Response to Omalizumab as an Add-On Therapy in the Treatment of Allergic Asthma in Adult Chinese Patients—A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Cases Data
2.2. Methods and Clinical Data
2.3. Statistical Methods
3. Results
3.1. Baseline and Clinical Characteristics
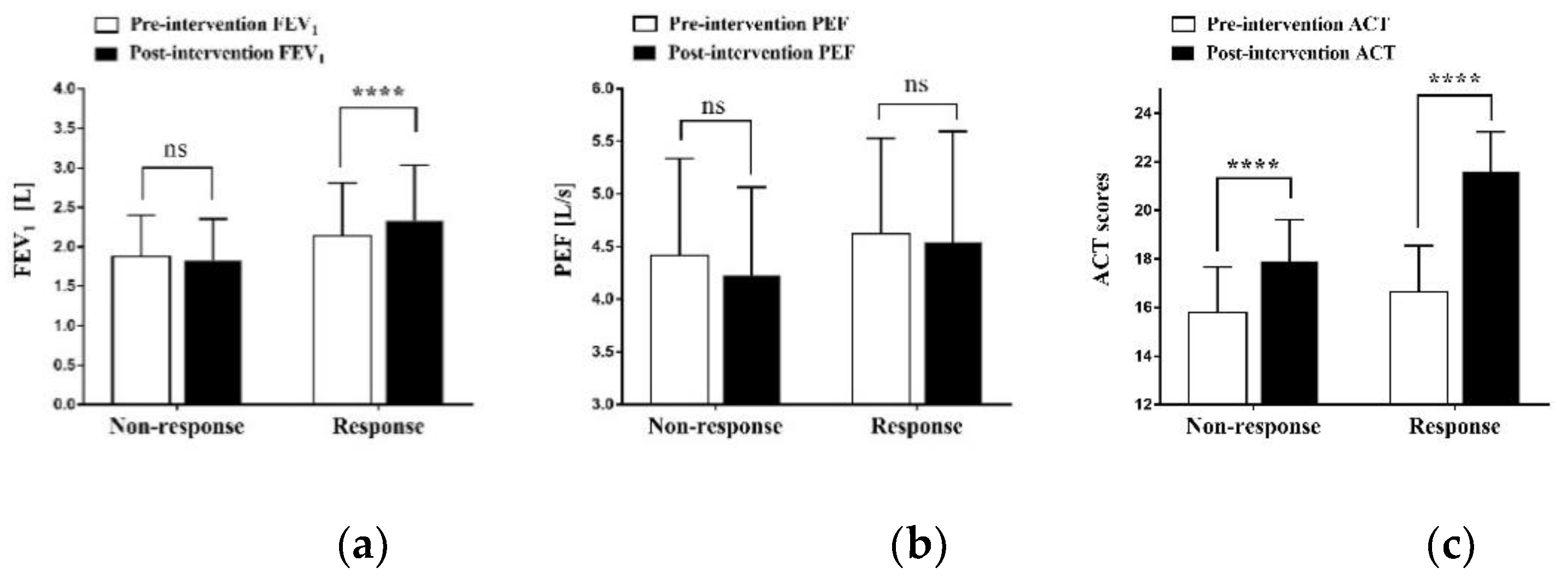
3.2. Efficacy Assessment after 16 Weeks
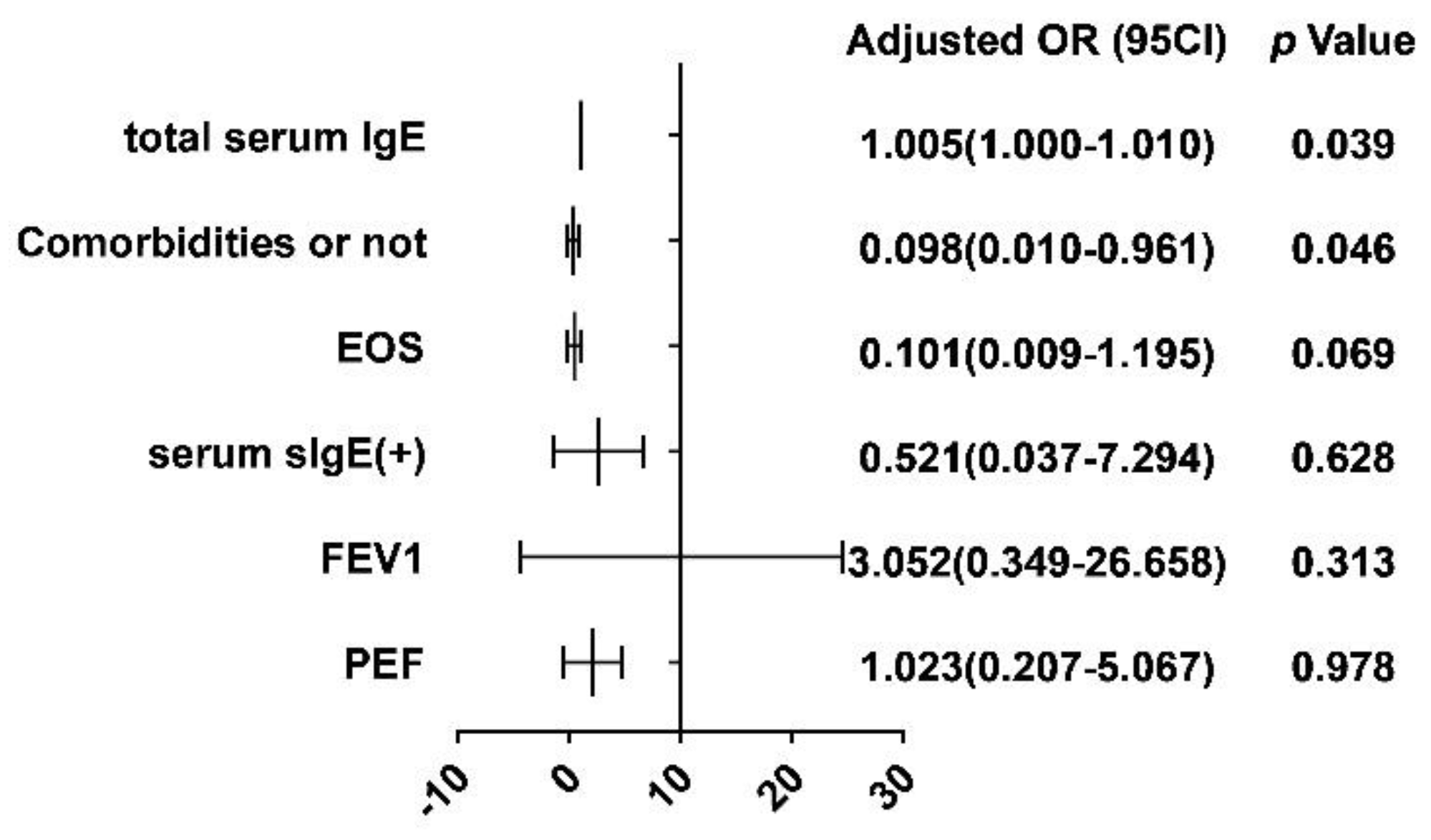
3.3. Predicting the Response to Omalizumab
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. 2022. Available online: https://ginasthma.org/ (accessed on 3 May 2022).
- Wenzel, S.E. Asthma phenotypes: The evolution from clinical to molecular approaches. Nat. Med. 2012, 18, 716–725. [Google Scholar] [CrossRef] [PubMed]
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Yang, T.; Xu, J.; Yang, L.; Zhao, J.; Zhang, X.; Bai, C.; Kang, J.; Ran, P.; Shen, H.; et al. Prevalence, risk factors, and management of asthma in China: A national cross-sectional study. Lancet 2019, 394, 407–418. [Google Scholar] [CrossRef]
- Haselkorn, T.; Chen, H.; Miller, D.P.; Fish, J.E.; Peters, S.P.; Weiss, S.T.; Jones, C.A. Asthma control and activity limitations: Insights from the Real-world Evaluation of Asthma Control and Treatment (REACT) study. Ann. Allergy Asthma Immunol. 2010, 104, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Su, N.; Lin, J.; Chen, P.; Li, J.; Wu, C.; Yin, K.; Liu, C.; Chen, Y.; Zhou, X.; Yuan, Y.; et al. Evaluation of asthma control and patient’s perception of asthma: Findings and analysis of a nationwide questionnaire-based survey in China. J. Asthma 2013, 50, 861–870. [Google Scholar] [CrossRef]
- Pelaia, G.; Canonica, G.W.; Matucci, A.; Paolini, R.; Triggiani, M.; Paggiaro, P. Targeted therapy in severe asthma today: Focus on immunoglobulin E. Drug Des. Dev. Ther. 2017, 11, 1979–1987. [Google Scholar] [CrossRef][Green Version]
- Pelaia, C.; Calabrese, C.; Terracciano, R.; de Blasio, F.; Vatrella, A.; Pelaia, G. Omalizumab, the first available antibody for biological treatment of severe asthma: More than a decade of real-life effectiveness. Ther. Adv. Respir. Dis. 2018, 12, 1753466618810192. [Google Scholar] [CrossRef]
- Humbert, M.; Beasley, R.; Ayres, J.; Slavin, R.; Hébert, J.; Bousquet, J.; Beeh, K.M.; Ramos, S.; Canonica, G.W.; Hedgecock, S.; et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005, 60, 309–316. [Google Scholar] [CrossRef]
- Kulus, M.; Hébert, J.; Garcia, E.; Fowler Taylor, A.; Fernandez Vidaurre, C.; Blogg, M. Omalizumab in children with inadequately controlled severe allergic (IgE-mediated) asthma. Curr. Med. Res. Opin. 2010, 26, 1285–1293. [Google Scholar] [CrossRef]
- Lee, J.K.; Amin, S.; Erdmann, M.; Kukaswadia, A.; Ivanovic, J.; Fischer, A.; Gendron, A. Real-World Observational Study on the Characteristics and Treatment Patterns of Allergic Asthma Patients Receiving Omalizumab in Canada. Patient Prefer. Adherence 2020, 14, 725–735. [Google Scholar] [CrossRef]
- Ducharme, F.M.; Ni Chroinin, M.; Greenstone, I.; Lasserson, T.J. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst. Rev. 2010, 14, CD005533. [Google Scholar] [CrossRef] [PubMed]
- Asthma group of Chinese Throacic Society. Guidelines for bronchial asthma prevent and management (2020 edition). Zhonghua Jie He He Hu Xi Za Zhi 2020, 43, 1023–1048. [Google Scholar] [CrossRef]
- Zhou, X.; Ding, F.M.; Lin, J.T.; Yin, K.S. Validity of asthma control test for asthma control assessment in Chinese primary care settings. Chest 2009, 135, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Schatz, M.; Kosinski, M.; Yarlas, A.S.; Hanlon, J.; Watson, M.E.; Jhingran, P. The minimally important difference of the Asthma Control Test. J. Allergy Clin. Immunol. 2009, 124, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, M.; White, J.; Manfrin, A. Novel pharmacist-led intervention secures the minimally important difference (MID) in Asthma Control Test (ACT) score: Better outcomes for patients and the healthcare provider. BMJ Open Respir. Res. 2018, 5, e000322. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, M.; Choudhry, S.; Tsai, H.J.; Thyne, S.; Navarro, D.; Nazario, S.; Rodriguez-Santana, J.R.; Casal, J.; Torres, A.; Chapela, R.; et al. Association between IgE levels and asthma severity among African American, Mexican, and Puerto Rican patients with asthma. J. Allergy Clin. Immunol. 2007, 120, 137–143. [Google Scholar] [CrossRef]
- Davila, I.; Valero, A.; Entrenas, L.M.; Valveny, N.; Herráez, L. Relationship between serum total IgE and disease severity in patients with allergic asthma in Spain. J. Investig. Allergol. Clin. Immunol. 2015, 25, 120–127. [Google Scholar]
- Korn, S.; Haasler, I.; Fliedner, F.; Becher, G.; Strohner, P.; Staatz, A.; Taube, C.; Buhl, R. Monitoring free serum IgE in severe asthma patients treated with omalizumab. Respir. Med. 2012, 106, 1494–1500. [Google Scholar] [CrossRef]
- Tajiri, T.; Matsumoto, H.; Gon, Y.; Ito, R.; Hashimoto, S.; Izuhara, K.; Suzukawa, M.; Ohta, K.; Ono, J.; Ohta, S.; et al. Utility of serum periostin and free IgE levels in evaluating responsiveness to omalizumab in patients with severe asthma. Allergy 2016, 71, 1472–1479. [Google Scholar] [CrossRef]
- Li, B.; Huang, M.; Huang, S.; Zeng, X.; Yuan, Y.; Peng, X.; Zhao, W.; Ye, Y.; Yu, C.; Liu, L.; et al. Prediction of clinical response to omalizumab in moderate-to-severe asthma patients using the change in total serum IgE level. J. Thorac. Dis. 2020, 12, 7097–7105. [Google Scholar] [CrossRef]
- Louis, R.; Pilette, C.; Michel, O.; Michils, A.; Brusselle, G.; Poskin, A.; Van Schoor, J.; Denhaerynck, K.; Vancayzeele, S.; Abraham, I.; et al. Variability in total serum IgE over 1 year in severe asthmatics. Allergy Asthma Clin. Immunol. 2019, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, R.G. Accuracy of US Food and Drug Administration-cleared IgE antibody assays in the presence of anti-IgE (omalizumab). J. Allergy Clin. Immunol. 2006, 117, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Semprini, R.; Shortt, N.; Ebmeier, S.; Semprini, A.; Varughese, R.; Holweg, C.T.J.; Matthews, J.G.; Fingleton, J.; Weatherall, M.; Beasley, R.; et al. Change in biomarkers of type-2 inflammation following severe exacerbations of asthma. Thorax 2019, 74, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Matucci, A.; Vultaggio, A.; Maggi, E.; Kasujee, I. Is IgE or eosinophils the key player in allergic asthma pathogenesis? Are we asking the right question? Respir. Res. 2018, 19, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Simpson, J.L.; Powell, H.; Yang, I.A.; Upham, J.W.; Reynolds, P.N.; Hodge, S.; James, A.L.; Jenkins, C.; Peters, M.J.; et al. Full blood count parameters for the detection of asthma inflammatory phenotypes. Clin. Exp. Allergy 2014, 44, 1137–1145. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, H.; Lv, Y.; Li, R.; Dong, H.; Liu, L.; Xia, Y.; Hou, C.; Cai, S.; Zou, F. Moderate accuracy of peripheral eosinophil count for predicting eosinophilic phenotype in steroid-naïve non-atopic adult asthmatics. Intern. Med. 2012, 51, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Hanania, N.A.; Wenzel, S.; Rosén, K.; Hsieh, H.J.; Mosesova, S.; Choy, D.F.; Lal, P.; Arron, J.R.; Harris, J.M.; Busse, W. Exploring the effects of omalizumab in allergic asthma: An analysis of biomarkers in the EXTRA study. Am. J. Respir. Crit. Care Med. 2013, 187, 804–811. [Google Scholar] [CrossRef]
- Casale, T.B.; Chipps, B.E.; Rosén, K.; Trzaskoma, B.; Haselkorn, T.; Omachi, T.A.; Greenberg, S.; Hanania, N.A. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy 2018, 73, 490–497. [Google Scholar] [CrossRef]
- Hendeles, L.; Sorkness, C.A. Anti-immunoglobulin E therapy with omalizumab for asthma. Ann. Pharmacother. 2007, 41, 1397–1410. [Google Scholar] [CrossRef]
- Wright, J.D.; Chu, H.M.; Huang, C.H.; Ma, C.; Chang, T.W.; Lim, C. Structural and Physical Basis for Anti-IgE Therapy. Sci. Rep. 2015, 5, 11581. [Google Scholar] [CrossRef]
- Pillai, P.; Chan, Y.C.; Wu, S.Y.; Ohm-Laursen, L.; Thomas, C.; Durham, S.R.; Menzies-Gow, A.; Rajakulasingam, R.K.; Ying, S.; Gould, H.J.; et al. Omalizumab reduces bronchial mucosal IgE and improves lung function in non-atopic asthma. Eur. Respir. J. 2016, 48, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Su, N.; Liu, G.; Yin, K.; Zhou, X.; Shen, H.; Chen, P.; Chen, R.; Liu, C.; Wu, C.; et al. The impact of concomitant allergic rhinitis on asthma control: A cross-sectional nationwide survey in China. J. Asthma 2014, 51, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Guerra, S.; Sherrill, D.L.; Baldacci, S.; Carrozzi, L.; Pistelli, F.; Di Pede, F.; Viegi, G. Rhinitis is an independent risk factor for developing cough apart from colds among adults. Allergy 2005, 60, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Ziyab, A.H. Prevalence and Risk Factors of Asthma, Rhinitis, and Eczema and Their Multimorbidity among Young Adults in Kuwait: A Cross-Sectional Study. Biomed. Res. Int. 2017, 2017, 2184193. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Chen, Y.; Pang, Y.; Wang, X.; Dai, D.; Zhuang, Y.; Shi, H.; Zheng, M.; Zhang, R.; Jin, W.; et al. Prevalence and risk factors of allergic rhinitis and asthma in the southern edge of the plateau grassland region of northern China: A cross-sectional study. World Allergy Organ. J. 2021, 14, 100537. [Google Scholar] [CrossRef] [PubMed]
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef]
- Vignola, A.M.; Humbert, M.; Bousquet, J.; Boulet, L.P.; Hedgecock, S.; Blogg, M.; Fox, H.; Surrey, K. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy 2004, 59, 709–717. [Google Scholar] [CrossRef]
- Casale, T.B.; Luskin, A.T.; Busse, W.; Zeiger, R.S.; Trzaskoma, B.; Yang, M.; Griffin, N.M.; Chipps, B.E. Omalizumab Effectiveness by Biomarker Status in Patients with Asthma: Evidence From PROSPERO, A Prospective Real-World Study. J. Allergy Clin. Immunol. Pract. 2019, 7, 156–164.e1. [Google Scholar] [CrossRef]
- Mirsadraee, M.; Dehghan, S.; Ghaffari, S.; Mirsadraee, N. Long-term effect of antifungal therapy for the treatment of severe resistant asthma: An active comparator clinical trial. Curr. Med. Mycol. 2019, 5, 1–7. [Google Scholar] [CrossRef]
- Arrobas, A.; Barbosa, M.P.; Rabiais, S.; Vandewalle, B.; Félix, J. Cost-effectiveness of omalizumab in real world uncontrolled allergic asthma patients. Pulmonology 2021, 27, 124–133. [Google Scholar] [CrossRef]
- Adachi, M.; Kozawa, M.; Yoshisue, H.; Lee Milligan, K.; Nagasaki, M.; Sasajima, T.; Miyamoto, T.; Ohta, K. Real-world safety and efficacy of omalizumab in patients with severe allergic asthma: A long-term post-marketing study in Japan. Respir. Med. 2018, 141, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Taillé, C.; Mala, L.; Le Gros, V.; Just, J.; Molimard, M. Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: The STELLAIR study. Eur. Respir. J. 2018, 51, 1702523. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Sumi, K.; Yoshisue, H.; Nakamura, N.; Nagasaki, M.; Sasajima, T.; Matsumoto, H. Real-life safety and efficacy of omalizumab in Japanese patients with severe allergic asthma who were subjected to dosing table revision or expansion: A post-marketing surveillance. Pulm. Pharmacol. Ther. 2020, 64, 101950. [Google Scholar] [CrossRef] [PubMed]
- Riccio, A.M.; Dal Negro, R.W.; Micheletto, C.; De Ferrari, L.; Folli, C.; Chiappori, A.; Canonica, G.W. Omalizumab modulates bronchial reticular basement membrane thickness and eosinophil infiltration in severe persistent allergic asthma patients. Int. J. Immunopathol. Pharmacol. 2012, 25, 475–484. [Google Scholar] [CrossRef]
- Hanania, N.A.; Alpan, O.; Hamilos, D.L.; Condemi, J.J.; Reyes-Rivera, I.; Zhu, J.; Rosen, K.E.; Eisner, M.D.; Wong, D.A.; Busse, W. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: A randomized trial. Ann. Intern. Med. 2011, 154, 573–582. [Google Scholar] [CrossRef]
- Geng, B.; Dixon, A.; Ko, J.; Janampally, P.; Haselkorn, T.; Holweg, C.; Casale, T.; Jarjour, N. Impact of body mass index on omalizumab response in adults with moderate-to-severe allergic asthma. Ann Allergy Asthma Immunol. 2022, 128, 553–560. [Google Scholar] [CrossRef]
| Baseline IgE (IU/mL) | Weight (kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 21−25 | 26−30 | 31−40 | 41−50 | 51−60 | 61−70 | 71−80 | 81−90 | 91−125 | 126−150 | |
| 31−100 | 75 | 75 | 75 | 150 | 150 | 150 | 150 | 150 | 300 | 300 |
| 101−200 | 150 | 150 | 150 | 300 | 300 | 300 | 300 | 300 | 450 | 600 |
| 201−300 | 150 | 150 | 225 | 300 | 300 | 450 | 450 | 450 | 600 | 375 |
| 301−400 | 225 | 225 | 300 | 450 | 450 | 450 | 600 | 600 | 450 | 525 |
| 401−500 | 225 | 300 | 450 | 450 | 600 | 600 | 375 | 375 | 525 | 600 |
| 501−600 | 300 | 300 | 450 | 600 | 600 | 375 | 450 | 450 | 600 | |
| 601−700 | 300 | 225 | 450 | 600 | 375 | 450 | 450 | 525 | ||
| 701−800 | 225 | 225 | 300 | 375 | 450 | 450 | 525 | 600 | ||
| 801−900 | 225 | 225 | 300 | 375 | 450 | 525 | 600 | |||
| 901−1000 | 225 | 300 | 375 | 450 | 525 | 600 | ||||
| 1001−1100 | 225 | 300 | 375 | 450 | 600 | |||||
| 1101−1200 | 300 | 300 | 450 | 525 | 600 | DO NOT DOSE | ||||
| 1201−1300 | 300 | 375 | 450 | 525 | ||||||
| 1301−1500 | 300 | 375 | 525 | 600 | ||||||
 Subcutaneous doses to be administered every 4 weeks;
Subcutaneous doses to be administered every 4 weeks;  Subcutaneous doses to be administered every 2 weeks;
Subcutaneous doses to be administered every 2 weeks;  Insufficient data to recommend a dose.
Insufficient data to recommend a dose.| Characteristic | Total (n = 32) | Non-Response (n = 10) | Response (n = 22) | P 3 |
|---|---|---|---|---|
| Gender | 0.636 | |||
| Male | 13 (40.63%) | 4 (40.00%) | 9 (40.90%) | |
| Female | 19 (59.37%) | 6 (60.00%) | 13 (59.10%) | |
| Age (years) | 53.38 ± 13.61 | 57.90 ± 9.83 | 51.32 ± 14.77 | 0.205 |
| Weight | 62.11 ± 9.55 | 62.50 ± 9.62 | 61.94 ± 9.74 | 0.877 |
| BMI 2 | ||||
| Total | 23.44 ± 2.91 | 23.73 ± 2.55 | 23.31 ± 3.11 | 0.705 |
| Above normal | 26.24 ± 1.61 | 26.52 ± 0.70 | 26.11 ± 1.91 | 0.670 |
| Course (years) | 4.00 (2.00–10.00) | 4.50 (2.75–12.50) | 3.00 (2.00–11.00) | 0.580 |
| Comorbidities | ||||
| RS | 2 (6.25%) | 1 (10.00%) | 1 (4.55%) | |
| ACO | 6 (18.75%) | 5 (50.00%) | 1 (4.55%) | |
| GERD | 1 (3.13%) | 1 (10.00%) | 0 (0.00%) | |
| Allergy history | ||||
| Food | 3 (9.38%) | 0 (0.00%) | 3 (13.64%) | |
| Inhalation | 6 (18.75%) | 1 (10.00%) | 5 (22.73%) | |
| Drug | 7 (21.87%) | 3 (30.00%) | 4 (18.18%) | |
| Skin | 3 (9.38%) | 1 (10.00%) | 2 (9.09%) | |
| Taking OCS | 11 (34.38%) | 5 (50.00%) | 6 (27.27%) | 0.077 |
| OCS dose | 0.00 (0.00–13.75) | 17.50 (0.00–22.50) | 0.00 (0.00–2.50) | 0.024 |
| Total serum IgE | 503.80 (315.68–956.00) | 410.03 ± 304.63 | 630.25 (374.30–1035.50) | 0.016 |
| EOS | 0.43 (0.10–0.78) | 0.74 ± 0.47 | 0.39 (0.08–0.49) | 0.064 |
| EOS% | 5.20 (1.63–8.40) | 4.05 (1.73–14.18) | 5.20 (1.50–7.55) | 0.745 |
| FEV1 | 2.06 ± 0.63 | 1.88 ± 0.52 | 2.14 ± 0.67 | 0.285 |
| PEF | 4.56 ± 0.90 | 4.42 ± 0.92 | 4.62 ± 0.91 | 0.562 |
| ACT scores | 16.38 ± 1.91 | 15.80 ± 1.87 | 16.64 ± 1.92 | 0.258 |
| Exacerbations | 0.00 (0.00–1.00) | 2.00 (1.00–2.50) | 0.00 (0.00–1.00) | <0.001 |
| Before | After | Z 2 or t 3 | P | |
|---|---|---|---|---|
| Taking OCS | 11/32 (34.38%) | 7/32 (21.88%) | 12.250 | <0.001 |
| OCS dose | 0.00 (0.00−13.75) | 0.00 (0.00−20.00) | −2.986 | 0.003 |
| Total serum IgE | 503.80 (315.68−956.00) | 299.75 (168.13–571.68) | −3.871 | <0.001 |
| EOS | 0.43 (0.10−0.78) | 0.18 (0.07–0.76) | −2.974 | 0.003 |
| EOS% | 5.20 (1.63–8.40) | 2.10 (1.55−4.80) | −2.488 | 0.013 |
| FEV1 | 2.06 ± 0.63 | 2.17 ± 0.69 | −3.712 | 0.001 |
| PEF | 4.56 ± 0.90 | 4.44 ± 0.99 | 0.855 | 0.399 |
| ACT scores | 16.38 ± 0.34 | 20.44 ± 0.42 | −11.315 | <0.001 |
| Exacerbations | 0.00 (0.00−1.00) | 0.00 (0.00−1.00) | −3.051 | 0.002 |
| Non-Response (n = 10) | Response (n = 22) | |||||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Z 2 or t 3 | P | Before | After | Z 2 or t 3 | P | |
| Taking OCS | 6/10 (60.00%) | 5/10 (50.00%) | 0.200 | 0.655 | 5/22 (22.73%) | 2/22 (9.09%) | 20.455 | <0.001 |
| OCS dose | 17.50 (0.00−22.50) | 5.00 (0.00−14.38) | −2.260 | 0.026 | 0.00 (0.00−2.50) | 0.00 (0.00−0.00) | −2.060 | 0.039 |
| Total serum IgE | 410.03 ± 304.63 | 465.28 ± 301.78 | −1.165 | 0.274 | 630.25 (374.30−1035.50) | 272.10 (149.90–469.65) | −4.107 | <0.001 |
| EOS | 0.74 ± 0.47 | 0.77 ± 0.57 | −0.169 | 0.869 | 0.39 (0.08−0.49) | 0.13 (0.05−0.29) | −3.247 | 0.001 |
| EOS% | 4.05 (1.73−14.18) | 3.15 (1.88−12.35) | −0.714 | 0.475 | 5.20 (1.50−7.55) | 1.85 (1.38−3.08) | −2.420 | 0.016 |
| Exacerbations | 2.00 (1.00−2.50) | 1.00 (1.00−1.25) | −2.121 | 0.034 | 1.00 (1.00−1.25) | 0.00 (0.00−0.00) | −2.236 | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Cao, L.; Zhang, M.; Fei, C.; Deng, J. Response to Omalizumab as an Add-On Therapy in the Treatment of Allergic Asthma in Adult Chinese Patients—A Retrospective Study. Vaccines 2022, 10, 2068. https://doi.org/10.3390/vaccines10122068
Li N, Cao L, Zhang M, Fei C, Deng J. Response to Omalizumab as an Add-On Therapy in the Treatment of Allergic Asthma in Adult Chinese Patients—A Retrospective Study. Vaccines. 2022; 10(12):2068. https://doi.org/10.3390/vaccines10122068
Chicago/Turabian StyleLi, Na, Linfeng Cao, Ming Zhang, Chunyuan Fei, and Jingjing Deng. 2022. "Response to Omalizumab as an Add-On Therapy in the Treatment of Allergic Asthma in Adult Chinese Patients—A Retrospective Study" Vaccines 10, no. 12: 2068. https://doi.org/10.3390/vaccines10122068
APA StyleLi, N., Cao, L., Zhang, M., Fei, C., & Deng, J. (2022). Response to Omalizumab as an Add-On Therapy in the Treatment of Allergic Asthma in Adult Chinese Patients—A Retrospective Study. Vaccines, 10(12), 2068. https://doi.org/10.3390/vaccines10122068






