Prevalence of COVID-19 Infection among Patients with Diabetes and Their Vaccination Coverage Status in Saudi Arabia: A Cross-Sectional Analysis from a Hospital-Based Diabetes Registry
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design, Setting and Population
2.2. Sample Size and Method
2.3. Data Sources and Collection Procedure
2.3.1. The PSMMC Diabetes Registry
2.3.2. Health—Diseases Surveillance Network System (HESN)
2.3.3. National Vaccination Record via Seha Platform
2.4. Data Management and Analysis
3. Results
3.1. Sociodemographic Characteristics
3.2. Prevalence of COVID-19 Infection among Patients with Type 2 Diabetes
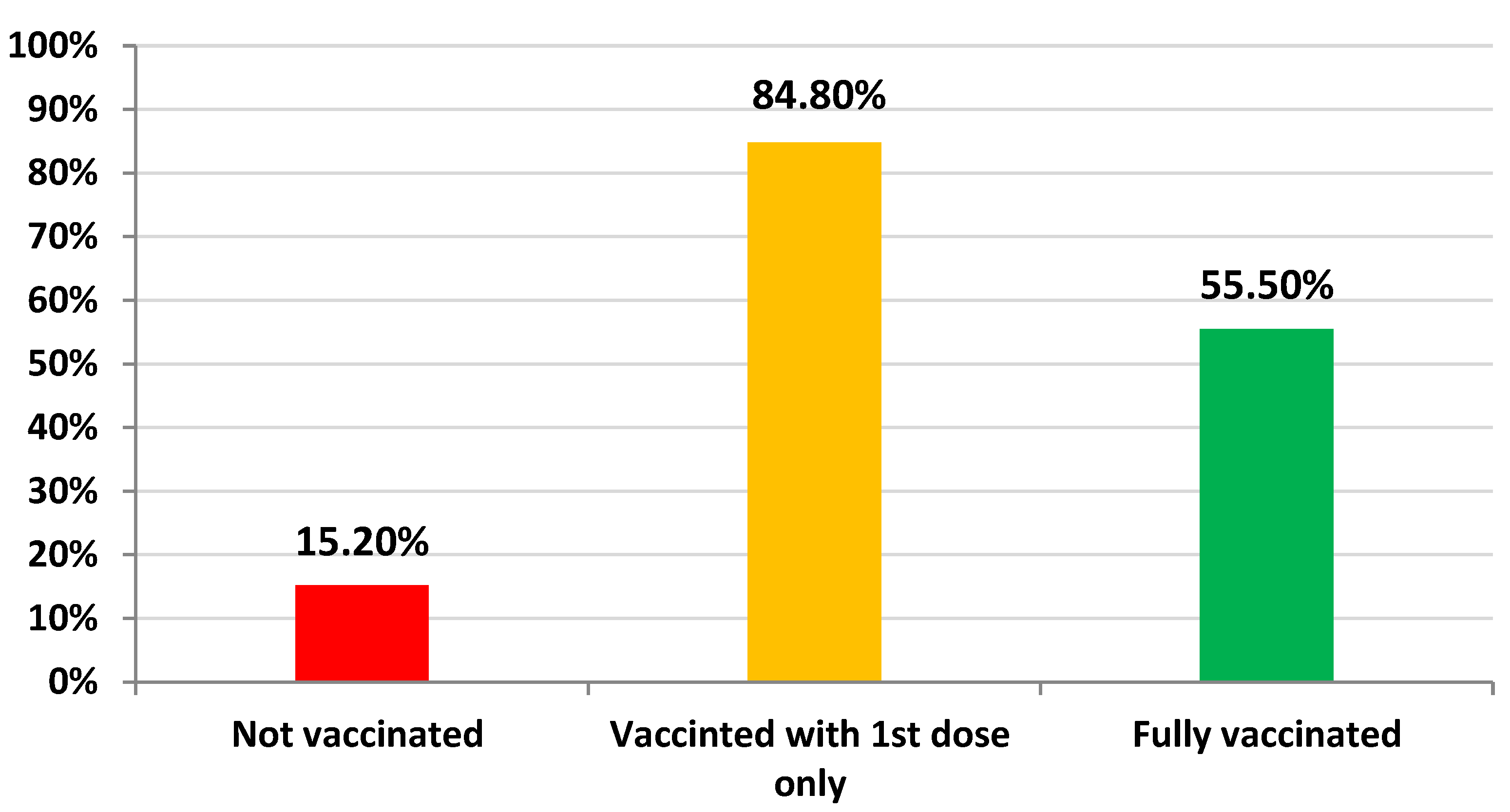
3.3. Rate of Vaccination among Patients with Type 2 Diabetes Mellitus
3.4. Association between Infection with COVID-19 and the Patients’ Sociodemographic Characteristics
3.5. Association between Vaccination Status, the Patients’ Sociodemographic Characteristics and Previous Infection
3.6. Association between Full Vaccination Status, the Patients’ Sociodemographic Characteristics and Previous Infection
3.7. Logistic Regression Analysis for the Impact of Factors Associated with the Unvaccinated Status
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef]
- Vilella, A.; Trilla, A. The COVID-19 Pandemic—An Epidemiological Perspective. Curr. Allergy Asthma Rep. 2021, 21, 1–6. [Google Scholar] [CrossRef]
- Das, K.; Pingali, M.S.; Paital, B.; Panda, F.; Pati, S.G.; Singh, A.; Varadwaj, P.K.; Samanta, S.K. A detailed review of the outbreak of COVID-19. Front. Biosci. -Landmark 2021, 26, 149–170. [Google Scholar] [CrossRef]
- Karan, A.; Wadhera, R.K. Healthcare system stress due to COVID-19: Evading an evolving crisis. J. Hosp. Med. 2021, 16, 127. [Google Scholar] [CrossRef]
- Trentini, F.; Marziano, V.; Guzzetta, G.; Tirani, M.; Cereda, D.; Poletti, P.; Piccarreta, R.; Barone, A.; Preziosi, G.; Arduini, F.; et al. The pressure on healthcare system and intensive care utilization during the COVID-19 outbreak in the Lombardy region: A retrospective observational study on 43,538 hospitalized patients. Am. J. Epidemiol. 2021, 191, 137–146. [Google Scholar] [CrossRef]
- Siow, W.T.; Liew, M.F.; Shrestha, B.R.; Muchtar, F.; See, K.C. Managing COVID-19 in resource-limited settings: Critical care considerations. Crit. Care 2020, 24, 167. [Google Scholar] [CrossRef]
- Goschin, Z.; Dimian, G.C. Healthcare under pressure: Modelling COVID-19 fatalities with multiscale geographically weighted regressions. Kybernetes 2021. [Google Scholar] [CrossRef]
- Hens, N.; Vranck, P.; Molenberghs, G. The COVID-19 epidemic, its mortality, and the role of non-pharmaceutical interventions. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 204–208. [Google Scholar] [CrossRef]
- Locatelli, I.; Trächsel, B.; Rousson, V. Estimating the basic reproduction number for COVID-19 in Western Europe. PLoS ONE 2021, 16, e0248731. [Google Scholar] [CrossRef]
- Hong, K.; Yum, S.J.; Kim, J.H.; Chun, B.C. Re-estimation of basic reproduction number of COVID-19 based on the epidemic curve by symptom onset date. Epidemiol. Infect. 2021, 149, e53. [Google Scholar] [CrossRef]
- Yu, C.-J.; Wang, Z.-X.; Xu, Y.; Hu, M.-X.; Chen, K.; Qin, G. Assessment of basic reproductive number for COVID-19 at global level: A meta-analysis. Medicine 2021, 100, e25837. [Google Scholar] [CrossRef] [PubMed]
- Shil, P.; Atre, N.M.; Patil, A.A.; Tandale, B.V.; Abraham, P. District-wise estimation of Basic reproduction number (R0) for COVID-19 in India in the initial phase. Spat. Inf. Res. 2021, 30, 37–45. [Google Scholar] [CrossRef]
- Rahman, B.; Sadraddin, E.; Porreca, A. The basic reproduction number of SARS-CoV-2 in Wuhan is about to die out, how about the rest of the world? Rev. Med. Virol. 2020, 30, e2111. [Google Scholar] [CrossRef]
- Ives, A.R.; Bozzuto, C. Estimating and explaining the spread of COVID-19 at the county level in the USA. Commun. Biol. 2021, 4, 60. [Google Scholar] [CrossRef]
- Beyrampour-Basmenj, H.; Milani, M.; Ebrahimi-Kalan, A.; Ben Taleb, Z.; Ward, K.; Dargahi Abbasabad, G.; Aliyari-serej, Z.; Ebrahimi Kalan, M. An Overview of the Epidemiologic, Diagnostic and Treatment Approaches of COVID-19: What do We Know. Public Health Rev. 2021, 42, 1604061. [Google Scholar] [CrossRef]
- Worldometers. Coronavirus (COVID-19) Mortality Rate. Available online: https://www.worldometers.info/coronavirus/coronavirus-death-rate/ (accessed on 13 November 2021).
- Booth, A.; Reed, A.B.; Ponzo, S.; Yassaee, A.; Aral, M.; Plans, D.; Labrique, A.; Mohan, D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS ONE 2021, 16, e0247461. [Google Scholar] [CrossRef]
- Wolff, D.; Nee, S.; Hickey, N.S.; Marschollek, M. Risk factors for COVID-19 severity and fatality: A structured literature review. Infection 2021, 49, 15–28. [Google Scholar] [CrossRef]
- Ahlström, B.; Frithiof, R.; Hultström, M.; Larsson, I.-M.; Strandberg, G.; Lipcsey, M. The swedish COVID-19 intensive care cohort: Risk factors of ICU admission and ICU mortality. Acta Anaesthesiol. Scand. 2021, 65, 525–533. [Google Scholar] [CrossRef]
- Peña, J.E.-d.l.; Rascón-Pacheco, R.A.; Ascencio-Montiel, I.d.J.; González-Figueroa, E.; Fernández-Gárate, J.E.; Medina-Gómez, O.S.; Borja-Bustamante, P.; Santillán-Oropeza, J.A.; Borja-Aburto, V.H. Hypertension, Diabetes and Obesity, Major Risk Factors for Death in Patients with COVID-19 in Mexico. Arch. Med. Res. 2021, 52, 443–449. [Google Scholar] [CrossRef]
- World Health Organization (WHO). WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 13 November 2021).
- World Health Organization (WHO). Saudi Arabia: WHO Coronavirus Disease (COVID-19) Dashboard with Vaccination Data. Available online: https://covid19.who.int/region/emro/country/sa (accessed on 13 November 2021).
- Algaissi, A.A.; Alharbi, N.K.; Hassanain, M.; Hashem, A.M. Preparedness and response to COVID-19 in Saudi Arabia: Building on MERS experience. J. Infect. Public Health 2020, 13, 834–838. [Google Scholar] [CrossRef]
- AlShoaibi, N.A.; Maghrabi, K.; Alanazi, H.; Harbi, M.A.; Alghamdi, S. Saudi Heart Rhythm Society Task Force on Management of Potential Arrhythmogenicity Associated with Pharmacotherapy for COVID-19. Ann. Saudi Med. 2020, 40, 365–372. [Google Scholar] [CrossRef]
- Alsofyani, M.A.; Malaekah, H.M.; Bashawyah, A.; Bawazeer, M.; Akkour, K.; Alsalmi, S.; Alkhairy, A.; Dajim, N.B.; Khalifah, S.; Almalki, I.A. Safety measures for COVID-19: A review of surgical preparedness at four major medical centres in Saudi Arabia. Patient Saf. Surg. 2020, 14, 1–14. [Google Scholar] [CrossRef]
- Alyamani, O.; Abushoshah, I.; Tawfeeq, N.A.; Al Dammas, F.; Algurashi, F.A. Considerations and recommendations for obstetric anesthesia care during COVID-19 pandemic-Saudi anesthesia society guidelines. Saudi J. Anaesth. 2020, 14, 359. [Google Scholar] [CrossRef]
- Badreldin, H.A.; Raslan, S.; Almudaiheem, H.; Alomari, B.; Almowaina, S.; Joharji, H.; Alawagi, M.; Al-Jedai, A. Pharmacists roles and responsibilities during epidemics and pandemics in Saudi Arabia: An opinion paper from the Saudi Society of clinical pharmacy. Saudi Pharm. J. SPJ 2020, 28, 1030. [Google Scholar] [CrossRef]
- Alkahtani, T.A.; Alakeel, A.; Alakeel, R.A.; Khorshid, F.A.; Alshammari, H.H.; Alguwaihes, A.M.; Almohideb, M.; Ali, E.M.; Bin-Jumah, M.; Abdel-Daim, M.M. The current reproduction number of COVID-19 in Saudi Arabia: Is the disease controlled? Environ. Sci. Pollut. Res. 2021, 28, 44812–44817. [Google Scholar] [CrossRef]
- Cleveland Clinics. Diabetes: Types, Risk Factors, Symptoms, Tests, Treatments & Prevention. Available online: https://my.clevelandclinic.org/health/diseases/7104-diabetes-mellitus-an-overview (accessed on 13 November 2021).
- Erener, S. Diabetes, infection risk and COVID-19. Mol. Metab. 2020, 39, 101044. [Google Scholar] [CrossRef]
- International Diabetes Federation (IDF) Diabetes Facts & Figures. Available online: https://idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (accessed on 13 November 2021).
- Carey, I.M.; Critchley, J.A.; DeWilde, S.; Harris, T.; Hosking, F.J.; Cook, D.G. Risk of infection in type 1 and type 2 diabetes compared with the general population: A matched cohort study. Diabetes Care 2018, 41, 513–521. [Google Scholar] [CrossRef]
- Critchley, J.A.; Carey, I.M.; Harris, T.; DeWilde, S.; Hosking, F.J.; Cook, D.G. Glycemic control and risk of infections among people with type 1 or type 2 diabetes in a large primary care cohort study. Diabetes Care 2018, 41, 2127–2135. [Google Scholar] [CrossRef]
- Norouzi, M.; Norouzi, S.; Ruggiero, A.; Khan, M.S.; Myers, S.; Kavanagh, K.; Vemuri, R. Type-2 Diabetes as a Risk Factor for Severe COVID-19 Infection. Microorganisms 2021, 9, 1211. [Google Scholar] [CrossRef]
- Singh, A.K.; Gupta, R.; Ghosh, A.; Misra, A. Diabetes in COVID-19: Prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 303–310. [Google Scholar] [CrossRef]
- Wu, Z.-H.; Tang, Y.; Cheng, Q. Diabetes increases the mortality of patients with COVID-19: A meta-analysis. Acta Diabetol. 2021, 58, 139–144. [Google Scholar] [CrossRef]
- Corona, G.; Pizzocaro, A.; Vena, W.; Rastrelli, G.; Semeraro, F.; Isidori, A.M.; Pivonello, R.; Salonia, A.; Sforza, A.; Maggi, M. Diabetes is most important cause for mortality in COVID-19 hospitalized patients: Systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2021, 22, 275–296. [Google Scholar] [CrossRef]
- Palaiodimos, L.; Chamorro-Pareja, N.; Karamanis, D.; Li, W.; Zavras, P.D.; Chang, K.M.; Mathias, P.; Kokkinidis, D.G. Diabetes is associated with increased risk for in-hospital mortality in patients with COVID-19: A systematic review and meta-analysis comprising 18,506 patients. Hormones 2021, 20, 305–314. [Google Scholar] [CrossRef]
- Varikasuvu, S.R.; Dutt, N.; Thangappazham, B.; Varshney, S. Diabetes and COVID-19: A pooled analysis related to disease severity and mortality. Prim. Care Diabetes 2021, 15, 24–27. [Google Scholar] [CrossRef]
- Alguwaihes, A.M.; Al-Sofiani, M.E.; Megdad, M.; Albader, S.S.; Alsari, M.H.; Alelayan, A.; Alzahrani, S.H.; Sabico, S.; Al-Daghri, N.M.; Jammah, A.A. Diabetes and COVID-19 among hospitalized patients in Saudi Arabia: A single-centre retrospective study. Cardiovasc. Diabetol. 2020, 19, 205. [Google Scholar] [CrossRef]
- Pal, R.; Bhadada, S.K.; Misra, A. COVID-19 vaccination in patients with diabetes mellitus: Current concepts, uncertainties and challenges. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 505–508. [Google Scholar] [CrossRef]
- Robert, A.A.; Al Saeed, A.; Al Dawish, M.A. COVID-19 among people with diabetes mellitus in Saudi Arabia: Current situation and new perspectives. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102231. [Google Scholar] [CrossRef]
- Frielitz, F.-S.; Wagner, I.V.; Schewe, D.M.; Bothe, K. COVID-19: Would compulsory vaccination be legally possible? Dtsch. Med. Wochenschr. (1946) 2021, 146, 206–208. [Google Scholar]
- Rawat, K.; Kumari, P.; Saha, L. COVID-19 vaccine: A recent update in pipeline vaccines, their design and development strategies. Eur. J. Pharmacol. 2020, 892, 173751. [Google Scholar] [CrossRef]
- Christie, A.; Brooks, J.T.; Hicks, L.A.; Sauber-Schatz, E.K.; Yoder, J.S.; Honein, M.A.; COVID, C.; Team, R. Guidance for implementing COVID-19 prevention strategies in the context of varying community transmission levels and vaccination coverage. Morb. Mortal. Wkly. Rep. 2021, 70, 1044. [Google Scholar] [CrossRef]
- Ndwandwe, D.; Wiysonge, C.S. COVID-19 vaccines. Curr. Opin. Immunol. 2021, 71, 111–116. [Google Scholar] [CrossRef]
- Moline, H.L.; Whitaker, M.; Deng, L.; Rhodes, J.C.; Milucky, J.; Pham, H.; Patel, K.; Anglin, O.; Reingold, A.; Chai, S.J.; et al. Effectiveness of COVID-19 Vaccines in Preventing Hospitalization Among Adults Aged ≥65 Years—COVID-NET, 13 States, February-April 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1088–1093. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Self, W.H.; Naioti, E.A.; Ginde, A.A.; Douin, D.J.; Olson, S.M.; Talbot, H.K.; Casey, J.D.; Mohr, N.M.; Zepeski, A.; et al. Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults—United States, March–July 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1156–1162. [Google Scholar] [CrossRef]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar] [CrossRef]
- Patel, N.; Bouchard, J.; Oliver, M.B.; Badowski, M.E.; Carreno, J.J. Early clinical trial data and real-world assessment of COVID-19 vaccines: Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2021, 41, 837–850. [Google Scholar] [CrossRef]
- Azar, W.S.; Njeim, R.; Fares, A.H.; Azar, N.S.; Azar, S.T.; El Sayed, M.; Eid, A.A. COVID-19 and diabetes mellitus: How one pandemic worsens the other. Rev. Endocr. Metab. Disord. 2020, 21, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Chee, Y.J.; Tan, S.K.; Yeoh, E. Dissecting the interaction between COVID-19 and diabetes mellitus. J. Diabetes Investig. 2020, 11, 1104–1114. [Google Scholar] [CrossRef]
- Soiza, R.L.; Scicluna, C.; Thomson, E.C. Efficacy and safety of COVID-19 vaccines in older people. Age Ageing 2021, 50, 279–283. [Google Scholar] [CrossRef]
- Saudi Press Agency. SFDA Approves Registration of Pfizer-BioNTech COVID-19 Vaccine. Available online: https://www.spa.gov.sa/viewfullstory.php?lang=en&newsid=2166947 (accessed on 13 November 2021).
- Saudi Press Agency. SFDA Approves Importing, Using Oxford-AstraZeneca COVID-19 Vaccine. Available online: https://www.spa.gov.sa/viewfullstory.php?lang=en&newsid=2192728 (accessed on 13 November 2021).
- Saudi Press Agency. SFDA Approves Registration of Moderna COVID-19 Vaccine. Available online: https://www.spa.gov.sa/viewfullstory.php?lang=en&newsid=2256283 (accessed on 13 November 2021).
- Assiri, A.; Al-Tawfiq, J.A.; Alkhalifa, M.; Al Duhailan, H.; Al Qahtani, S.; Dawas, R.A.; El Seoudi, A.A.; Alomran, N.; Omar, O.A.; Alotaibi, N. Launching COVID-19 vaccination in Saudi Arabia: Lessons learned, and the way forward. Travel Med. Infect. Dis. 2021, 43, 102119. [Google Scholar] [CrossRef]
- Ministry of Health. COVID-19 & Vaccines FAQS. Available online: https://www.moh.gov.sa/en/Ministry/HotTopics/Pages/COVID-19-Vaccine.aspx (accessed on 5 November 2021).
- Minsitry of Health SA. Launching the COVID-19 Vaccination Program in Saudi Arabia. Available online: https://www.moh.gov.sa/Ministry/MediaCenter/News/Pages/News-2020-12-17-007.aspx (accessed on 13 November 2021).
- Saudi Press Agency. Launching the COVID-19 Vaccination Program and the Stages of the Program. Available online: https://www.spa.gov.sa/2168181 (accessed on 13 November 2021).
- Unified National Platform. Registering for COVID-19 Vaccination and the Stages of the National Vaccination Program. Available online: https://www.my.gov.sa/wps/portal/snp/servicesDirectory/servicedetails/s9123 (accessed on 13 November 2021).
- Ministry of Health SA. Announcement of the Second Stage of the Vaccine Program. Available online: https://twitter.com/SaudiMOH/status/1362297708850143233?ref_src=twsrc%5Etfw (accessed on 13 November 2021).
- Ministry of Health SA. Number of COVID-19 Vaccination Centers in Saudi Arabia. Available online: https://twitter.com/SaudiMOH/status/1455569601941671937/photo/1 (accessed on 13 November 2021).
- Romer, D.; Jamieson, K.H. Conspiracy theories as barriers to controlling the spread of COVID-19 in the US. Soc. Sci. Med. 2020, 263, 113356. [Google Scholar] [CrossRef]
- Douglas, K.M. COVID-19 conspiracy theories. Group Processes Intergroup Relat. 2021, 24, 270–275. [Google Scholar] [CrossRef]
- Khairat, S.; Zou, B.; Adler-Milstein, J. Factors and Reasons Associated with Low COVID-19 Vaccine Uptake among Highly Hesitant Communities in the US. Am. J. Infect. Control. 2022, in press. [Google Scholar] [CrossRef] [PubMed]
- King, W.C.; Rubinstein, M.; Reinhart, A.; Mejia, R. Time trends, factors associated with, and reasons for COVID-19 vaccine hesitancy: A massive online survey of US adults from January-May 2021. PLoS ONE 2021, 16, e0260731. [Google Scholar] [CrossRef] [PubMed]
- Alshehry, A.S.; Cruz, J.P.; Alquwez, N.; Alsharari, A.F.; Tork, H.M.; Almazan, J.U.; Alshammari, F.; Alabdulaziz, H.; Alsolami, F.; Tumala, R.B. Predictors of nursing students’ intention to receive COVID-19 vaccination: A multi-university study in Saudi Arabia. J. Adv. Nurs. 2022, 78, 446–457. [Google Scholar] [CrossRef]
- Loomba, S.; de Figueiredo, A.; Piatek, S.J.; de Graaf, K.; Larson, H.J. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat. Hum. Behav. 2021, 5, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health SA. Half of the Population Received Two Doses of the Vaccine. Available online: https://www.aleqt.com/2021/09/17/article_2173101.html (accessed on 13 November 2021).
- Noushad, M.; Nassani, M.Z.; Koppolu, P.; Alsalhani, A.B.; Samran, A.; Alqerban, A.; Abusalim, G.S.; Barakat, A.; Alshalhoub, M.B.; Rastam, S. Predictors of COVID-19 vaccine intention among the saudi arabian population: A cross-sectional survey. Vaccines 2021, 9, 892. [Google Scholar] [CrossRef]
- Aldossari, K.K.; Alharbi, M.B.; Alkahtani, S.M.; Alrowaily, T.Z.; Alshaikhi, A.M.; Twair, A.A. COVID-19 vaccine hesitancy among patients with diabetes in Saudi Arabia. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102271. [Google Scholar] [CrossRef]
- Al-Mansour, K.; Alyahya, S.; AbuGazalah, F.; Alabdulkareem, K. Factors Affecting COVID-19 Vaccination among the General Population in Saudi Arabia. Healthcare 2021, 9, 1218. [Google Scholar] [CrossRef]
- Ministry of Health SA. Announcement of Starting Administering the 2nd Dose for Persons of 60 and Above. Available online: https://www.moh.gov.sa/Ministry/MediaCenter/News/Pages/News-2021-05-28-001.aspx (accessed on 13 November 2021).
- Ministry of Health SA. Announcement of Starting Administering the 2nd Dose for Persons of 50 Years and Above. Available online: https://www.alwatan.com.sa/article/1079508 (accessed on 13 November 2021).
- Ministry of Health SA. Approval of Mixing Vaccines in Saudi Arabia. Available online: https://www.moh.gov.sa/Ministry/MediaCenter/News/Pages/News-2021-06-23-007.aspx (accessed on 13 November 2021).
- Saudi Press Agency. Announcement of Approval of Vaccines for the Age Group 12–18 with Pfizer/BioNteck Vaccine. Available online: https://www.spa.gov.sa/2246602 (accessed on 13 November 2021).
- Ministry of Health SA. Announcement of Starting Administering the 2nd Dose for Persons of 40 Years and Above. Available online: https://www.moh.gov.sa/Ministry/MediaCenter/News/Pages/News-2021-07-05-008.aspx (accessed on 13 November 2021).
- Ministry of Health SA. Announcement of Administering the 2nd Dose for All Eligible Persons from All Age Groups. Available online: https://twitter.com/SaudiMOH/status/1414201732285018115 (accessed on 13 November 2021).
- Saudi Press Agency. Approval of Taking the COVID-19 Vaccines after 10 Days from Recovery of the Infection. Available online: https://www.spa.gov.sa/2266653 (accessed on 13 November 2021).
- Saudi Food and Drug Authority. Approval of Pfizer/BioNteck Vaccine for the Age Group 5–11. Available online: https://sfda.gov.sa/ar/news/85832 (accessed on 13 November 2021).
- Al-Amer, R.; Maneze, D.; Everett, B.; Montayre, J.; Villarosa, A.R.; Dwekat, E.; Salamonson, Y. COVID-19 vaccination intention in the first year of the pandemic: A systematic review. J. Clin. Nurs. 2021, 31, 62–86. [Google Scholar] [CrossRef]
- Al-Metwali, B.Z.; Al-Jumaili, A.A.; Al-Alag, Z.A.; Sorofman, B. Exploring the acceptance of COVID-19 vaccine among healthcare workers and general population using health belief model. J. Eval. Clin. Pract. 2021, 27, 1112–1122. [Google Scholar] [CrossRef]
- Al-Mohaithef, M.; Padhi, B.K. Determinants of COVID-19 Vaccine Acceptance in Saudi Arabia: A Web-Based National Survey. J. Multidiscip. Healthc. 2020, 13, 1657–1663. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohaithef, M.; Padhi, B.K.; Ennaceur, S. Socio-Demographics Correlate of COVID-19 Vaccine Hesitancy During the Second Wave of COVID-19 Pandemic: A Cross-Sectional Web-Based Survey in Saudi Arabia. Front. Public Health 2021, 9, 698106. [Google Scholar] [CrossRef]
- Ashok, N.; Krishnamurthy, K.; Singh, K.; Rahman, S.; Majumder, M.A.A. High COVID-19 Vaccine Hesitancy Among Healthcare Workers: Should Such a Trend Require Closer Attention by Policymakers? Cureus 2021, 13, e17990. [Google Scholar] [CrossRef] [PubMed]
- Gagneux-Brunon, A.; Detoc, M.; Bruel, S.; Tardy, B.; Rozaire, O.; Frappe, P.; Botelho-Nevers, E. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: A cross-sectional survey. J. Hosp. Infect. 2021, 108, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Konopińska, J.; Obuchowska, I.; Lisowski, Ł.; Dub, N.; Kozera, M.; Rękas, M. Intention to Get COVID-19 Vaccinations among Ophthalmology Residents in Poland: A Cross-Sectional Survey. Vaccines 2021, 9, 371. [Google Scholar] [CrossRef] [PubMed]
- Magadmi, R.M.; Kamel, F.O. Beliefs and barriers associated with COVID-19 vaccination among the general population in Saudi Arabia. BMC Public Health 2021, 21, 1438. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.A.; McFadden, S.M.; Elharake, J.; Omer, S.B. Determinants of COVID-19 vaccine acceptance in the US. EClinicalMedicine 2020, 26, 100495. [Google Scholar] [CrossRef]
- Pacella-LaBarbara, M.L.; Park, Y.L.; Patterson, P.D.; Doshi, A.; Guyette, M.K.; Wong, A.H.; Chang, B.P.; Suffoletto, B.P. COVID-19 Vaccine Uptake and Intent Among Emergency Healthcare Workers: A Cross-Sectional Survey. J. Occup Environ. Med. 2021, 63, 852–856. [Google Scholar] [CrossRef]
- Paul, E.; Steptoe, A.; Fancourt, D. Attitudes towards vaccines and intention to vaccinate against COVID-19: Implications for public health communications. Lancet Reg. Health-Eur. 2021, 1, 100012. [Google Scholar] [CrossRef]
- Ruiz, J.B.; Bell, R.A. Predictors of intention to vaccinate against COVID-19: Results of a nationwide survey. Vaccine 2021, 39, 1080–1086. [Google Scholar] [CrossRef]
- Sherman, S.M.; Smith, L.E.; Sim, J.; Amlôt, R.; Cutts, M.; Dasch, H.; Rubin, G.J.; Sevdalis, N. COVID-19 vaccination intention in the UK: Results from the COVID-19 vaccination acceptability study (CoVAccS), a nationally representative cross-sectional survey. Hum. Vaccines Immunother. 2021, 17, 1612–1621. [Google Scholar] [CrossRef] [PubMed]
- Batra, U.; Nathany, S.; Bansal, N.; Sharma, M. COVID-19 vaccination status in Indian patients with cancer: An observational study. Cancer Res. Stat. Treat. 2021, 4, 219–223. [Google Scholar] [CrossRef]
- Lim, S.; Bae, J.H.; Kwon, H.-S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Lima-Martínez, M.M.; Boada, C.C.; Madera-Silva, M.D.; Marín, W.; Contreras, M. COVID-19 y diabetes mellitus: Una relación bidireccional. Clin. E Investig. En Arterioscler. 2021, 33, 151. [Google Scholar] [CrossRef]
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (COVID-19). Diabetes/Metab. Res. Rev. 2021, 37, e3377. [Google Scholar] [CrossRef]
- Khubchandani, J.; Sharma, S.; Price, J.H.; Wiblishauser, M.J.; Sharma, M.; Webb, F.J. COVID-19 vaccination hesitancy in the United States: A rapid national assessment. J. Community Health 2021, 46, 270–277. [Google Scholar] [CrossRef]
- Solís Arce, J.S.; Warren, S.S.; Meriggi, N.F.; Scacco, A.; McMurry, N.; Voors, M.; Syunyaev, G.; Malik, A.A.; Aboutajdine, S.; Adeojo, O.; et al. COVID-19 vaccine acceptance and hesitancy in low- and middle-income countries. Nat. Med. 2021, 27, 1385–1394. [Google Scholar] [CrossRef]
- Potdar, M.; Potdar, S.; Potdar, M. A study of gender disparities towards COVID-19 vaccination drive in Maharashtra State, India. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102297. [Google Scholar] [CrossRef]
- Green, M.S.; Abdullah, R.; Vered, S.; Nitzan, D. A study of ethnic, gender and educational differences in attitudes toward COVID-19 vaccines in Israel–implications for vaccination implementation policies. Isr. J. Health Policy Res. 2021, 10, 26. [Google Scholar] [CrossRef]
- Diesel, J.; Sterrett, N.; Dasgupta, S.; Kriss, J.L.; Barry, V.; Vanden Esschert, K.; Whiteman, A.; Cadwell, B.L.; Weller, D.; Qualters, J.R.; et al. COVID-19 Vaccination Coverage Among Adults—United States, December 14, 2020–May 22, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 922–927. [Google Scholar] [CrossRef]

| No. | Milestone | Date | Reference |
|---|---|---|---|
| 1 | Launching the national COVID1-19 vaccination program (1st stage) | 17 December 2020 | [59] |
| 2 | Announcement of the 2nd stage of the program | 18 February 2021 | [62] |
| 3 | Announcement of the administration of 2nd dose for ≥60 | 28 May 2021 | [74] |
| 4 | Announcement of the administration of 2nd dose for ≥50 | 24 June 2021 | [75] |
| 5 | Approval of mixing vaccines in Saudi Arabia | 23 June 2021 | [76] |
| 6 | Approval of Pfizer/BioNTech vaccine for ages 12–18 | 27 June 2021 | [77] |
| 7 | Announcement of the administration of 2nd dose for ≥40 | 5 July 2021 | [78] |
| 8 | Announcement of the administration of 2nd dose for all eligible persons of all ages | 11 July 2021 | [79] |
| 9 | Approval of receiving the vaccine after 10 days from recovery of COVID-19 infection (two doses regardless of the prior infection) | 29 July 2021 | [80] |
| 10 | Approval of Pfizer/BioNTech vaccine for the ages 5–11 | 3 November 2021 | [81] |
| Variable | History of COVID-19 Infection | p Value | |
|---|---|---|---|
| Yes (n = 1981) % (n) | No (n = 9592) % (n) | ||
| Gender | 0.817 | ||
| Male | 17.2% (872) | 82.8% (4195) | |
| Female | 17.0% (1109) | 83.0% (5397) | |
| Age | 0.098 | ||
| ≤40 | 18.6% (168) | 81.4% (734) | |
| 41–50 | 15.5% (340) | 84.5% (1850) | |
| 51–60 | 16.8% (651) | 83.2% (3231) | |
| 61–70 | 18.1% (528) | 81.9% (2389) | |
| 71–80 | 16.9% (224) | 83.1% (1101) | |
| >80 | 19.6% (70) | 80.4% (287) | |
| Variable | Vaccination Status (1st Dose Only) | p Value | |
|---|---|---|---|
| Yes (n = 9811) % (n) | No (n = 1762) % (n) | ||
| Gender | <0.001 | ||
| Male | 88.5% (4482) | 11.5% (585) | |
| Female | 81.9% (5329) | 18.1% (1177) | |
| Age | <0.001 | ||
| ≤40 | 88.7% (800) | 11.3% (102) | |
| 41–50 | 88.1% (1929) | 11.9% (261) | |
| 51–60 | 86.0% (3338) | 14.0% (544) | |
| 61–70 | 83.6% (2438) | 16.4% (479) | |
| 71–80 | 79.5% (1053) | 20.5% (272) | |
| >80 | 70.9% (253) | 29.1% (104) | |
| History of previous infection with COVID-19 | <0.001 | ||
| Yes | 73.3% (1452) | 26.7% (529) | |
| No | 87.1% (8359) | 12.9% (1233) | |
| Variable | Fully Vaccination Status (Completed Two Doses) | p Value | |
|---|---|---|---|
| Yes (n = 6422) % (n) | No (n = 5151) % (n) | ||
| Gender | <0.001 | ||
| Male | 61.0% (3091) | 39.0% (1976) | |
| Female | 51.2% (3331) | 48.8% (3175) | |
| Age | <0.001 | ||
| ≤40 | 49.6% (447) | 50.4% (455) | |
| 41–50 | 52.6% (1153) | 47.4% (1037) | |
| 51–60 | 55.4% (2150) | 44.6% (1732) | |
| 61–70 | 59.0% (1721) | 41.0% (1196) | |
| 71–80 | 58.6% (776) | 41.4% (549) | |
| >80 | 49.0% (175) | 51.0% (182) | |
| History of previous infection with COVID-19 | <0.001 | ||
| Yes | 14.6% (290) | 85.4% (1691) | |
| No | 63.9% (6132) | 36.1% (3460) | |
| Variable | Adjusted Odds Ratio (95% CI) | p Value |
|---|---|---|
| Gender | ||
| Male | 1 | p < 0.001 |
| Female | 1.705 (1.528–1.902) | |
| Age | p < 0.001 | |
| ≤40 | 1 | |
| 41–50 | 1.035 (0.808–1.324) | 0.786 |
| 51–60 | 1.155 (0.918–1.453) | 0.219 |
| 61–70 | 1.390 (1.102–1.753) | 0.006 |
| 71–80 | 1.924 (1.499–2.470) | p < 0.001 |
| >80 | 3.081 (2.252–4.214) | p < 0.001 |
| History of previous infection with COVID-19 | ||
| Yes | 2.501 (2.223–2.813) | p < 0.001 |
| No | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tourkmani, A.M.; Bin Rsheed, A.M.; AlEissa, M.S.; Alqahtani, S.M.; AlOtaibi, A.F.; Almujil, M.S.; AlKhshan, I.H.; ALNassar, T.N.; ALOtaibi, M.N.; Alrasheedy, A.A. Prevalence of COVID-19 Infection among Patients with Diabetes and Their Vaccination Coverage Status in Saudi Arabia: A Cross-Sectional Analysis from a Hospital-Based Diabetes Registry. Vaccines 2022, 10, 310. https://doi.org/10.3390/vaccines10020310
Tourkmani AM, Bin Rsheed AM, AlEissa MS, Alqahtani SM, AlOtaibi AF, Almujil MS, AlKhshan IH, ALNassar TN, ALOtaibi MN, Alrasheedy AA. Prevalence of COVID-19 Infection among Patients with Diabetes and Their Vaccination Coverage Status in Saudi Arabia: A Cross-Sectional Analysis from a Hospital-Based Diabetes Registry. Vaccines. 2022; 10(2):310. https://doi.org/10.3390/vaccines10020310
Chicago/Turabian StyleTourkmani, Ayla M., Abdulaziz Mansour Bin Rsheed, Mohammad Saad AlEissa, Sulaiman Mohammed Alqahtani, Azzam Fahad AlOtaibi, Mohammed S. Almujil, Ibraheem H. AlKhshan, Turki N. ALNassar, Mansour N. ALOtaibi, and Alian A. Alrasheedy. 2022. "Prevalence of COVID-19 Infection among Patients with Diabetes and Their Vaccination Coverage Status in Saudi Arabia: A Cross-Sectional Analysis from a Hospital-Based Diabetes Registry" Vaccines 10, no. 2: 310. https://doi.org/10.3390/vaccines10020310
APA StyleTourkmani, A. M., Bin Rsheed, A. M., AlEissa, M. S., Alqahtani, S. M., AlOtaibi, A. F., Almujil, M. S., AlKhshan, I. H., ALNassar, T. N., ALOtaibi, M. N., & Alrasheedy, A. A. (2022). Prevalence of COVID-19 Infection among Patients with Diabetes and Their Vaccination Coverage Status in Saudi Arabia: A Cross-Sectional Analysis from a Hospital-Based Diabetes Registry. Vaccines, 10(2), 310. https://doi.org/10.3390/vaccines10020310






