COVID-19 Epidemiology, Immunity, and Vaccine Development in Children: A Review
Abstract
1. Introduction
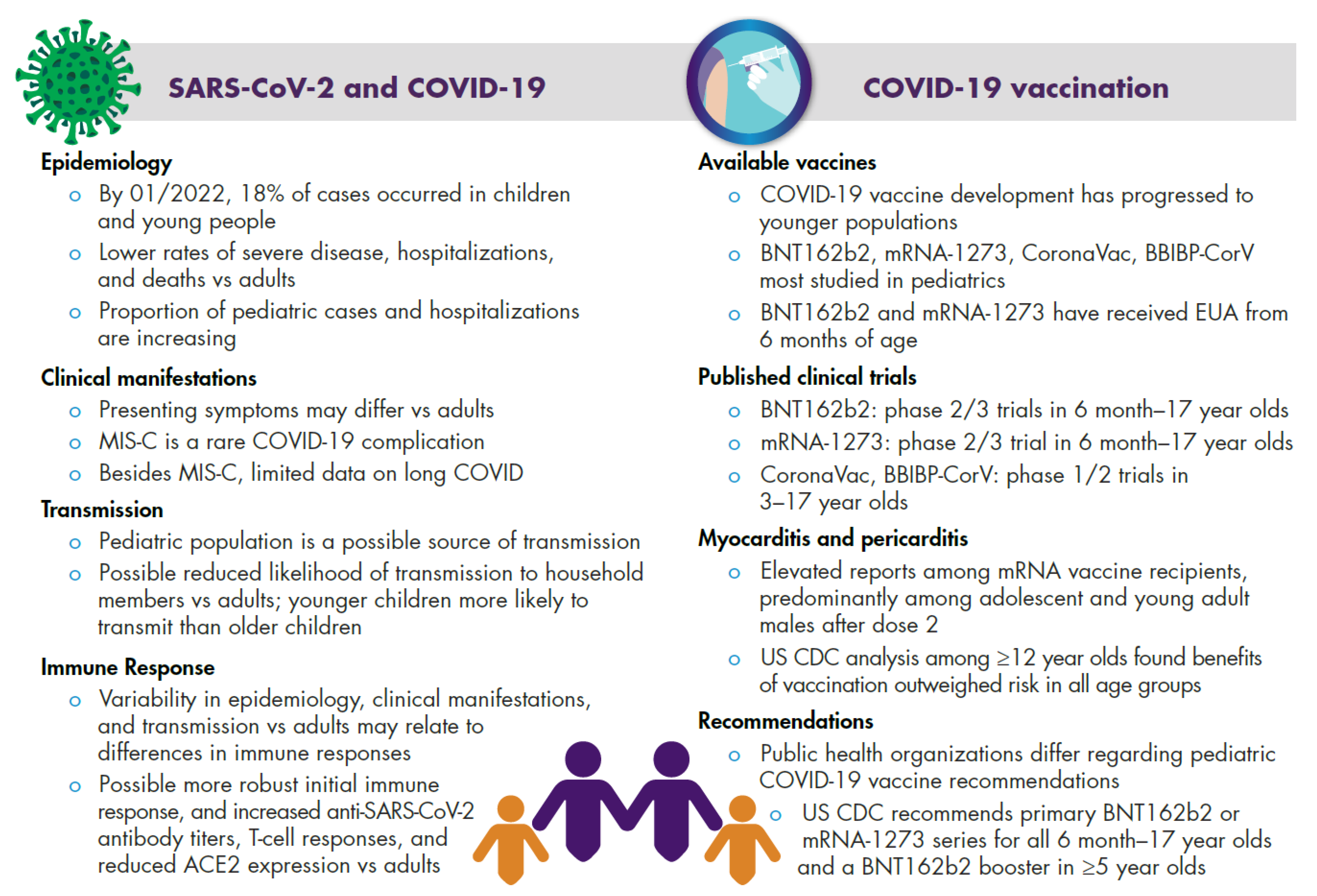
2. COVID-19 in Children and Adolescents
2.1. Epidemiology
2.2. Clinical Manifestations
2.3. Transmission
2.4. Immune Response
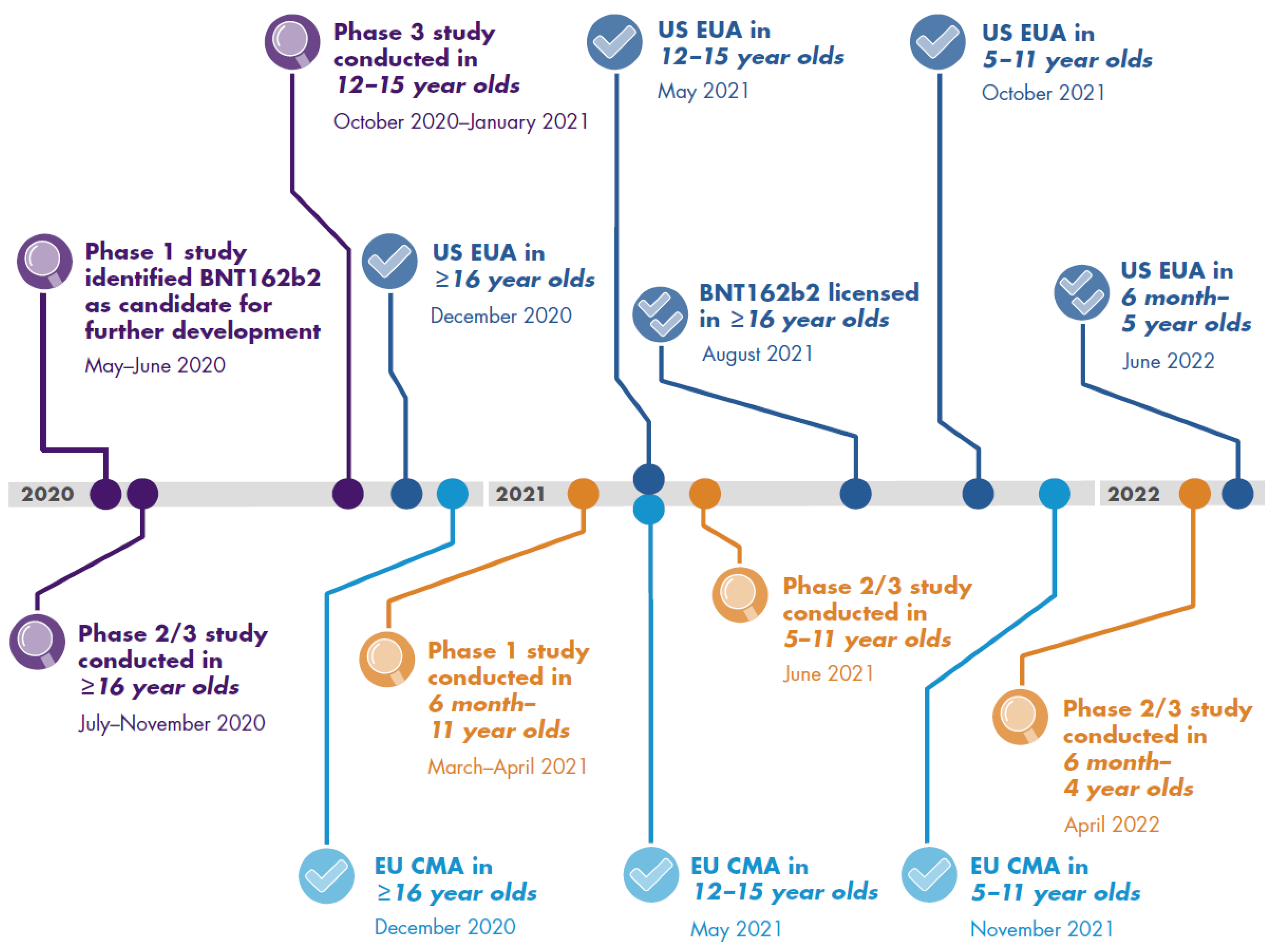
3. Pediatric COVID-19 Vaccine Development
3.1. Pediatric Clinical Trials
3.1.1. Clinical Trial Data on Immunogenicity and Vaccine Efficacy
3.1.2. Clinical Trial Safety Data
3.2. Real-World Evidence
3.2.1. Real-World Vaccine Effectiveness
3.2.2. Real-World Safety
3.2.3. Myocarditis and Pericarditis
3.3. Vaccination Recommendations
3.4. Parental Vaccine Hesitancy
| CDC [83] | JCVI [84,85,86,87,95] | WHO [14] | |||||
|---|---|---|---|---|---|---|---|
| Children 6 Months–4 Years Old | Children 5–11 Years Old | Adolescents 12–17 Years Old | Children 5–11 Years Old | Adolescents 12–17 Years Old | Children 5–11 Years Old | Adolescents 12–17 Years Old | |
| Primary vaccination | Recommended for all individuals Dosing: three 3 µg BNT162b2 doses (≥3 weeks between doses 1 and 2 and ≥8 weeks between doses 2 and 3); two 25 µg mRNA-1273 doses (≥4–8 weeks between doses) | Recommended for all individuals Dosing: two 10 µg BNT162b2 doses ≥3–8 weeks apart for those 5–11 years old; two 25 µg mRNA-1273 doses ≥4 weeks apart for those 5 years old; and two 50 µg mRNA-1273 doses ≥4–8 weeks apart for those 6–11 years old | Recommended for all individuals Dosing: two 30 µg BNT162b2 doses ≥3–8 weeks apart; two 100 µg mRNA-1273 doses ≥4–8 weeks apart | Should be offered to those in a clinical risk group or household contacts of immunosuppressed individuals Dosing: two 10 µg BNT162b2 doses 8 weeks apart | Should be offered to all individuals Dosing: two 30 µg BNT162b2 doses ≥8 (at-risk individuals) or ≥12 weeks (healthy individuals) apart | Countries should consider their specific epidemiologic and social context when considering vaccination of children and adolescents, with priority given to vaccination of adults and at-risk groups | |
| Additional dose | No recommendations given | Recommended for moderately and severely immunocompromised individuals Timing: ≥28 days after second dose | Not recommended | Should be offered to those who were severely immunosuppressed at the time of their first or second dose Timing: generally ≥8 weeks after second dose | No recommendations given | No recommendations given | |
| Booster dose | Not recommended | Recommended (for BNT162b2 only) Timing: ≥5 months after last primary dose | Recommended (for BNT162b2 only) Timing: ≥5 months after last primary dose | Not recommended | 12–15 years of age: should be offered to those in a clinical risk group, household contact of immunosuppressed individuals, or severely immunocompromised and received a third dose 16–17 years of age: should be offered to all individuals Timing: ≥3 months after last primary dose | No recommendations given | No recommendations given |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 23 November 2022).
- World Health Organization. COVID-19 Disease in Children and Adolescents. Scientific Brief. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Children_and_adolescents-2021.1 (accessed on 31 May 2021).
- Rider, E.A.; Ansari, E.; Varrin, P.H.; Sparrow, J. Mental health and wellbeing of children and adolescents during the COVID-19 pandemic. BMJ 2021, 374, n1730. [Google Scholar] [CrossRef]
- US Centers for Disease Control and Prevention. Health Department-Reported Cases of Multisystem Inflammatory Syndrome in Children (MIS-C) in the United States. Available online: https://www.cdc.gov/mis-c/cases/index.html (accessed on 15 August 2022).
- US Centers for Disease Control and Prevention. Information for Healthcare Providers about Multisystem Inflammatory Syndrome in Children (MIS-C). Available online: https://www.cdc.gov/mis/mis-c/hcp/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fmis%2Fhcp%2Findex.html (accessed on 30 December 2021).
- Buonsenso, D.; Munblit, D.; De Rose, C.; Sinatti, D.; Ricchiuto, A.; Carfi, A.; Valentini, P. Preliminary evidence on long COVID in children. Acta Paediatr. 2021, 110, 2208–2211. [Google Scholar] [CrossRef]
- Stephenson, T.; Shafran, R.; De Stavola, B.; Rojas, N.; Aiano, F.; Amin-Chowdhury, Z.; McOwat, K.; Simmons, R.; Zavala, M.; CLoCk Consortium; et al. Long COVID and the mental and physical health of children and young people: National matched cohort study protocol (the CLoCk study). BMJ Open 2021, 11, e052838. [Google Scholar] [CrossRef] [PubMed]
- US Centers for Disease Control and Prevention. COVID-NET COVID-19-associated hospitalization surveillance network. Available online: https://gis.cdc.gov/grasp/COVIDNet/COVID19_3.html (accessed on 15 August 2021).
- US Centers for Disease Control and Prevention. COVID-19 Weekly Cases and Deaths per 100,000 Population by Age, Race/Ethnicity, and Sex. Available online: https://covid.cdc.gov/covid-data-tracker/#demographicsovertime (accessed on 15 August 2022).
- UK Health and Security Agency. SARS-CoV-2 Variants of Concern and Variants Under Investigation in England: Technical Briefing 34. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050236/technical-briefing-34-14-january-2022.pdf (accessed on 11 February 2022).
- UNICEF. COVID-19 Confirmed Cases and Deaths. Available online: https://data.unicef.org/resources/covid-19-confirmed-cases-and-deaths-dashboard/ (accessed on 15 August 2021).
- Our World in Data. Coronavirus (COVID-19) Vaccinations: Vaccinations by Age. Available online: https://ourworldindata.org/covid-vaccinations?country=~OWID_WRL#vaccinations-by-age (accessed on 8 February 2022).
- US Centers for Disease Control and Prevention. Demographic Trends of People Receiving COVID-19 Vaccinations in the United States. Available online: https://covid.cdc.gov/covid-data-tracker/#vaccination-demographics-trends (accessed on 15 August 2022).
- World Health Organization. Interim Statement on COVID-19 Vaccination for Children and Adolescents. Available online: https://www.who.int/news/item/24-11-2021-interim-statement-on-covid-19-vaccination-for-children-and-adolescents (accessed on 5 January 2021).
- Hodcroft, E.B. CoVariants: SARS-CoV-2 Mutations and Variants of Interest. Available online: https://covariants.org/ (accessed on 7 January 2022).
- Assaker, R.; Colas, A.E.; Julien-Marsollier, F.; Bruneau, B.; Marsac, L.; Greff, B.; Tri, N.; Fait, C.; Brasher, C.; Dahmani, S. Presenting symptoms of COVID-19 in children: A meta-analysis of published studies. Br. J. Anaesth. 2020, 125, e330–e332. [Google Scholar] [CrossRef]
- Liguoro, I.; Pilotto, C.; Bonanni, M.; Ferrari, M.E.; Pusiol, A.; Nocerino, A.; Vidal, E.; Cogo, P. SARS-CoV-2 infection in children and newborns: A systematic review. Eur. J. Pediatr. 2020, 179, 1029–1046. [Google Scholar] [CrossRef]
- Woodruff, R.C.; Campbell, A.P.; Taylor, C.A.; Chai, S.J.; Kawasaki, B.; Meek, J.; Anderson, E.J.; Weigel, A.; Monroe, M.L.; Reeg, L.; et al. Risk Factors for Severe COVID-19 in Children. Pediatrics 2022, 149, e2021053418. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E.; Wallace, M.; Moulia, D.L.; Twentyman, E.; Roper, L.E.; Hall, E.; Link-Gelles, R.; Godfrey, M.; Woodworth, K.R.; Anderson, T.C.; et al. Interim Recommendations of the Advisory Committee on Immunization Practices for Use of Moderna and Pfizer-BioNTech COVID-19 Vaccines in Children Aged 6 Months-5 Years-United States, June 2022. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 859–868. [Google Scholar] [CrossRef]
- Fleming-Dutra, K.E. COVID-19 Epidemiology in Children Ages 6 Months–4 Years. In Proceedings of the Advisory Committee on Immunization Practices Meeting, CDC, Atlanta, GA, USA, 17–18 June 2022. [Google Scholar]
- Oliveira, E.A.; Colosimo, E.A.; Simões, S.A.C.; Mak, R.H.; Martelli, D.B.; Silva, L.R.; Martelli-Júnior, H.; Oliveira, M.C.L. Clinical characteristics and risk factors for death among hospitalised children and adolescents with COVID-19 in Brazil: An analysis of a nationwide database. Lancet Child Adolesc. Health 2021, 5, 559–568. [Google Scholar] [CrossRef]
- Viner, R.M.; Ward, J.L.; Hudson, L.D.; Ashe, M.; Patel, S.V.; Hargreaves, D.; Whittaker, E. Systematic review of reviews of symptoms and signs of COVID-19 in children and adolescents. Arch. Dis. Child. 2020, 106, 802–807. [Google Scholar] [CrossRef]
- Wald, E.R.; Schmit, K.M.; Gusland, D.Y. A Pediatric Infectious Disease Perspective on COVID-19. Clin. Infect. Dis. 2021, 72, 1660–1666. [Google Scholar] [CrossRef]
- Tunҫ, E.M.; Shin, C.K.J.; Usoro, E.; Thomas-Smith, S.E.; Trehan, I.; Migita, R.T.; Keilman, A.E. Croup during the Coronavirus Disease 2019 Omicron Variant Surge. J. Pediatr. 2022, 247, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, F.T.; Gutierrez-Sacristan, A.; Keller, M.S.; Liu, M.; Hong, C.; Bonzel, C.L.; Tan, A.L.M.; Aronow, B.J.; Boeker, M.; Booth, J.; et al. International Analysis of Electronic Health Records of Children and Youth Hospitalized with COVID-19 Infection in 6 Countries. JAMA Netw. Open 2021, 4, e2112596. [Google Scholar] [CrossRef]
- Barda, N.; Dagan, N.; Ben-Shlomo, Y.; Kepten, E.; Waxman, J.; Ohana, R.; Hernan, M.A.; Lipsitch, M.; Kohane, I.; Netzer, D.; et al. Safety of the BNT162b2 mRNA COVID-19 vaccine in a nationwide setting. N. Engl. J. Med. 2021, 385, 1078–1090. [Google Scholar] [CrossRef]
- Godfred-Cato, S.; Bryant, B.; Leung, J.; Oster, M.E.; Conklin, L.; Abrams, J.; Roguski, K.; Wallace, B.; Prezzato, E.; Koumans, E.H.; et al. COVID-19-associated multisystem inflammatory syndrome in children-United States, March-July 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 1074–1080. [Google Scholar] [CrossRef]
- US Centers for Disease Control and Prevention. ACIP Presentation Slides: November 2–3, 2021 Meeting. Available online: https://www.cdc.gov/vaccines/acip/meetings/slides-2021-11-2-3.html (accessed on 23 November 2022).
- Bellon, M.; Baggio, S.; Bausch, F.J.; Spechbach, H.; Salamun, J.; Genecand, C.; Tardin, A.; Kaiser, L.; L’Huillier, A.G.; Eckerle, I. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral load kinetics in symptomatic children, adolescents, and adults. Clin. Infect. Dis. 2021, 73, e1384–e1386. [Google Scholar] [CrossRef] [PubMed]
- Bahar, B.; Jacquot, C.; Mo, Y.D.; De Biasi, R.L.; Campos, J.; Delaney, M. Kinetics of viral clearance and antibody production across age groups in children with severe acute respiratory syndrome coronavirus 2 infection. J. Pediatr. 2020, 227, 31–37. [Google Scholar] [CrossRef]
- Wolfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Muller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- L’Huillier, A.G.; Torriani, G.; Pigny, F.; Kaiser, L.; Eckerle, I. Culture-Competent SARS-CoV-2 in Nasopharynx of Symptomatic Neonates, Children, and Adolescents. Emerg. Infect. Dis. 2020, 26, 2494–2497. [Google Scholar] [CrossRef]
- Zhu, Y.; Bloxham, C.J.; Hulme, K.D.; Sinclair, J.E.; Tong, Z.W.M.; Steele, L.E.; Noye, E.C.; Lu, J.; Xia, Y.; Chew, K.Y.; et al. A Meta-analysis on the Role of Children in Severe Acute Respiratory Syndrome Coronavirus 2 in Household Transmission Clusters. Clin. Infect. Dis. 2021, 72, e1146–e1153. [Google Scholar] [CrossRef]
- Goldstein, E.; Lipsitch, M.; Cevik, M. On the effect of age on the transmission of SARS-CoV-2 in households, schools, and the community. J. Infect. Dis. 2021, 223, 362–369. [Google Scholar] [CrossRef]
- US Centers for Disease Control and Prevention Science Brief: Transmission of SARS-CoV-2 in K-12 Schools and Early Care and Education Programs–Updated. Available online: https://www.cdc.gov/coronavirus/2019-ncov/science/science-briefs/transmission_k_12_schools.html (accessed on 31 May 2021).
- Paul, L.A.; Daneman, N.; Schwartz, K.L.; Science, M.; Brown, K.A.; Whelan, M.; Chan, E.; Buchan, S.A. Association of Age and Pediatric Household Transmission of SARS-CoV-2 Infection. JAMA Pediatr. 2021, 175, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Kabeerdoss, J.; Pilania, R.K.; Karkhele, R.; Kumar, T.S.; Danda, D.; Singh, S. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: Immunological mechanisms, clinical manifestations and management. Rheumatol. Int. 2021, 41, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, L.R.; Rose, E.B.; Horwitz, S.M.; Collins, J.P.; Newhams, M.M.; Son, M.B.F.; Newburger, J.W.; Kleinman, L.C.; Heidemann, S.M.; Martin, A.A.; et al. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N. Engl. J. Med. 2020, 383, 334–346. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Curtis, N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch. Dis. Child. 2020, 106, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.A.; Sy, S.; Galen, B.; Goldstein, D.Y.; Orner, E.; Keller, M.J.; Herold, K.C.; Herold, B.C. Natural mucosal barriers and COVID-19 in children. JCI Insight 2021, 6, e148694. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; Connors, T.J.; Zhu, Y.; Baldwin, M.R.; Lin, W.H.; Wontakal, S.; Szabo, P.A.; Wells, S.B.; Dogra, P.; Gray, J.; et al. Distinct antibody responses to SARS-CoV-2 in children and adults across the COVID-19 clinical spectrum. Nat. Immunol. 2021, 22, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Costa, V.; Racine-Brzostek, S.E.; Acker, K.P.; Yee, J.; Chen, Z.; Karbaschi, M.; Zuk, R.; Rand, S.; Sukhu, A.; et al. Association of Age With SARS-CoV-2 Antibody Response. JAMA Netw. Open 2021, 4, e214302. [Google Scholar] [CrossRef]
- Ng, K.W.; Faulkner, N.; Cornish, G.H.; Rosa, A.; Harvey, R.; Hussain, S.; Ulferts, R.; Earl, C.; Wrobel, A.G.; Benton, D.J.; et al. Preexisting and de novo humoral immunity to SARS-CoV-2 in humans. Science 2020, 370, 1339–1343. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 15 August 2022).
- UNICEF. COVID-19 Vaccine Market Dashboard. Available online: https://www.unicef.org/supply/covid-19-vaccine-market-dashboard (accessed on 15 August 2022).
- Frenck, R.W., Jr.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef]
- Walter, E.B.; Talaat, K.R.; Sabharwal, C.; Gurtman, A.; Lockhart, S.; Paulsen, G.C.; Barnett, E.D.; Munoz, F.M.; Maldonado, Y.; Pahud, B.A.; et al. Evaluation of the BNT162b2 COVID-19 Vaccine in Children 5 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 35–46. [Google Scholar] [CrossRef]
- Ali, K.; Berman, G.; Zhou, H.; Deng, W.; Faughnan, V.; Coronado-Voges, M.; Ding, B.; Dooley, J.; Girard, B.; Hillebrand, W.; et al. Evaluation of mRNA-1273 SARS-CoV-2 vaccine in adolescents. N. Engl. J. Med. 2021, 385, 2241–2251. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Song, Y.; Li, C.; Yang, W.; Ma, Q.; Jiang, Z.; Li, M.; Lian, X.; Jiao, W.; Wang, L.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: A double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021, 21, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: A randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect. Dis. 2022, 22, 196–208. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. EMA Recommends COVID-19 Vaccine Moderna for Authorisation in the EU. Available online: https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-moderna-authorisation-eu (accessed on 7 January 2021).
- European Medicines Agency. First COVID-19 Vaccine Approved for Children Aged 12 to 15 in EU. Available online: https://www.ema.europa.eu/en/news/first-covid-19-vaccine-approved-children-aged-12-15-eu (accessed on 23 November 2022).
- European Medicines Agency. Comirnaty COVID-19 Vaccine: EMA Recommends Approval for Children Aged 5 to 11. Available online: https://www.ema.europa.eu/en/news/comirnaty-covid-19-vaccine-ema-recommends-approval-children-aged-5-11 (accessed on 23 November 2022).
- US Food and Drug Administration. Letter of Authorization-Pfizer-BioNTech COVID-19 Vaccine. Available online: https://www.fda.gov/media/150386/download (accessed on 31 May 2022).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration Emergency Use Authorization of the Pfizer-BioNTech COVID-19 Vaccine to Prevent Coronavirus Disease 2019 (COVID-19). Available online: https://www.fda.gov/media/159312/download (accessed on 15 August 2022).
- European Medicines Agency. EMA Recommends First COVID-19 Vaccine for Authorisation in the EU. Available online: https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu (accessed on 23 November 2022).
- Full Prescribing Information; Spikevax (mRNA-1273); Moderna US Inc.: Cambridge, MA, USA, 2022.
- Summary of Product Characteristics; Spikevax (mRNA-1273); Moderna Biotech Spain, S.L.: Madrid, Spain, 2022.
- US Food and Drug Administration. Fact Sheet for Healthcare Providers Administering Vaccine (Vaccination Providers). Emergency Use Authorization (EUA) of the Moderna COVID-19 Vaccine to Prevent Coronavirus Disesase 2019 (COVID-19). Available online: https://www.fda.gov/media/159307/download (accessed on 15 August 2022).
- World Health Organization. WHO Validates Sinovac COVID-19 Vaccine for Emergency Use and Issues Interim Policy Recommendations. Available online: https://www.who.int/news/item/01-06-2021-who-validates-sinovac-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations (accessed on 31 May 2021).
- World Health Organization. WHO Lists Additional COVID-19 Vaccine for Emergency Use and Issues Interim Policy Recommendations. Available online: https://www.who.int/news/item/07-05-2021-who-lists-additional-covid-19-vaccine-for-emergency-use-and-issues-interim-policy-recommendations (accessed on 31 May 2021).
- Creech, C.B.; Anderson, E.; Berthaud, V.; Yildirim, I.; Atz, A.M.; Baez, I.M.; Finkelstein, D.; Pickrell, P.; Kirstein, J.; Yut, C.; et al. Evaluation of mRNA-1273 COVID-19 Vaccine in Children 6 to 11 Years of Age. N. Engl. J. Med. 2022, 386, 2011–2023. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Pfizer Briefing Materials for June 14-15, 2022 Vaccines and Related Biological Products Advisory Committee; US Food and Drug Administration: Silver Spring, MD, USA, 2022.
- US Food & Drug Administration. Real-World Evidence: Real-World Data (RWD) and Real-World Evidence (RWE) Are Playing an Increasing Role in Health Care Decisions. Available online: https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence (accessed on 23 November 2022).
- Glatman-Freedman, A.; Hershkovitz, Y.; Kaufman, Z.; Dichtiar, R.; Keinan-Boker, L.; Bromberg, M. Effectiveness of BNT162b2 vaccine in adolescents during outbreak of SARS-CoV-2 Delta variant infection, Israel, 2021. Emerg. Infect. Dis. 2021, 27, 2919–2922. [Google Scholar] [CrossRef]
- Reis, B.Y.; Barda, N.; Leshchinsky, M.; Kepten, E.; Hernan, M.A.; Lipsitch, M.; Dagan, N.; Balicer, R.D. Effectiveness of BNT162b2 Vaccine against Delta Variant in Adolescents. N. Engl. J. Med. 2021, 385, 2101–2103. [Google Scholar] [CrossRef]
- Olson, S.M.; Newhams, M.M.; Halasa, N.B.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Irby, K.; Walker, T.C.; Schwartz, S.P.; Pannaraj, P.S.; et al. Effectiveness of Pfizer-BioNTech mRNA Vaccination Against COVID-19 Hospitalization among Persons Aged 12–18 Years-United States, June-September 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1483–1488. [Google Scholar] [CrossRef]
- Zambrano, L.D.; Newhams, M.M.; Olson, S.M.; Halasa, N.B.; Price, A.M.; Boom, J.A.; Sahni, L.C.; Kamidani, S.; Tarquinio, K.M.; Maddux, A.B.; et al. Effectiveness of BNT162b2 (Pfizer-BioNTech) mRNA Vaccination Against Multisystem Inflammatory Syndrome in Children among Persons Aged 12–18 Years-United States, July-December 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 52–58. [Google Scholar] [CrossRef]
- Powell, A.A.; Kirsebom, F.; Stowe, J.; McOwat, K.; Saliba, V.; Ramsay, M.E.; Lopez-Bernal, J.; Andrews, N.; Ladhani, S.N. Effectiveness of BNT162b2 against COVID-19 in adolescents. Lancet Infect. Dis. 2022, 22, 581–583. [Google Scholar] [CrossRef]
- Hause, A.M.; Gee, J.; Baggs, J.; Abara, W.E.; Marquez, P.; Thompson, D.; Su, J.R.; Licata, C.; Rosenblum, H.G.; Myers, T.R.; et al. COVID-19 vaccine safety in adolescents aged 12–17 years-United States, December 14, 2020–July 16, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1053–1058. [Google Scholar] [CrossRef]
- Hause, A.M.; Baggs, J.; Marquez, P.; Myers, T.R.; Gee, J.; Su, J.R.; Zhang, B.; Thompson, D.; Shimabukuro, T.T.; Shay, D.K. COVID-19 vaccine safety in children aged 5–11 years-United States, November 3–December 19, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Wallace, M.; Hadler, S.C.; Langley, G.; Su, J.R.; Oster, M.E.; Broder, K.R.; Gee, J.; Weintraub, E.; Shimabukuro, T.; et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: Update from the Advisory Committee on Immunization Practices-United States, June 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, H. Pfizer-BioNTech COVID-19 Vaccine and Myocarditis in Individuals Aged 16–29 Years: Benefits-Risk Discussion: Advisory Committee on Immunization Practices (ACIP): August 30, 2021. Available online: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-08-30/06-COVID-Rosenblum-508.pdf (accessed on 23 November 2022).
- Boehmer, T.K.; Kompaniyets, L.; Lavery, A.M.; Hsu, J.; Ko, J.Y.; Yusuf, H.; Romano, S.D.; Gundlapalli, A.V.; Oster, M.E.; Harris, A.M. Association between COVID-19 and myocarditis using hospital-based administrative data-United States, March 2020–January 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1228–1232. [Google Scholar] [CrossRef] [PubMed]
- US Department of Health and Human Services. Guide to Interpreting VAERS Data. Available online: https://vaers.hhs.gov/data/dataguide.html (accessed on 23 November 2022).
- Mevorach, D.; Anis, E.; Cedar, N.; Hasin, T.; Bromberg, M.; Goldberg, L.; Parnasa, E.; Dichtiar, R.; Hershkovitz, Y.; Ash, N.; et al. Myocarditis after BNT162b2 Vaccination in Israeli Adolescents. N. Engl. J. Med. 2022, 386, 998–999. [Google Scholar] [CrossRef] [PubMed]
- Patone, M.; Mei, X.W.; Handunnetthi, L.; Dixon, S.; Zaccardi, F.; Shankar-Hari, M.; Watkinson, P.; Khunti, K.; Harnden, A.; Coupland, C.A.C.; et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat. Med. 2022, 28, 410–422. [Google Scholar] [CrossRef]
- Truong, D.T.; Dionne, A.; Muniz, J.C.; McHugh, K.E.; Portman, M.A.; Lambert, L.M.; Thacker, D.; Elias, M.D.; Li, J.S.; Toro-Salazar, O.H.; et al. Clinically Suspected Myocarditis Temporally Related to COVID-19 Vaccination in Adolescents and Young Adults: Suspected Myocarditis after COVID-19 Vaccination. Circulation 2022, 145, 345–356. [Google Scholar] [CrossRef]
- Oster, M.E.; Shay, D.K.; Su, J.R.; Gee, J.; Creech, C.B.; Broder, K.R.; Edwards, K.; Soslow, J.H.; Dendy, J.M.; Schlaudecker, E.; et al. Myocarditis Cases Reported after mRNA-Based COVID-19 Vaccination in the US from December 2020 to August 2021. JAMA 2022, 327, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Kelleman, M.; West, Z.; Peter, A.; Dove, M.; Butto, A.; Oster, M.E. Comparison of Multisystem Inflammatory Syndrome in Children-Related Myocarditis, Classic Viral Myocarditis, and COVID-19 Vaccine-Related Myocarditis in Children. J. Am. Heart Assoc. 2022, 11, e024393. [Google Scholar] [CrossRef] [PubMed]
- US Centers for Disease Control and Prevention. Interim COVID-19 Immunization Schedule for 6 Months of Age and Older. Available online: https://www.cdc.gov/vaccines/covid-19/downloads/COVID-19-immunization-schedule-ages-6months-older.pdf (accessed on 15 August 2022).
- UK Health Security Agency. Press Release: JCVI Advice on COVID-19 Booster Vaccines for Those Aged 18 to 39 and a Second Dose for Ages 12 to 15. Available online: https://www.gov.uk/government/news/jcvi-advice-on-covid-19-booster-vaccines-for-those-aged-18-to-39-and-a-second-dose-for-ages-12-to-15 (accessed on 4 January 2021).
- UK Department of Health and Social Care. Independent Report: Joint Committee on Vaccination and Immunisation (JCVI) Advice on COVID-19 Vaccination in People Aged 16 to 17 Years: 15 November 2021. Available online: https://www.gov.uk/government/publications/covid-19-vaccination-in-children-and-young-people-aged-16-to-17-years-jcvi-statement-november-2021/joint-committee-on-vaccination-and-immunisation-jcvi-advice-on-covid-19-vaccination-in-people-aged-16-to-17-years-15-november-2021 (accessed on 4 January 2021).
- UK Health Security Agency. Press Release: JCVI Issues New Vaccination Advice for Children and Young People. Available online: https://www.gov.uk/government/news/jcvi-issues-new-vaccination-advice-for-children-and-young-people (accessed on 4 January 2021).
- Public Health England. Press Release: JCVI Issues Advice on Third Dose Vaccination for Severely Immunosuppressed. Available online: https://www.gov.uk/government/news/jcvi-issues-advice-on-third-dose-vaccination-for-severely-immunosuppressed (accessed on 4 January 2021).
- Fazel, M.; Puntis, S.; White, S.R.; Townsend, A.; Mansfield, K.L.; Viner, R.; Herring, J.; Pollard, A.J.; Freeman, D. Willingness of children and adolescents to have a COVID-19 vaccination: Results of a large whole schools survey in England. EClinicalMedicine 2021, 40, 101144. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.B.; Bell, R.A. Parental COVID-19 Vaccine Hesitancy in the United States. Public Health Rep. 2022, 137, 1162–1169. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Proma, T.S.; Tasnim, Z.; Islam, M.A.; Urmi, T.A.; Ahmed, S.; Sarkar, A.S.; Bonna, A.S.; Khan, U.S. Parental COVID-19 vaccine hesitancy for children with neurodevelopmental disorders: A cross-sectional survey. Trop. Med. Health 2022, 50, 24. [Google Scholar] [CrossRef] [PubMed]
- Shati, A.A.; Al-Qahtani, S.M.; Alsabaani, A.A.; Mahmood, S.E.; Alqahtani, Y.A.; AlQahtani, K.M.; Aldarami, M.S.; AlAmri, F.D.; Alqahtani, A.S.; AlHadi, A.M.; et al. Perceptions of Parents towards COVID-19 Vaccination in Children, Aseer Region, Southwestern Saudi Arabia. Vaccines 2022, 10, 1222. [Google Scholar] [CrossRef] [PubMed]
- Temsah, M.H.; Alhuzaimi, A.N.; Aljamaan, F.; Bahkali, F.; Al-Eyadhy, A.; Alrabiaah, A.; Alhaboob, A.; Bashiri, F.A.; Alshaer, A.; Temsah, O.; et al. Parental attitudes and hesitancy about COVID-19 vs. routine childhood vaccinations: A national survey. Front. Public Health 2021, 9, 752323. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wen, W.; Chen, C.; Tang, J.; Wang, C.; Zhou, M.; Cheng, Y.; Zhang, X.; Wang, M.; Feng, Z.; et al. Explore the attitudes of children and adolescent parents towa3s the vaccination of COVID-19 in China. Ital. J. Pediatr. 2022, 48, 122. [Google Scholar] [CrossRef] [PubMed]
- Massie, J.; Paxton, G.A.; Crawford, N.; Danchin, M.H. Vaccination of young people from 12 years of age for COVID-19 against parents’ wishes. Med. J. Aust. 2022, 216, 455–457. [Google Scholar] [CrossRef]
- UK Health Security Agency. COVID-19 vaccination: A Guide for Eligible Children and Young People Aged 12 to 17 (Version 3). Available online: https://www.gov.uk/government/publications/covid-19-vaccination-resources-for-children-and-young-people/covid-19-vaccination-a-guide-for-eligible-children-and-young-people-aged-12-to-17 (accessed on 25 March 2022).
| Vaccine (Type) | Characteristic | Details |
|---|---|---|
| Infants, children, and adolescents | ||
| CoronaVac (inactivated vaccine) [49] | ClinicalTrials.gov identifier | NCT04551547 |
| Phase (design) | 1/2 (randomized, controlled) | |
| Age group | 3–17 years | |
| Dose (schedule) | 1.5 or 3.0 µg (2 doses 28 days apart) | |
| Number of participants | 72 (phase 1) and 480 (phase 2) | |
| Immunogenicity | Humoral responses were induced, with neutralizing antibody titers induced by the 3.0 µg dose higher than those of the 1.5 µg dose (the data support the 3.0 µg dose for further development in this age group) | |
| Safety | The most common reactions were injection site pain and fever; most were mild or moderate and transient | |
| CoronaVac (inactivated vaccine) | ClinicalTrials.gov identifier | NCT04992260 |
| Phase (design) | 3 (randomized, placebo-controlled) | |
| Age group | 6 months–17 years | |
| Number of participants | ~14,000 | |
| Endpoints | Efficacy of RT-PCR–confirmed symptomatic COVID-19 (primary), safety, immunogenicity | |
| Primary completion date | May 2022 (estimated) | |
| BBIBP-CorV (inactivated vaccine) [50] | Clinical trial identifier | ChiCTR2000032459 |
| Phase (design) | 1/2 (randomized, controlled) | |
| Age group | 3–17 years | |
| Dose (schedule) | 2, 4, or 8 µg (3 doses on days 0, 28, and 56) | |
| Number of participants | 288 (phase 1) and 720 (phase 2) | |
| Immunogenicity | All doses elicited robust humoral responses; the 4 µg dose on a 2-dose regimen (21 days apart) will be further studied in this age group | |
| Safety | The most common reactions were injection site pain and fever; most were mild or moderate | |
| Adolescents | ||
| BNT162b2 (mRNA vaccine) [46] | ClinicalTrials.gov identifier | NCT04368728 |
| Phase (design) | 3 (randomized, placebo-controlled) | |
| Age group | 12–15 years | |
| Dose (schedule) | 30 µg (2 doses 21 days apart) | |
| Number of participants | 2260 | |
| Follow-up | At the time of the report, 58% of participants had ≥2 months of follow-up after dose 2 | |
| Vaccine efficacy | Observed vaccine efficacy of 100% (95% CI: 75.3, 100) | |
| Immunogenicity | Immune response was noninferior to that observed in 16–25-year-olds | |
| Safety | Injection site pain was the most common local reaction, and headache and fatigue were the most common systemic events; these events were mostly mild to moderate in severity and transient | |
| mRNA-1273 (mRNA vaccine) [48] | ClinicalTrials.gov identifier | NCT04649151 |
| Phase (design) | 2/3 (randomized, placebo-controlled) | |
| Age group | 12–17 years | |
| Dose (schedule) | 100 µg (2 doses 28 days apart) | |
| Number of participants | 3726 | |
| Follow-up | 83 days | |
| Vaccine efficacy | Using CDC definition of COVID-19 with onset of 14 days after dose 2: 93% (95% CI: 47.9, 99.9) In the per-protocol population with an onset of 14 days after dose 2: 56% (95% CI: 16.8, 76.4) | |
| Immunogenicity | Immune response was noninferior to that observed in 18–25-year-olds | |
| Safety | Injection site pain was the most common local reaction, and fatigue and headache were the most frequently reported systemic events; these were most commonly grade 1/2 and transient | |
| Children | ||
| BNT162b2 (mRNA vaccine) [47] | ClinicalTrials.gov identifier | NCT04816643 |
| Phase (design) | 2/3 (randomized, placebo-controlled) | |
| Age group | 5–11 years | |
| Dose (schedule) | 10 µg (2 doses 21 days apart) | |
| Number of participants | 2268 | |
| Follow-up | Median of 2.3 months (range 0–2.5 months) | |
| Vaccine efficacy | Observed vaccine efficacy of 90.7% (95% CI: 67.7, 98.3) | |
| Immunogenicity | Immune response was noninferior to that observed in 16–25-year-olds | |
| Safety | Injection-site pain was the most common local reaction, and fatigue and headache were the most frequently reported systemic events; these events were mostly mild to moderate in severity and transient | |
| mRNA-1273 (mRNA vaccine) [64] | ClinicalTrials.gov identifier | NCT04796896 |
| Phase (design) | 2/3 (randomized, placebo-controlled) | |
| Age group | 6–11 years | |
| Dose (schedule) | 50 µg (2 doses 28 days apart) | |
| Number of participants | 4016 | |
| Follow-up | Median 82 days after dose 2 | |
| Vaccine efficacy | Using CDC definition of COVID-19 with onset of 14 days after dose 2: 88% (95% CI: 70.0, 95.8) | |
| Immunogenicity | Immune response was noninferior to that observed in 18–25-year-olds who received 2 doses at the 100 µg dose level | |
| Safety | The most common local adverse reaction was injection site pain, and the most common systemic adverse reactions were headache and fatigue | |
| Infants | ||
| BNT162b2 (mRNA vaccine) [57] | ClinicalTrials.gov identifier | NCT04816643 |
| Phase (design) | 2/3 (randomized, placebo-controlled) | |
| Age group | 6 months–4 years | |
| Dose (schedule) | Dose 1 and 2 administered 3 weeks apart; dose 3 administered ≥8 weeks after dose 2 | |
| Number of participants | 1776 (6–23 months of age); 2750 (2–4 years of age) | |
| Follow-up | Median 1.3–1.4 months after dose 3 | |
| Vaccine efficacy | N/A | |
| Immunogenicity | Immune response after 3 doses was noninferior to that observed after 2 doses in 16–25-year-olds | |
| Safety | In participants 6–23 months of age, adverse reactions after any dose included irritability (68%), decreased appetite (39%), injection site tenderness (26%), injection site redness (18%), fever (14%), injection site swelling (7%), and lymphadenopathy (0.2%). In participants 2–4 years of age, these included injection site pain (47%), fatigue (45%), injection site redness (19%), fever (11%), headache (9%), injection site swelling (8%), chills (6%), muscle pain (5%), joint pain (2%), and lymphadenopathy (0.1%). | |
| mRNA-1273 (mRNA vaccine) [61] | ClinicalTrials.gov identifier | NCT04796896 |
| Phase (design) | 2/3 (randomized, placebo-controlled) | |
| Age group | 6 months–5 years | |
| Dose (schedule) | 25 µg (2 doses 1 month apart) | |
| Number of participants | 6388 | |
| Follow-up | Median of 68–71 days after dose 2 | |
| Immunogenicity | Immune response was noninferior to that observed in 18–25-year-olds who received 2 doses at the 100 µg dose level | |
| Safety | The most common adverse reactions in those 6–23 months of age were irritability/crying (64–68%), pain (37–46%), sleepiness (35–37%), and loss of appetite (30–32%). In those 24–36 months of age, these were pain (53–68%), irritability/crying (54–55%), and sleepiness (24–31%). In those 37 months–5 years of age, these were pain (65–73%) and fatigue (40–48%). | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fergie, J.; Moran, M.M.; Cane, A.; Pather, S.; Türeci, Ӧ.; Srivastava, A. COVID-19 Epidemiology, Immunity, and Vaccine Development in Children: A Review. Vaccines 2022, 10, 2039. https://doi.org/10.3390/vaccines10122039
Fergie J, Moran MM, Cane A, Pather S, Türeci Ӧ, Srivastava A. COVID-19 Epidemiology, Immunity, and Vaccine Development in Children: A Review. Vaccines. 2022; 10(12):2039. https://doi.org/10.3390/vaccines10122039
Chicago/Turabian StyleFergie, Jaime, Mary M. Moran, Alejandro Cane, Shanti Pather, Ӧzlem Türeci, and Amit Srivastava. 2022. "COVID-19 Epidemiology, Immunity, and Vaccine Development in Children: A Review" Vaccines 10, no. 12: 2039. https://doi.org/10.3390/vaccines10122039
APA StyleFergie, J., Moran, M. M., Cane, A., Pather, S., Türeci, Ӧ., & Srivastava, A. (2022). COVID-19 Epidemiology, Immunity, and Vaccine Development in Children: A Review. Vaccines, 10(12), 2039. https://doi.org/10.3390/vaccines10122039






