A Sandwich ELISA for Quality Control of PCV2 Virus-like Particles Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Animals
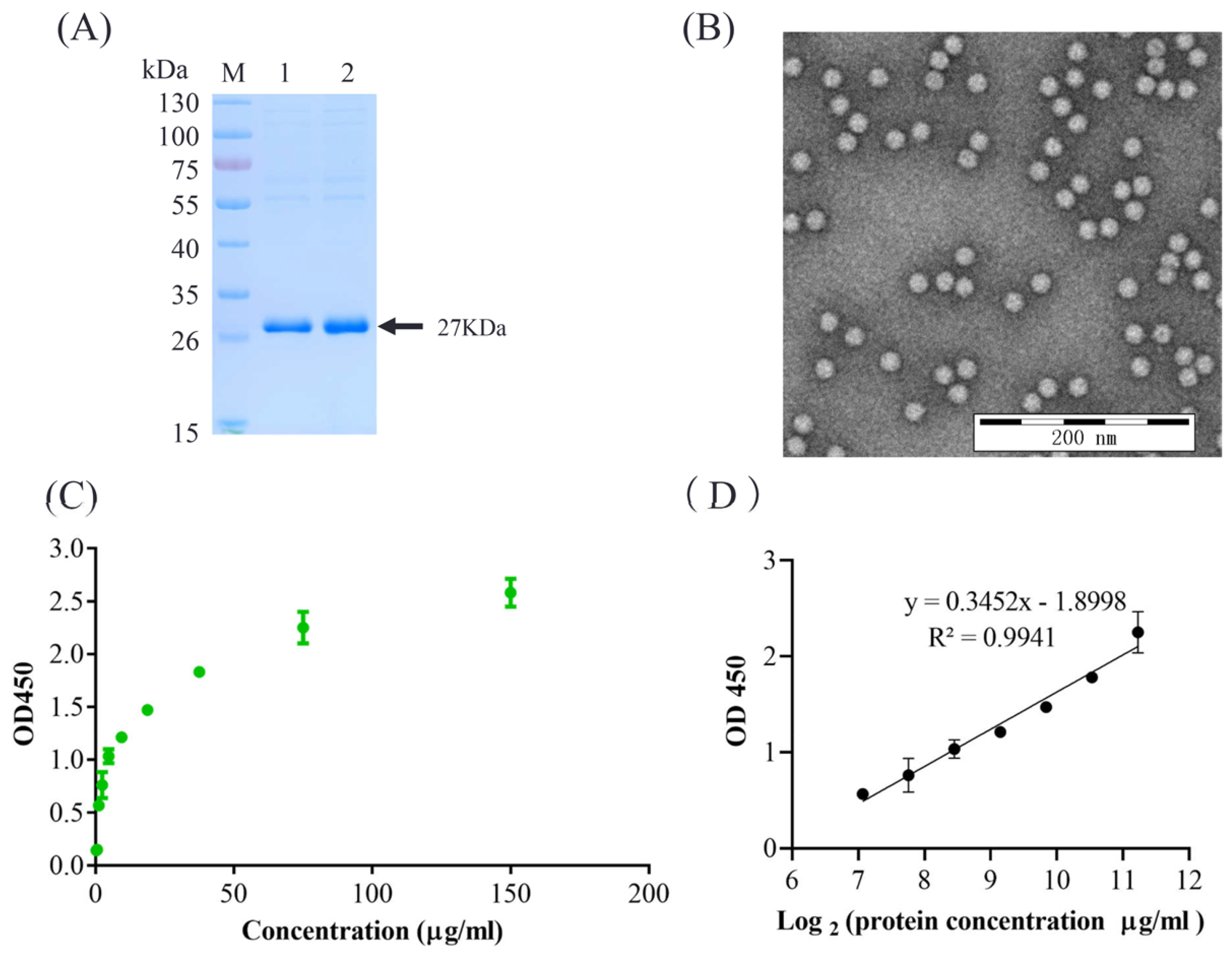
2.2. Preparation of PCV2 VLPs
2.3. Generation of Antibodies
2.4. Horseradish Peroxidase (HRP) Labeled mAb
2.5. Quantification of Antigen
2.6. Forced Degradation
2.7. ELISA for the Specificity of mAbs
2.8. Development and Optimization of the Sandwich ELISA Procedure
2.9. SDS-PAGE and Western Blot (WB) Analyses
2.10. IFA
2.11. Statistical Analysis
3. Results
3.1. Generation and Characterization of mAbs
3.2. Development of the Sandwich ELISA Method
3.3. Characterization of the Sandwich ELISA
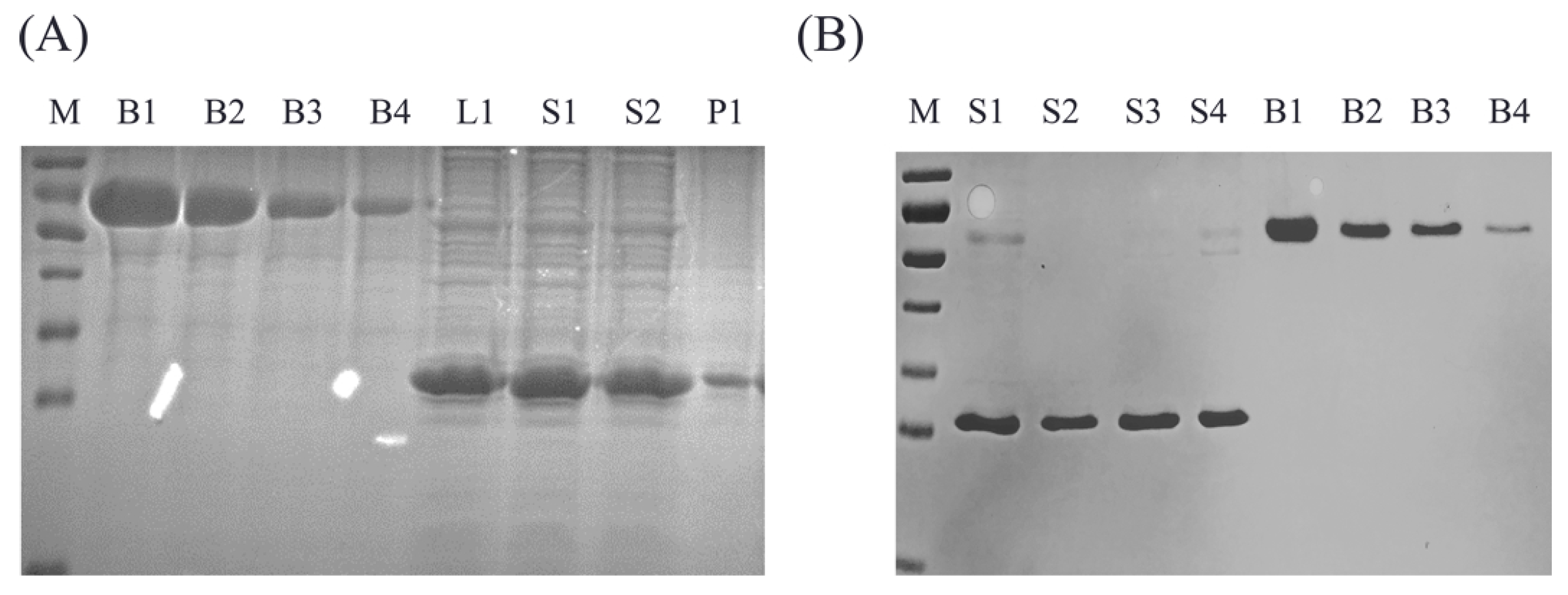
3.4. Application to Samples of Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tischer, I.; Gelderblom, H.; Vettermann, W.; Koch, M.A. A very small porcine virus with circular single-stranded DNA. Nature 1982, 295, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.; Opriessnig, T.; Meng, X.J.; Pelzer, K.; Buechner-Maxwell, V. Porcine circovirus type 2 and porcine circovirus-associated disease. J. Vet. Intern. Med. 2009, 23, 1151–1163. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Allan, G.M.; Domingo, M. Porcine circovirus diseases. Anim. Health Res. Rev. 2005, 6, 119–142. [Google Scholar] [CrossRef]
- Allan, G.M.; McNeilly, F.; Kennedy, S.; Daft, B.; Ellis, J.A.; Haines, D.M.; Meehan, B.M.; Adair, B.M. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the USA and Europe. J. Vet. Diagn. Investig. 1998, 10, 3–10. [Google Scholar] [CrossRef]
- Nawagitgul, P.; Morozov, I.; Bolin, S.R.; Harms, P.A.; Sorden, S.D.; Paul, P.S. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J. Gen. Virol. 2000, 81, 2281–2287. [Google Scholar] [CrossRef]
- Wu, P.C.; Chen, T.Y.; Chi, J.N.; Chien, M.S.; Huang, C. Efficient expression and purification of porcine circovirus type 2 virus-like particles in Escherichia coli. J. Biotechnol. 2016, 220, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Yao, L.; Hao, H.; Fu, X.; Yang, Z.; Du, E. Enhanced production of porcine circovirus type 2 (PCV2) virus-like particles in Sf9 cells by translational enhancers. Biotechnol. Lett. 2015, 37, 1765–1771. [Google Scholar] [CrossRef]
- Xi, X.; Mo, X.; Xiao, Y.; Yin, B.; Lv, C.; Wang, Y.; Sun, Z.; Yang, Q.; Yao, Y.; Xuan, Y. Production of Escherichia coli-based virus-like particle vaccine against porcine circovirus type 2 challenge in piglets: Structure characterization and protective efficacy validation. J. Biotechnol. 2016, 223, 8–12. [Google Scholar] [CrossRef]
- Yin, S.; Sun, S.; Yang, S.; Shang, Y.; Cai, X.; Liu, X. Self-assembly of virus-like particles of porcine circovirus type 2 capsid protein expressed from Escherichia coli. Virol. J. 2010, 7, 166. [Google Scholar] [CrossRef]
- Metz, B.; Van Den Dobbelsteen, G.; Van Els, C.; Van Der Gun, J.; Levels, L.; van Der Pol, L.; Rots, N.; Kersten, G. Quality-control issues and approaches in vaccine development. Expert Rev. Vaccines 2009, 8, 227–238. [Google Scholar] [CrossRef]
- Zhou, W.; Bi, J.; Janson, J.C.; Dong, A.; Li, Y.; Zhang, Y.; Huang, Y.; Su, Z. Ion-exchange chromatography of hepatitis B virus surface antigen from a recombinant Chinese hamster ovary cell line. J. Chromatogr. A 2005, 1095, 119–125. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, S.; Yu, H.; Xia, N.; Modis, Y. Virus-like particle-based human vaccines: Quality assessment based on structural and functional properties. Trends Biotechnol. 2013, 31, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Metz, B.; Hendriksen, C.F.; Jiskoot, W.; Kersten, G.F. Reduction of animal use in human vaccine quality control: Opportunities and problems. Vaccine 2002, 20, 2411–2430. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.; Basketter, D.; Creton, S.; Pelkonen, O.; Van Benthem, J.; Zuang, V.; Andersen, K.E.; Angers-Loustau, A.; Aptula, A.; Bal-Price, A. Alternative (non-animal) methods for cosmetics testing: Current status and future prospects—2010. Arch. Toxicol. 2011, 85, 367–485. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, R.; Lewis, R.; Shinners, B.; Kubai, L.; Akhtar, N. Angiogenesis assays: A critical overview. Clin. Chem. 2003, 49, 32–40. [Google Scholar] [CrossRef]
- Steppert, P.; Burgstaller, D.; Klausberger, M.; Tover, A.; Berger, E.; Jungbauer, A. Quantification and characterization of virus-like particles by size-exclusion chromatography and nanoparticle tracking analysis. J. Chromatogr. A 2017, 1487, 89–99. [Google Scholar] [CrossRef]
- Yang, Y.; Li, H.; Li, Z.; Zhang, Y.; Zhang, S.; Chen, Y.; Yu, M.; Ma, G.; Su, Z. Size-exclusion HPLC provides a simple, rapid, and versatile alternative method for quality control of vaccines by characterizing the assembly of antigens. Vaccine 2015, 33, 1143–1150. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Y.; Zou, X.; Li, C.; Zhu, Y.; Qin, Y.; Li, Y.; Sheng, Y.N.; Liu, Y.; Peng, G. Characterization of the antigens in inactivated porcine circovirus type 2 vaccines and virus-like particle vaccines by high-performance size-exclusion chromatography coupled with multi-angle laser light scattering. Sheng Wu Gong Cheng Xue Bao Chin. J. Biotechnol. 2022, 38, 2948–2958. [Google Scholar]
- Le, C.T.; Grambsch, P.M.; Giebink, G.S. Quality control and the identification of vaccine responders using ELISA-derived antibody data. Stat. Med. 2003, 22, 2935–2942. [Google Scholar] [CrossRef]
- Galfre, G.; Milstein, C. Preparation of monoclonal antibodies: Strategies and procedures. Methods Enzymol. 1981, 73, 3–46. [Google Scholar] [CrossRef]
- Li, D.; Morisseau, C.; McReynolds, C.B.; Duflot, T.; Bellien, J.; Nagra, R.M.; Taha, A.Y.; Hammock, B.D. Development of improved double-nanobody sandwich ELISAs for human soluble epoxide hydrolase detection in peripheral blood mononuclear cells of diabetic patients and the prefrontal cortex of multiple sclerosis patients. Anal. Chem. 2020, 92, 7334–7342. [Google Scholar] [CrossRef] [PubMed]
- Lua, L.H.; Connors, N.K.; Sainsbury, F.; Chuan, Y.P.; Wibowo, N.; Middelberg, A.P. Bioengineering virus-like particles as vaccines. Biotechnol. Bioeng. 2014, 111, 425–440. [Google Scholar] [CrossRef] [PubMed]


| Sample | Inter-Batch Recovery | CV/% | |||
|---|---|---|---|---|---|
| Repeat1 | Repeat2 | Repeat3 | Mean Value | ||
| 40 g/mL | 113.54 | 101.41 | 121.36 | 112.10 | 8.96 |
| 10 g/mL | 107.94 | 108.26 | 114.05 | 110.08 | 3.12 |
| 2 g/mL | 99.99 | 110.34 | 105.04 | 105.12 | 4.92 |
| Sample | Inter-Batch Recovery | CV/% | |||
|---|---|---|---|---|---|
| Repeat1 | Repeat2 | Repeat3 | Mean Value | ||
| 40 g/mL | 104.01 | 106.68 | 111.9 | 107.53 | 3.73 |
| 10 g/mL | 102.22 | 105.14 | 100.84 | 102.73 | 2.72 |
| 2 g/mL | 111.90 | 115.38 | 106.57 | 111.28 | 5.16 |
| Sample | Grayscale Analysis g/mL | ELISA g/mL | Compliance Rate |
|---|---|---|---|
| L1 | 752.9155 | 652.14 | 0.8667 |
| S1 | 818.0191 | 741.85 | 0.907 |
| S2 | 638.9822 | 593.48 | 0.9288 |
| P1 | 59.41282 | 7.81 | 0.1314 |
| Sample | Grayscale Analysis g/mL | ELISA g/mL | Compliance Rate |
|---|---|---|---|
| S1 | 365.83 | 354.61 | 0.969 |
| S2 | 277.57 | 262.98 | 0.947 |
| S3 | 331.72 | 347.69 | 1.048 |
| S4 | 303.62 | 327.19 | 1.078 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Wang, S.; Fang, Z.; Zhao, M.; Gao, Y.; An, T.; Tu, Y.; Wang, H.; Cai, X. A Sandwich ELISA for Quality Control of PCV2 Virus-like Particles Vaccine. Vaccines 2022, 10, 2175. https://doi.org/10.3390/vaccines10122175
Sun M, Wang S, Fang Z, Zhao M, Gao Y, An T, Tu Y, Wang H, Cai X. A Sandwich ELISA for Quality Control of PCV2 Virus-like Particles Vaccine. Vaccines. 2022; 10(12):2175. https://doi.org/10.3390/vaccines10122175
Chicago/Turabian StyleSun, Mingxia, Shanghui Wang, Zheng Fang, Man Zhao, Yanfei Gao, Tongqing An, Yabin Tu, Haiwei Wang, and Xuehui Cai. 2022. "A Sandwich ELISA for Quality Control of PCV2 Virus-like Particles Vaccine" Vaccines 10, no. 12: 2175. https://doi.org/10.3390/vaccines10122175
APA StyleSun, M., Wang, S., Fang, Z., Zhao, M., Gao, Y., An, T., Tu, Y., Wang, H., & Cai, X. (2022). A Sandwich ELISA for Quality Control of PCV2 Virus-like Particles Vaccine. Vaccines, 10(12), 2175. https://doi.org/10.3390/vaccines10122175






