olmeda, M.; Castaño, L.; Bertran, M.J.; García-Pérez, J.; Campins, M.; Portolés, A.; González-Pérez, M.; Morales, M.T.G.; et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet 2021, 398, 121–130. [Google Scholar] [CrossRef]
- Barros-Martins, J.; Hammerschmidt, S.I.; Cossmann, A.; Odak, I.; Stankov, M.V.; Ramos, G.M.; Dopfer-Jablonka, A.; Heidemann, A.; Ritter, C.; Friedrichsen, M.; et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021, 27, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Dimeglio, C.; Herin, F.; Da-Silva, I.; Jougla, I.; Pradere, C.; Porcheron, M.; Martin-Blondel, G.; Chapuy-Regaud, S.; Izopet, J. Heterologous ChAdOx1-S/BNT162b2 Vaccination: Neutralizing Antibody Response to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2021, ciab705. [Google Scholar] [CrossRef]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir. Med. 2021, 9, 1255–1265. [Google Scholar] [CrossRef]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef]
- Tenbusch, M.; Schumacher, S.; Vogel, E.; Priller, A.; Held, J.; Steininger, P.; Beileke, S.; Irrgang, P.; Brockhoff, R.; Salmanton-García, J.; et al. Heterologous prime–boost vaccination with ChAdOx1 nCoV-19 and BNT162b. Lancet Infect. Dis. 2021, 21, 1212–1213. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; A Clutterbuck, E.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- A Powell, A.; Power, L.; Westrop, S.; McOwat, K.; Campbell, H.; Simmons, R.; E Ramsay, M.; Brown, K.; Ladhani, S.N.; Amirthalingam, G. Real-world data shows increased reactogenicity in adults after heterologous compared to homologous prime-boost COVID-19 vaccination, March−June 2021, England. Eurosurveillance 2021, 26, 2100634. [Google Scholar] [CrossRef]
- Stuart, A.S.V.; Shaw, R.H.; Liu, X.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; A Clutterbuck, E.; Collins, A.M.; et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): A single-blind, randomised, phase 2, non-inferiority trial. Lancet 2021, 399, 36–49. [Google Scholar] [CrossRef]
- Vallée, A.; Vasse, M.; Mazaux, L.; Bonan, B.; Amiel, C.; Zia-Chahabi, S.; Chan-Hew-Wai, A.; Farfour, E.; Camps, E.; Touche, P.; et al. An Immunogenicity Report for the Comparison between Heterologous and Homologous Prime-Boost Schedules with ChAdOx1-S and BNT162b2 Vaccines. J. Clin. Med. 2021, 10, 3817. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.; Chen YM, A.; Chan, H.P.; Chang, C.C.; Chuang, K.P.; Lee, C.H. The Effects of Heterologous Immunization with Prime-Boost COVID-19 Vaccination against SARS-CoV. Vaccines 2021, 9, 1163. [Google Scholar] [CrossRef] [PubMed]
- Benning, L.; Töllner, M.; Hidmark, A.; Schaier, M.; Nusshag, C.; Kälble, F.; Reichel, P.; Buylaert, M.; Grenz, J.; Ponath, G.; et al. Heterologous ChAdOx1 nCoV-19/BNT162b2 Prime-Boost Vaccination Induces Strong Humoral Responses among Health Care Workers. Vaccines 2021, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Groß, R.; Zanoni, M.; Seidel, A.; Conzelmann, C.; Gilg, A.; Krnavek, D.; Erdemci-Evin, S.; Mayer, B.; Hoffmann, M.; Pöhlmann, S.; et al. Heterologous ChAdOx1 nCoV-19 and BNT162b2 prime-boost vaccination elicits potent neutralizing antibody responses and T cell reactivity against prevalent SARS-CoV-2 variants. EBioMedicine 2021, 75, 103761. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Effectiveness of heterologous ChAdOx1 nCoV-19 and mRNA prime-boost vaccination against symptomatic Covid-19 infection in Sweden: A nationwide cohort study. Lancet Reg. Health Eur. 2021, 11, 100249. [Google Scholar] [CrossRef]
- Poukka, E.; Baum, U.; Palmu, A.A.; Lehtonen, T.O.; Salo, H.; Nohynek, H.; Leino, T. Cohort study of Covid-19 vaccine effectiveness among healthcare workers in Finland, December 2020–October. Vaccine 2021, 40, 701–705. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Effectiveness of COVID-19 Vaccination Against Risk of Symptomatic Infection, Hospitalization, and Death Up to 9 Months: A Swedish Total-Population Cohort Study. Lancet 2021. preprints. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Setayeshgar, S.; Febriani, Y.; Ouakki, M.; Zou, M.; Talbot, D. Two-dose SARS-CoV-2 vaccine effectiveness with mixed schedules and extended dosing intervals: Test-negative design studies from British Columbia and Quebec, Canada. medRxiv 2021. [Google Scholar] [CrossRef]
- Martínez-Baz, I.; Trobajo-Sanmartín, C.; Miqueleiz, A.; Guevara, M.; Fernández-Huerta, M.; Burgui, C.; Casado, I.; Portillo, M.E.; Navascués, A.; Ezpeleta, C.; et al. Product-specific COVID-19 vaccine effectiveness against secondary infection in close contacts, Navarre, Spain, April to August. Eurosurveillance 2021, 26, 2100894. [Google Scholar] [CrossRef]
- Pozzetto, B.; Legros, V.; Djebali, S.; Barateau, V.; Guibert, N.; Villard, M.; Peyrot, L.; Allatif, O.; Fassier, J.-B.; Massardier-Pilonchéry, A.; et al. Immunogenicity and efficacy of heterologous ChAdOx1–BNT162b2 vaccination. Nature 2021, 600, 701–706. [Google Scholar] [CrossRef]
- EMA. Heterologous Primary and Booster COVID-19 Vaccination—Evidence Based Regulatory Considerations; EMA/349565/2021; Biological Health Threats and Vaccine Strategy Office, EMA Pandemic Task Force for COVID-19 (COVID-ETF): Amsterdam, The Netherlands, 2021. [Google Scholar]
- Chiu, N.-C.; Chi, H.; Tu, Y.-K.; Huang, Y.-N.; Tai, Y.-L.; Weng, S.-L.; Chang, L.; Huang, D.T.-N.; Huang, F.-Y.; Lin, C.-Y. To mix or not to mix? A rapid systematic review of heterologous prime–boost covid-19 vaccination. Expert Rev. Vaccines 2021, 20, 1211–1220. [Google Scholar] [CrossRef] [PubMed]
- Rudberg, A.-S.; Havervall, S.; Månberg, A.; Falk, A.J.; Aguilera, K.; Ng, H.; Gabrielsson, L.; Salomonsson, A.-C.; Hanke, L.; Murrell, B.; et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat. Commun. 2020, 11, 5064. [Google Scholar] [CrossRef] [PubMed]
- Havervall, S.; Rosell, A.; Phillipson, M.; Mangsbo, S.M.; Nilsson, P.; Hober, S.; Thålin, C. Symptoms and Functional Impairment Assessed 8 Months after Mild COVID-19 among Health Care Workers. JAMA 2021, 325, 2015–2016. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Sweden, SmiNet. Public Health Agency of Sweden, SmiNet. Available online: www.folkhalsomyndigheten.se/smittskydd-beredskap/overvakning-och-rapportering/sminet/ (accessed on 1 October 2021).
- Havervall, S.; Marking, U.; Greilert-Norin, N.; Ng, H.; Gordon, M.; Salomonsson, A.-C.; Hellström, C.; Pin, E.; Blom, K.; Mangsbo, S.; et al. Antibody responses after a single dose of ChAdOx1 nCoV-19 vaccine in healthcare workers previously infected with SARS-CoV-2. eBioMedicine 2021, 70, 103523. [Google Scholar] [CrossRef] [PubMed]
- Varnaitė, R.; García, M.; Glans, H.; Maleki, K.T.; Sandberg, J.T.; Tynell, J.; Christ, W.; Lagerqvist, N.; Asgeirsson, H.; Ljunggren, H.-G.; et al. Expansion of SARS-CoV-2–Specific Antibody-Secreting Cells and Generation of Neutralizing Antibodies in Hospitalized COVID-19 Patients. J. Immunol. 2020, 205, 2437–2446. [Google Scholar] [CrossRef]
- Havervall, S.; Ng, H.; Falk, A.J.; Greilert-Norin, N.; Månberg, A.; Marking, U.; Laurén, I.; Gabrielsson, L.; Salomonsson, A.; Aguilera, K.; et al. Robust humoral and cellular immune responses and low risk for reinfection at least 8 months following asymptomatic to mild COVID-19. J. Intern. Med. 2021, 291, 72–80. [Google Scholar] [CrossRef]
- Mangsbo, S.M.; Havervall, S.; Laurén, I.; Lindsay, R.; Falk, A.J.; Marking, U.; Lord, M.; Buggert, M.; Dönnes, P.; Christoffersson, G.; et al. An evaluation of a FluoroSpot assay as a diagnostic tool to determine SARS-CoV-2 specific T cell responses. PLoS ONE 2021, 16, e0258041. [Google Scholar] [CrossRef]
- Schirmbeck, R.; Reimann, J.; Kochanek, S.; Kreppel, F. The Immunogenicity of Adenovirus Vectors Limits the Multispecificity of CD8 T-cell Responses to Vector-encoded Transgenic Antigens. Mol. Ther. 2008, 16, 1609–1616. [Google Scholar] [CrossRef]
- Rössler, A.; Riepler, L.; Bante, D.; von Laer, D.; Kimpel, J. SARS-CoV-2 Omicron Variant Neutralization in Serum from Vaccinated and Convalescent Persons. N. Engl. J. Med. 2022, 386, 698–700. [Google Scholar] [CrossRef]
- Eyre, D.W.; Lumley, S.F.; Wei, J.; Cox, S.; James, T.; Justice, A.; Jesuthasan, G.; O’Donnell, D.; Howarth, A.; Hatch, S.B.; et al. Quantitative SARS-CoV-2 anti-spike responses to Pfizer–BioNTech and Oxford–AstraZeneca vaccines by previous infection status. Clin. Microbiol. Infect. 2021, 27, 1516.e7–1516.e14. [Google Scholar] [CrossRef] [PubMed]
| BNT/BNT n = 210 | ChAd/BNT n = 241 | ChAd/ChAd n = 113 | |
|---|---|---|---|
| Age (IQR) | 50 (40–58) | 48 (39–56) | 50 (39–56) |
| Sex | |||
| Female | 180 (86%) | 211 (88%) | 99 (88%) |
| Male | 30 (14%) | 30 (12%) | 14 (12%) |
| Dose interval, days (IQR) | 21 (21–28) | 91 (86–94) | 83.0 (77–87) |
| Infection prior to vaccine | |||
| Naïve | 210 (100%) | 174 (72%) | 73 (65%) |
| Recovered | 0 | 67 (28%) | 40 (35%) |
| 2 Weeks to 3 Months | 3 to 6 Months | 2 Weeks to 6 Months | |
|---|---|---|---|
| Naïve | |||
| ChAd/BNT | 1.3 months (1.2–1.4) | 2.7 months (2.2–3.4) | 1.9 months (1.7–2.1) |
| ChAd/ChAd | 2.5 months (2.2–3) | 3.7 months (2.9–5.1) | 3.1 months (2.8–3.6) |
| Recovered | |||
| ChAd/BNT | 1.6 months (1.4–1.9) | 4.7 months (3–10.6) | 2.7 months (2.3–3.3) |
| ChAd/ChAd | 2 months (1.7–2.4) | 9.8 months (5.1–129.7) | 3.8 months (3.1–4.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marking, U.; Havervall, S.; Greilert-Norin, N.; Ng, H.; Blom, K.; Nilsson, P.; Phillipson, M.; Hober, S.; Nilsson, C.; Mangsbo, S.; et al. Duration of SARS-CoV-2 Immune Responses Up to Six Months Following Homologous or Heterologous Primary Immunization with ChAdOx1 nCoV-19 and BNT162b2 mRNA Vaccines. Vaccines 2022, 10, 359. https://doi.org/10.3390/vaccines10030359
Marking U, Havervall S, Greilert-Norin N, Ng H, Blom K, Nilsson P, Phillipson M, Hober S, Nilsson C, Mangsbo S, et al. Duration of SARS-CoV-2 Immune Responses Up to Six Months Following Homologous or Heterologous Primary Immunization with ChAdOx1 nCoV-19 and BNT162b2 mRNA Vaccines. Vaccines. 2022; 10(3):359. https://doi.org/10.3390/vaccines10030359
Chicago/Turabian StyleMarking, Ulrika, Sebastian Havervall, Nina Greilert-Norin, Henry Ng, Kim Blom, Peter Nilsson, Mia Phillipson, Sophia Hober, Charlotta Nilsson, Sara Mangsbo, and et al. 2022. "Duration of SARS-CoV-2 Immune Responses Up to Six Months Following Homologous or Heterologous Primary Immunization with ChAdOx1 nCoV-19 and BNT162b2 mRNA Vaccines" Vaccines 10, no. 3: 359. https://doi.org/10.3390/vaccines10030359
APA StyleMarking, U., Havervall, S., Greilert-Norin, N., Ng, H., Blom, K., Nilsson, P., Phillipson, M., Hober, S., Nilsson, C., Mangsbo, S., Christ, W., Klingström, J., Gordon, M., Åberg, M., & Thålin, C. (2022). Duration of SARS-CoV-2 Immune Responses Up to Six Months Following Homologous or Heterologous Primary Immunization with ChAdOx1 nCoV-19 and BNT162b2 mRNA Vaccines. Vaccines, 10(3), 359. https://doi.org/10.3390/vaccines10030359






