Immunization of Nile Tilapia (Oreochromis niloticus) Broodstock with Tilapia Lake Virus (TiLV) Inactivated Vaccines Elicits Protective Antibody and Passive Maternal Antibody Transfer
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Vaccine Preparation
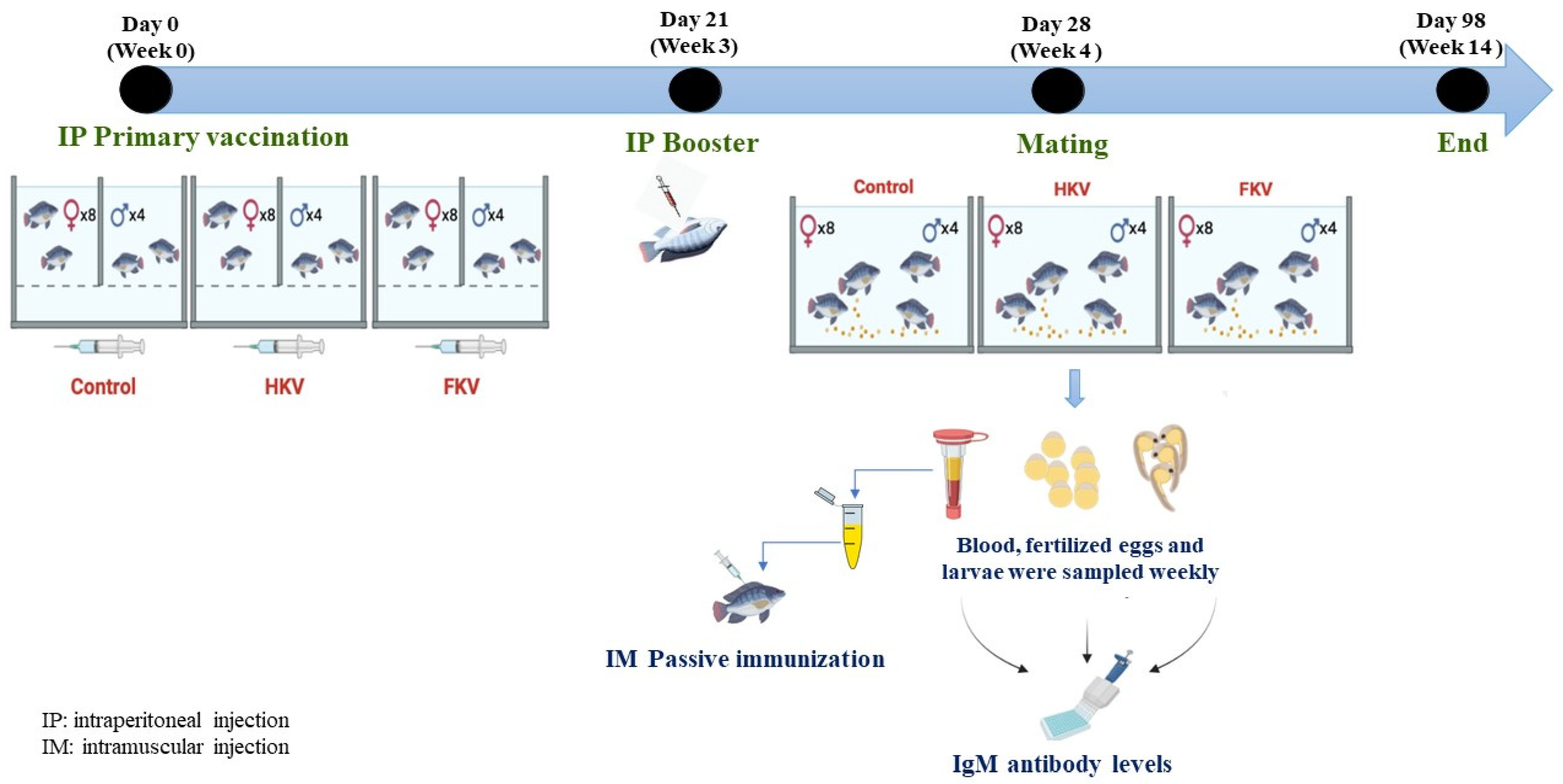
2.3. Immunization, Breeding and Sampling
2.4. Egg and Larvae Collection
2.5. Measurement of Anti-TiLV IgM Antibody Levels by ELISA
2.6. Passive Immunization
2.7. Statistical Analysis
3. Results
3.1. Measurement of Systemic Anti-TiLV IgM Levels by ELISA
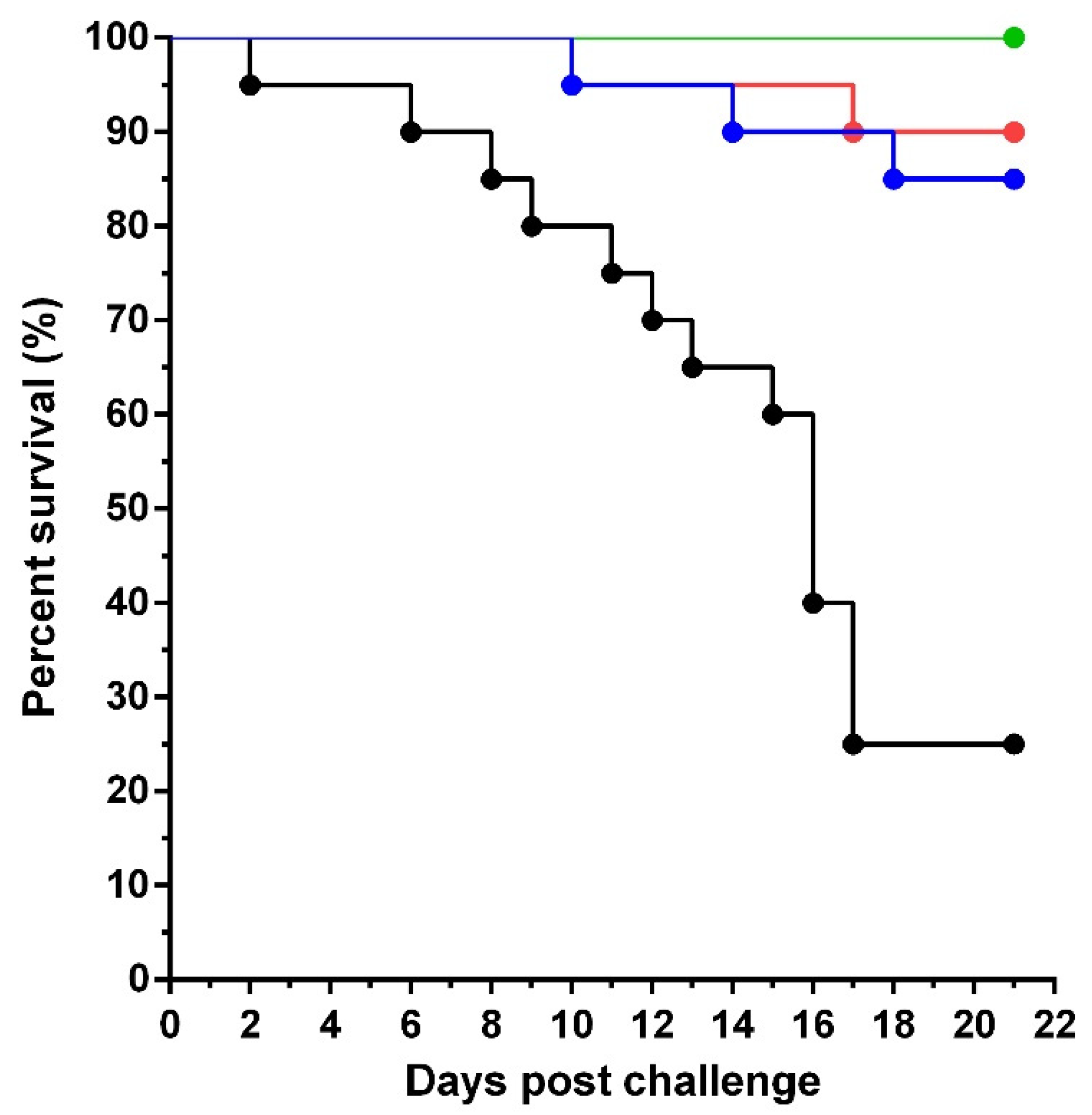
3.2. Passive Immunization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO Tilapia Sector Expected to Resume Rapid Growth after Temporary Slowdown in 2020. 2021. Available online: https://www.fao.org/in-action/globefish/market-reports/resource-detail/en/c/1379264/ (accessed on 10 December 2021).
- Fitzsimmons, K. Market Stability: Why Tilapia Supply and Demand have Avoided the Boom and Bust of other Commodities Tilapia. In Proceedings of the 4th International Trade and Technical Conference and Position on Tilapia, Kuala Lumpur, Malaysia, 2–4 April 2015; Available online: https://www.infopesca.org/sites/default/files/complemento/actividadesrecientes/adjuntos/1369/6%20Malaysia-Industry%20and%20Market%20Status%20of%20Tialpia.pdf (accessed on 15 November 2021).
- Machimbirike, V.I.; Jansen, M.D.; Senapin, S.; Khunrae, P.; Rattanarojpong, T.; Dong, H.T. Viral infections in tilapines: More than just tilapia lake virus. Aquaculture 2019, 503, 508–518. [Google Scholar] [CrossRef]
- Bacharach, E.; Mishra, N.; Briese, T.; Eldar, A.; Lipkin, W.I.; Kuhn, J.H.; Lipkin, W.I. ICTV Taxonomic Proposal 2016.016a-dM.A.v2.Tilapinevirus. Create the Unassigned Genus Tilapinevirus. 2016. Available online: https://talk.ictvonline.org/ICTV/proposals/2016.016a-dM.A.v2.Tilapinevirus.pdf (accessed on 27 July 2021).
- Eyngor, M.; Zamostiano, R.; Tsofack, J.E.K.; Berkowitz, A.; Bercovier, H.; Tinman, S.; Lev, M.; Hurvitz, A.; Galeotti, M.; Bacharach, E.; et al. Identification of a novel RNA virus lethal to tilapia. J. Clin. Microbiol. 2014, 52, 4137–4146. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, H.W.; Kabuusu, R.; Beltran, S.; Reyes, E.; Lince, J.A.; del Pozo, J. Syncytial hepatitis of farmed tilapia, Oreochromis niloticus (L.): A case report. J. Fish Dis. 2014, 37, 583–589. [Google Scholar] [CrossRef]
- Del-Pozo, J.; Mishra, N.; Kabuusu, R.; Cheetham, S.; Eldar, A.; Bacharach, E.; Lipkin, W.I.; Ferguson, H.W. Syncytial Hepatitis of Tilapia (Oreochromis niloticus L.) is Associated With Orthomyxovirus-Like Virions in Hepatocytes. Vet. Pathol. 2017, 54, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Bacharach, E.; Mishra, N.; Briese, T.; Zody, M.C.; Tsofack, J.E.K.; Zamostiano, R.; Berkowitz, A.; Ng, J.; Nitido, A.; Corvelo, A.; et al. Characterization of a novel orthomyxo-like virus causing mass die-offs of Tilapia. MBio 2016, 7, e00431-16. [Google Scholar] [CrossRef]
- Dong, H.T.; Siriroob, S.; Meemetta, W.; Santimanawong, W.; Gangnonngiw, W.; Pirarat, N.; Khunrae, P.; Rattanarojpong, T.; Vanichviriyakit, R.; Senapin, S. Emergence of tilapia lake virus in Thailand and an alternative semi-nested RT-PCR for detection. Aquaculture 2017, 476, 111–118. [Google Scholar] [CrossRef]
- Surachetpong, W.; Janetanakit, T.; Nonthabenjawan, N.; Tattiyapong, P.; Sirikanchana, K.; Amonsin, A. Outbreaks of Tilapia Lake Virus Infection. Emerg. Infect. Dis. 2017, 23, 1031–1033. [Google Scholar] [CrossRef]
- Dinh-Hung, N.; Sangpo, P.; Kruangkum, T.; Kayansamruaj, P.; Rung-ruangkijkrai, T.; Senapin, S.; Rodkhum, C.; Dong, H.T. Dissecting the localization of Tilapia tilapinevirus in the brain of the experimentally infected Nile tilapia, Oreochromis niloticus (L.). J. Fish Dis. 2021, 44, 1053–1064. [Google Scholar] [CrossRef]
- Tattiyapong, P.; Dachavichitlead, W.; Surachetpong, W. Experimental infection of Tilapia Lake Virus (TiLV) in Nile tilapia (Oreochromis niloticus) and red tilapia (Oreochromis spp.). Vet. Microbiol. 2017, 207, 170–177. [Google Scholar] [CrossRef]
- Amal, M.N.A.; Koh, C.B.; Nurliyana, M.; Suhaiba, M.; Nor-Amalina, Z.; Santha, S.; Diyana-Nadhirah, K.P.; Yusof, M.T.; Ina-Salwany, M.Y.; Zamri-Saad, M. A case of natural co-infection of Tilapia Lake Virus and Aeromonas veronii in a Malaysian red hybrid tilapia (Oreochromis niloticus × O. mossambicus) farm experiencing high mortality. Aquaculture 2018, 485, 12–16. [Google Scholar] [CrossRef]
- Dong, H.T.; Ataguba, G.A.; Khunrae, P.; Rattanarojpong, T.; Senapin, S. Evidence of TiLV infection in tilapia hatcheries in Thailand from 2012 to 2017 reveals probable global spread of the disease. Aquaculture 2017, 479, 579–583. [Google Scholar] [CrossRef]
- Dong, H.T.; Senapin, S.; Gangnonngiw, W.; Nguyen, V.V.; Rodkhum, C.; Debnath, P.P.; Delamare-Deboutteville, J.; Mohan, C.V. Experimental infection reveals transmission of tilapia lake virus (TiLV) from tilapia broodstock to their reproductive organs and fertilized eggs. Aquaculture 2020, 515, 734541. [Google Scholar] [CrossRef]
- Yamkasem, J.; Tattiyapong, P.; Kamlungdee, A.; Surachetpong, W. Evidence of potential vertical transmission of tilapia lake virus. J. Fish Dis. 2019, 42, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Bacharach, E.; Eldar, A. Tilapia Lake Virus Vaccines. U.S. Patent US2016/0354458A1, 9 August 2016. Available online: https://patents.google.com/patent/US20160354458A1/en (accessed on 15 July 2021).
- Zeng, W.; Wang, Y.; Hu, H.; Wang, Q.; Bergmann, S.M.; Wang, Y.; Li, B.; Lv, Y.; Li, H.; Yin, J.; et al. Cell Culture-Derived Tilapia Lake Virus-Inactivated Vaccine Containing Montanide Adjuvant Provides High Protection against Viral Challenge for Tilapia. Vaccines 2021, 9, 86. [Google Scholar] [CrossRef]
- Zeng, W.; Wang, Y.; Chen, X.; Wang, Q.; Bergmann, S.M.; Yang, Y.; Wang, Y.; Li, B.; Lv, Y.; Li, H.; et al. Potency and efficacy of VP20-based vaccine against tilapia lake virus using different prime-boost vaccination regimens in tilapia. Aquaculture 2021, 539, 736654. [Google Scholar] [CrossRef]
- Mai, T.T.; Kayansamruaj, P.; Taengphu, S.; Senapin, S.; Jorge, J.Z.C. Efficacy of heat-killed and formalin-killed vaccines against Tilapia tilapinevirus in juvenile Nile tilapia (Oreochromis niloticus). J. Fish Dis. 2021, 44, 2097–2109. [Google Scholar] [CrossRef]
- Baxter, D. Active and passive immunity, vaccine types, excipients and licensing. Occup. Med. 2007, 57, 552–556. [Google Scholar] [CrossRef]
- Secombes, C.J.; Belmonte, R. Overview of the fish adaptive immune system. Birkhauser Adv. Infect. Dis. 2016, 2, 35–52. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Z.; Wang, H. Maternal immunity in fish. Dev. Comp. Immunol. 2013, 39, 72–78. [Google Scholar] [CrossRef]
- Rajan, B.; Løkka, G.; Koppang, E.O.; Austbø, L. Passive Immunization of Farmed Fish. J. Immunol. 2017, 198, 4195–4202. [Google Scholar] [CrossRef]
- Kanlis, G.; Takashima, F.; Suzuki, Y.; Tauchi, M.; Numata, T.; Shirojo, Y.; Kawano, K. Immunoglobulin Concentration and Specific Antibody Activity in Oocytes and Eggs of Immunized Red Sea Bream. Fish. Sci. 1995, 61, 791–795. [Google Scholar] [CrossRef]
- Picchietti, S.; Abelli, L.; Buonocore, F.; Randelli, E.; Fausto, A.M.; Scapigliati, G.; Mazzini, M. Immunoglobulin protein and gene transcripts in sea bream (Sparus aurata L.) oocytes. Fish Shellfish Immunol. 2006, 20, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Kai, Y.H.; Su, H.M.; Tai, K.T.; Chi, S.C. Vaccination of grouper broodfish (Epinephelus tukula) reduces the risk of vertical transmission by nervous necrosis virus. Vaccine 2010, 28, 996–1001. [Google Scholar] [CrossRef] [PubMed]
- Nurani, F.S.; Sukenda, S.; Nuryati, S. Maternal immunity of tilapia broodstock vaccinated with polyvalent vaccine and resistance of their offspring against Streptococcus agalactiae. Aquac. Res. 2020, 51, 1513–1522. [Google Scholar] [CrossRef]
- Taengphu, S. Concentration and quantification of Tilapia tilapinevirus from water using a simple iron flocculation coupled with probe-based RT-qPCR. bioRxiv 2021. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. The American. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Amadou Ly, M.; Ngom, A.; Bamba Fall, A.; Diouf, O. Broodstock Sex Ratio Improves Fry Production in Nile Tilapia (Oreochromis Niloticus) in Northern Senegal. Int. J. Adv. Res. 2021, 9, 72–76. [Google Scholar] [CrossRef]
- Soonthonsrima, T.; Wangman, P.; Chaivisuthangkura, P.; Pengsuk, C.; Sithigorngul, P.; Longyant, S. Generation of mouse monoclonal antibodies specific to tilapia immunoglobulin using fish immunoglobulin/BSA complex for monitoring of the immune response in Nile tilapia Oreochromis niloticus. Aquac. Res. 2019, 50, 277–283. [Google Scholar] [CrossRef]
- Frey, A.; Di, J.; Zurakowski, D. A statistically defined endpoint titer determination method for immunoassays. J. Immunol. Methods 1998, 221, 35–41. [Google Scholar] [CrossRef]
- Tattiyapong, P.; Dechavichitlead, W.; Waltzek, T.B.; Surachetpong, W. Tilapia develop protective immunity including a humoral response following exposure to tilapia lake virus. Fish Shellfish Immunol. 2020, 106, 666–674. [Google Scholar] [CrossRef]
- Forthal, D.N. Functions of antibodies. Microbiol. Spectr. 2014, 2, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, P.K.; Gregg, J.L.; Grady, C.A.; LaPatra, S.E.; Winton, J.R. Passive immunization of Pacific herring against viral hemorrhagic septicemia. J. Aquat. Anim. Health 2011, 23, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Towers, L. Hatchery Management and Tilapia Fingerling Production. 2015. Available online: https://thefishsite.com/articles/hatchery-management-and-tilapia-fingerling-production (accessed on 19 December 2021).
- Abu-Elala, N.M.; Samir, A.; Wasfy, M.; Elsayed, M. Efficacy of Injectable and Immersion Polyvalent Vaccine against Streptococcal Infections in Broodstock and Offspring of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2019, 88, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Pasaribu, W.; Sukenda, S.; Nuryati, S. The Efficacy of Nile Tilapia (Oreochromis niloticus) Broodstock and Larval Immunization against Streptococcus agalactiae and Aeromonas hydrophila. Fishes 2018, 3, 16. [Google Scholar] [CrossRef]
- Huang, S.-M.; Cheng, J.-H.; Tu, C.; Chen, T.-I.; Lin, C.-T.; Chang, S.-K. A Bivalent Inactivated Vaccine of Viral Nervous Necrosis Virus and Grouper Iridovirus Applied To Grouper Broodfish (Epinephelus Coioides) Reduces the Risk of Vertical Transmission. Taiwan Vet. J. 2017, 43, 171–176. [Google Scholar] [CrossRef]
- Takemura, A. Changes in an immunoglobulin M (IgM)-like protein during larval stages in tilapia, Oreochromis mossambicus. Aquaculture 1993, 115, 233–241. [Google Scholar] [CrossRef]
- Breuil, G.; Vassiloglou, B.; Pepin, J.F.; Romestand, B. Ontogeny of IgM-bearing cells and changes in the immunoglobulin M-like protein level (IgM) during larval stages in sea bass (Dicentrarchus labrax). Fish Shellfish Immunol. 1997, 7, 29–43. [Google Scholar] [CrossRef]
- Olsen, Y.A.; Press, C.M.L. Degradation kinetics of immunoglobulin in the egg, alevin and fry of Atlantic salmon, Salmo salar L., and the localisation of immunoglobulin in the egg. Fish Shellfish Immunol. 1997, 7, 81–91. [Google Scholar] [CrossRef]
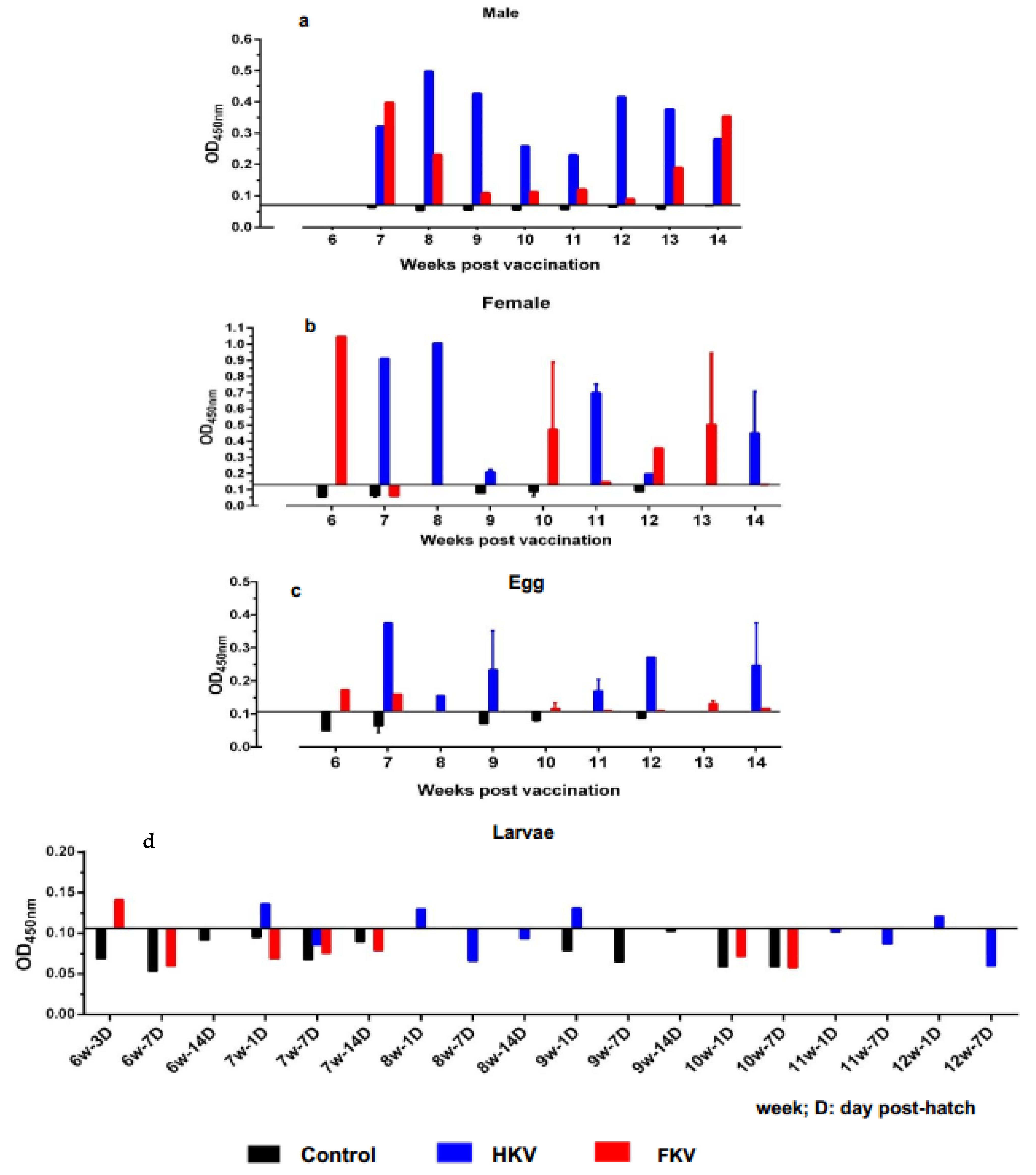
| Time | 6 Wppv | 7 Wppv | 8 Wppv | 9 Wppv | 10 Wppv | 11 Wppv | 12 Wppv | 13 Wppv | 14 Wppv | |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | ||||||||||
| Control | 2 FE 1 L (3D-7D-14D) | 2 FE 1 L (1D-7D-14D) | 1 FE 1L (1D-7D-14D) | 2 FE 1 L (1D-7D) | 1 FE | |||||
| HKV | 1 FE 1 L (1D-7D) | 1 FE 1 L (1D-7D-14D) | 2 FE 1 L (1D) | 2 FE 1 L (1D-7D) | 1 FE 1 L (1D-7D) | 2 FE | ||||
| FKV | 1 FE 1 L (3D-7D) | 1 FE 1 L (1D-7D-14D) | 2 FE 1 L (1D-7D) | 1 FE | 1 FE | 2 FE | 1 FE | |||
| Samples | Structures | Cut-Off Values |
|---|---|---|
| Female broodstock sera | Mean OD450 + 2.077 × SD | 0.070 |
| Male broodstock sera | Mean OD450 + 2.01 × SD | 0.130 |
| Egg supernatant | Mean OD450 + 2.01 × SD | 0.104 |
| Larval supernatant | Mean OD450 + 1.923 × SD | 0.106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mai, T.T.; Kayansamruaj, P.; Soontara, C.; Kerddee, P.; Nguyen, D.-H.; Senapin, S.; Costa, J.Z.; del-Pozo, J.; Thompson, K.D.; Rodkhum, C.; et al. Immunization of Nile Tilapia (Oreochromis niloticus) Broodstock with Tilapia Lake Virus (TiLV) Inactivated Vaccines Elicits Protective Antibody and Passive Maternal Antibody Transfer. Vaccines 2022, 10, 167. https://doi.org/10.3390/vaccines10020167
Mai TT, Kayansamruaj P, Soontara C, Kerddee P, Nguyen D-H, Senapin S, Costa JZ, del-Pozo J, Thompson KD, Rodkhum C, et al. Immunization of Nile Tilapia (Oreochromis niloticus) Broodstock with Tilapia Lake Virus (TiLV) Inactivated Vaccines Elicits Protective Antibody and Passive Maternal Antibody Transfer. Vaccines. 2022; 10(2):167. https://doi.org/10.3390/vaccines10020167
Chicago/Turabian StyleMai, Thao Thu, Pattanapon Kayansamruaj, Chayanit Soontara, Pattarawit Kerddee, Dinh-Hung Nguyen, Saengchan Senapin, Janina Z. Costa, Jorge del-Pozo, Kim D. Thompson, Channarong Rodkhum, and et al. 2022. "Immunization of Nile Tilapia (Oreochromis niloticus) Broodstock with Tilapia Lake Virus (TiLV) Inactivated Vaccines Elicits Protective Antibody and Passive Maternal Antibody Transfer" Vaccines 10, no. 2: 167. https://doi.org/10.3390/vaccines10020167
APA StyleMai, T. T., Kayansamruaj, P., Soontara, C., Kerddee, P., Nguyen, D.-H., Senapin, S., Costa, J. Z., del-Pozo, J., Thompson, K. D., Rodkhum, C., & Dong, H. T. (2022). Immunization of Nile Tilapia (Oreochromis niloticus) Broodstock with Tilapia Lake Virus (TiLV) Inactivated Vaccines Elicits Protective Antibody and Passive Maternal Antibody Transfer. Vaccines, 10(2), 167. https://doi.org/10.3390/vaccines10020167









