SARS-CoV-2 Specific Humoral Immune Responses after BNT162b2 Vaccination in Hospital Healthcare Workers
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Antibody Detection
2.3. Statistical Analysis
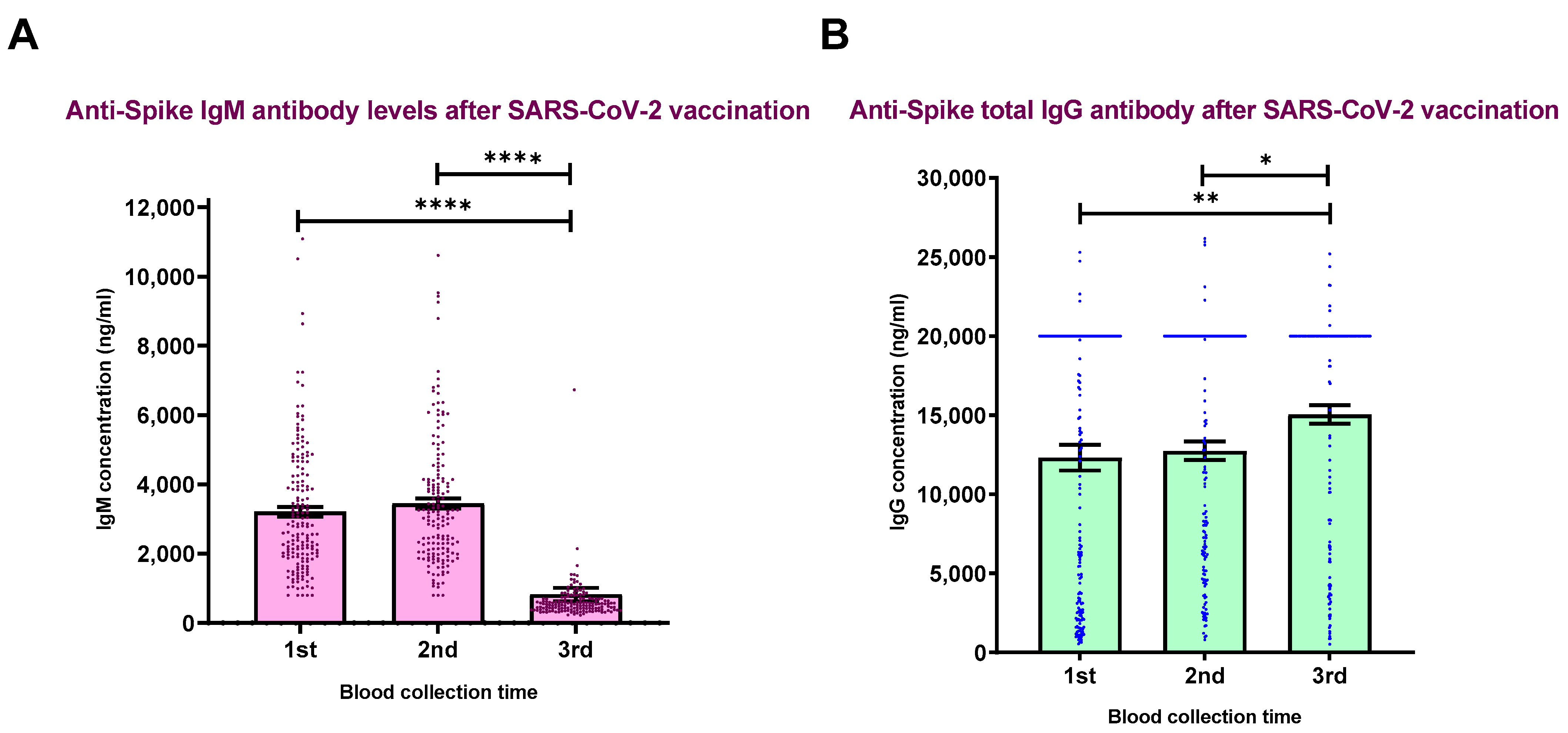
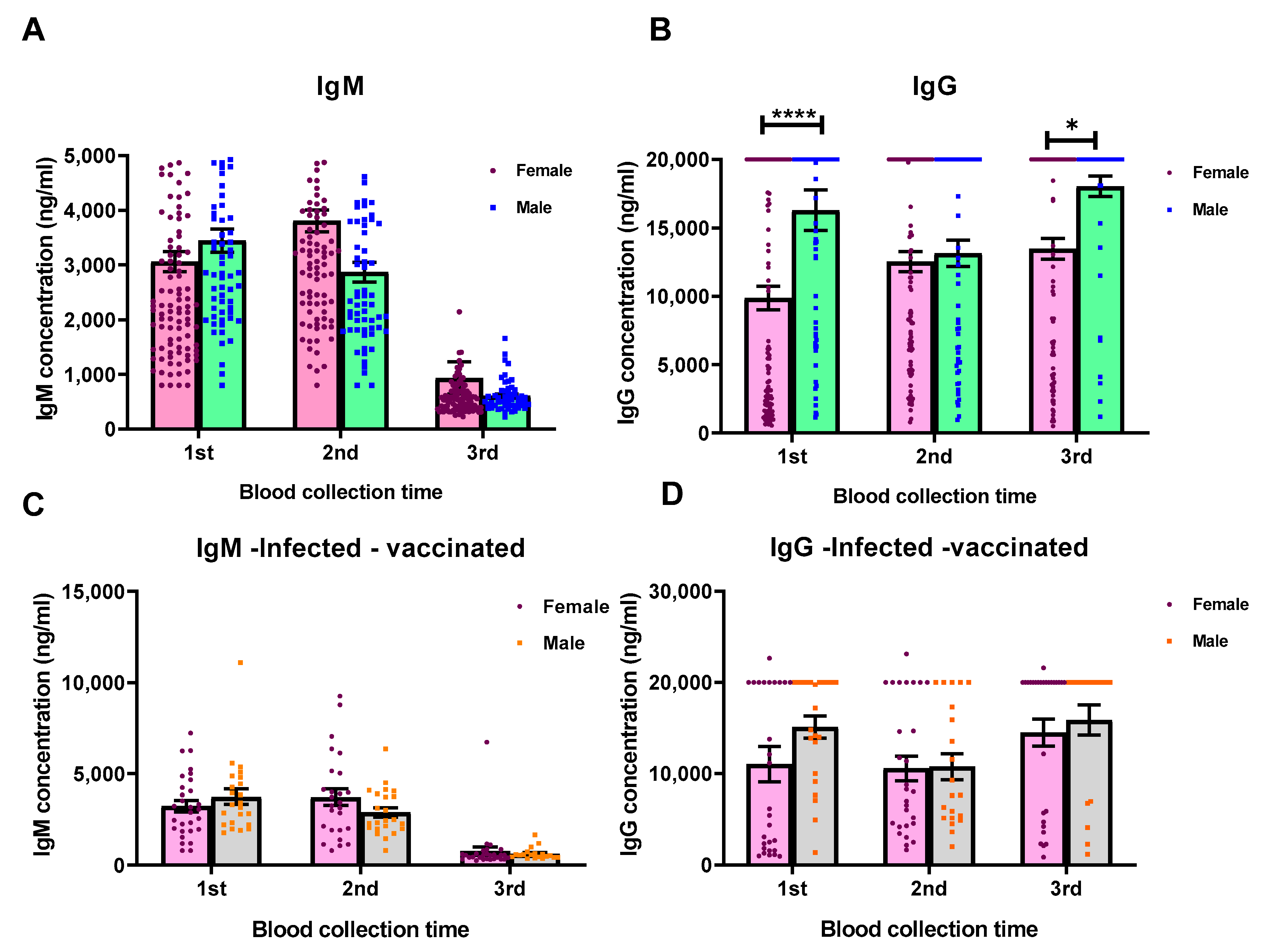
3. Results
3.1. Participant Characteristics
3.2. Humoral Immune Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pascarella, G.; Strumia, A.; Piliego, C.; Bruno, F.; Del Buono, R.; Costa, F.; Scarlata, S.; Agrò, F.E. COVID-19 diagnosis and management: A comprehensive review. J. Intern. Med. 2020, 288, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Prado, E.; Simbana-Rivera, K.; Gomez-Barreno, L.; Rubio-Neira, M.; Guaman, L.P.; Kyriakidis, N.C.; Muslin, C.; Jaramillo, A.M.G.; Barba-Ostria, C.; Cevallos-Robalino, D. Clinical molecular, epidemiological characterization of the SARS-CoV-2 virus the Coronavirus Disease 2019 (COVID-19) a comprehensive literature review. Diagn. Microbiol. Infect. Dis. 2020, 98, 115094. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Jakhmola, S.; Indari, O.; Jha, H.C.; Chen, Z.S.; Tripathi, V.; Pérez de la Lastra, J. Potential Therapeutic Targets and Vaccine Development for SARS-CoV-2/COVID-19 Pandemic Management: A Review on the Recent Update. Front. Immunol. 2021, 12, 658519. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, H.K. Delivery Routes for COVID-19 Vaccines. Vaccines 2021, 9, 524. [Google Scholar] [CrossRef] [PubMed]
- Triggle, C.R.; Bansal, D.; Ding, H.; Islam, M.M.; Farag, E.; Hadi, H.A.; Sultan, A.A. A Comprehensive Review of Viral Characteristics, Transmission, Pathophysiology, Immune Response, and Management of SARS-CoV-2 and COVID-19 as a Basis for Controlling the Pandemic. Front. Immunol. 2021, 12, 631139. [Google Scholar] [CrossRef] [PubMed]
- Boechat, J.L.; Chora, I.; Morais, A.; Delgado, L. The immune response to SARS-CoV-2 and COVID-19 immunopathology–Current perspectives. Pulmonology 2021, 27, 423–437. [Google Scholar] [CrossRef]
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- De Leon-Rodriguez, S.G.; Hernandez-Rico, B.; Olmo-Vazquez, G.D.; Cruz-Davalos, I.; Bonifaz, L.C. SARS-CoV-2: Previous coronaviruses, immune response, and development of vaccines. Bol. Med. Hosp. Infant. Mex. 2020, 77, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus Worldometers. 2022. Available online: https://www.worldometers.info/coronavirus/ (accessed on 7 October 2020).
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Covidportal. Information about Available Vaccines: Ministry of Health 2022. Available online: https://covid.gov.cz/en/situations/information-about-vaccine/information-about-available-vaccines (accessed on 20 October 2022).
- Ema Ema. COVID-19 Vaccines: Authorised 2021. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/public-health-threats/coronavirus-disease-covid-19/treatments-vaccines/vaccines-covid-19/covid-19-vaccines-authorised#authorised-covid-19-vaccines-section (accessed on 20 October 2022).
- Bloomfield, M.; Pospisilova, I.; Cabelova, T.; Sediva, A.; Ibrahimova, M.; Borecka, K. Searching for COVID-19 Antibodies in Czech Children-A Needle in the Haystack. Front Pediatr. 2020, 8, 597736. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus Worldometer Czech Republic. 2022. Available online: https://www.worldometers.info/coronavirus/country/czech-republic/ (accessed on 20 October 2022).
- Institute of Health Information and Statistics of the Czech Republic IoBaA. COVID-19: Overview of the current situation in the Czech Republic: Ministry of Health of Czech Republic; 2022. Available online: https://onemocneni-aktualne.mzcr.cz/covid-19 (accessed on 20 October 2022).
- Covidportal. Vaccination Timeline: Ministry of Health; 2022. Available online: https://covid.gov.cz/en/situations/register-vaccination/vaccination-timeline (accessed on 20 October 2022).
- Expats.cz. A comprehensive guide to vaccination in the Czech Republic: Updated 14 September 2021. Available online: https://www.expats.cz/czech-news/article/vaccination-in-the-czech-republic-all-you-need-to-know (accessed on 20 October 2022).
- Kageyama, T.; Ikeda, K.; Tanaka, S.; Taniguchi, T.; Igari, H.; Onouchi, Y.; Kaneda, A.; Matsushita, K.; Hanaoka, H.; Nakada, T.-A.; et al. Antibody responses to BNT162b2 mRNA COVID-19 vaccine and their predictors among healthcare workers in a tertiary referral hospital in Japan. Clin. Microbiol. Infect. 2021, 27, 1861. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R.; Farber, D.L. COVID-19 vaccines: Modes of immune activation and future challenges. Nat. Rev. Immunol. 2021, 21, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Azzi, L.; Dalla Gasperina, D.; Veronesi, G.; Shallak, M.; Ietto, G.; Iovino, D.; Baj, A.; Gianfagna, F.; Maurino, V.; Focosi, D.; et al. Mucosal immune response in BNT162b2 COVID-19 vaccine recipients. EBioMedicine 2021, 75, 103788. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.S.; O’Halloran, J.A.; Kalaidina, E.; Kim, W.; Schmitz, A.J.; Zhou, J.Q. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021, 596, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Iguacel, I.; Maldonado, A.L.; Ruiz-Cabello, A.L.; Casaus, M.; Moreno, L.A.; Martinez-Jarreta, B. Association between COVID-19 Vaccine Side Effects and Body Mass Index in Spain. Vaccines 2021, 9, 1321. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Klaser, K.; May, A.; Polidori, L.; Capdevila, J.; Louca, P. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID Symptom Study app in the UK: A prospective observational study. Lancet Infect. Dis. 2021, 21, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Martin, S.W.; Rose, C.E., Jr.; Biagini, R.E.; Franzke, L.H.; Smith, J.P.; Bs, D.L.S.; Robertson, S.A.; McNeil, M.M. Evaluation of body mass index, pre-vaccination serum progesterone levels and anti-anthrax protective antigen immunoglobulin G on injection site adverse events following anthrax vaccination in women. Pharmacoepidemiol. Drug Saf. 2008, 17, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Pondo, T.; Rose, C.E., Jr.; Martin, S.W.; Keitel, W.A.; Keyserling, H.L.; Babcock, J.; Parker, S.; Jacobson, R.M.; Poland, G.A.; McNeil, M.M. Evaluation of sex, race, body mass index and pre-vaccination serum progesterone levels and post-vaccination serum anti-anthrax protective immunoglobulin G on injection site adverse events following anthrax vaccine adsorbed (AVA) in the CDC AVA human clinical trial. Vaccine 2014, 32, 3548–3554. [Google Scholar] [PubMed]
- Regula, P.; Rosenstreich, D.; Jerschow, E.; Ramesh, M.; Ferastraoaru, D.; Oh, J. Safety and efficacy of graded dosing of Pfizer-BioNTech mRNA COVID-19 vaccine after an immediate hypersensitivity reaction to first dose. J. Allergy Clin. Immunol. Glob. 2022, 1, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Shavit, R.; Maoz-Segal, R.; Offengenden, I.; Yahia, S.H.; Maayan, D.M.; Lifshitz, Y.; Niznik, S.; Deutch, M.; Elbaz, E.; Genaim, H.; et al. Assessment of Immediate Allergic Reactions After Immunization with the Pfizer BNT162b2 Vaccine Using Intradermal Skin Testing With the COVID-19 Vaccines. J. Allergy Clin. Immunol. Pract. 2022, 10, 2677–2684. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Abrams, E.M.; Golden, D.B.K.; Blumenthal, K.G.; Wolfson, A.R.; Stone, C.A., Jr. Risk of Second Allergic Reaction to SARS-CoV-2 Vaccines: A Systematic Review and Meta-analysis. JAMA Intern Med. 2022, 182, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.; Farzan, S.; Coscia, G.; Rosenthal, D.W.; McInerney, A.; Jongco, A.M. Allergic reactions to coronavirus disease 2019 vaccines and addressing vaccine hesitancy: Northwell Health experience. Ann. Allergy Asthma Immunol. 2022, 128, 161–168.e1. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.M.; Snow, T.T.; Lee, A.S.; Shah, M.M.; Heider, A.; Blomkalns, A.; Betts, B.; Buzzanco, A.S.; Gonzalez, J.; Chinthrajah, R.S.; et al. Assessment of Allergic and Anaphylactic Reactions to mRNA COVID-19 Vaccines with Confirmatory Testing in a US Regional Health System. JAMA Netw Open. 2021, 4, e2125524. [Google Scholar] [CrossRef] [PubMed]
- Troelnikov, A.; Perkins, G.; Yuson, C.; Ahamdie, A.; Balouch, S.; Hurtado, P.R.; Hissaria, P. Basophil reactivity to BNT162b2 is mediated by PEGylated lipid nanoparticles in patients with PEG allergy. J. Allergy Clin. Immunol. 2021, 148, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Risma, K.A. COVID-19 mRNA vaccine allergy. Curr. Opin. Pediatr. 2021, 33, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Turk, V.E. Anaphylaxis associated with the mRNA COVID-19 vaccines: Approach to allergy investigation. Clin. Immunol. 2021, 227, 108748. [Google Scholar] [CrossRef]
- Herman, S.M.; Lui, E.; Kim, H. The hidden allergen: Triton X-100, a derivative of polyethylene glycol. J. Allergy Clin. Immunol. Pract. 2021, 9, 2941. [Google Scholar] [CrossRef] [PubMed]
- Busa, R.; Sorrentino, M.C.; Russelli, G.; Amico, G.; Miceli, V.; Miele, M. Specific Anti-SARS-CoV-2 Humoral and Cellular Immune Responses After Booster Dose of BNT162b2 Pfizer-BioNTech mRNA-Based Vaccine: Integrated Study of Adaptive Immune System Components. Front Immunol. 2022, 13, 856657. [Google Scholar] [CrossRef]
- Franzese, M.C.L.; Silva, R.; Santini, S.A.; Cinquanta, L.; Ottomano, C.; Salvatore, M.; Incoronato, M. SARS-CoV-2 antibody responses before and after a third dose of the BNT162b2 vaccine in Italian healthcare workers aged ≤60 years: One year of surveillance. Front Immunol. 2022, 13, 947187. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, B.; Chabrolles, H.; Archimbaud, C.; Brebion, A.; Cosme, J.; Dutheil, F.; Lambert, C.; Junda, M.; Mirand, A.; Ollier, A.; et al. Decline of Humoral and Cellular Immune Responses Against SARS-CoV-2 6 Months After Full BNT162b2 Vaccination in Hospital Healthcare Workers. Front Immunol. 2022, 13, 842912. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, J.S.; Fukunaga, A.; Yamamoto, S.; Tanaka, A.; Matsuda, K.; Kimura, M. SARS-CoV-2 specific T cell and humoral immune responses upon vaccination with BNT162b2: A 9 months longitudinal study. Sci. Rep. 2022, 12, 15447. [Google Scholar] [CrossRef] [PubMed]
- Morgiel, E.; Szmyrka, M.; Madej, M.; Sebastian, A.; Sokolik, R.; Andrasiak, I.; Chodyra, M.; Walas-Antoszek, M.; Korman, L.; Świerkot, J. Complete (Humoral and Cellular) Response to Vaccination against COVID-19 in a Group of Healthcare Workers-Assessment of Factors Affecting Immunogenicity. Vaccines 2022, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.; Andree, M.; Moskorz, W.; Drexler, I.; Walotka, L.; Grothmann, R.; Ptok, J.; Hillebrandt, J.; Ritchie, A.; Rabl, D.; et al. Age-dependent Immune Response to the Biontech/Pfizer BNT162b2 Coronavirus Disease 2019 Vaccination. Clin. Infect. Dis. 2021, 73, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, M.; Campillo, N.E.; Canelles, M. COVID-19 Vaccine Race: Analysis of Age-Dependent Immune Responses against SARS-CoV-2 Indicates that more than Just One Strategy May Be Needed. Curr. Med. Chem. 2021, 28, 3964–3979. [Google Scholar] [CrossRef] [PubMed]
- Collier, D.A.; Ferreira, I.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E. Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Anastassopoulou, C.; Antoni, D.; Manoussopoulos, Y.; Stefanou, P.; Argyropoulou, S.; Vrioni, G. Age and sex associations of SARS-CoV-2 antibody responses post BNT162b2 vaccination in healthcare workers: A mixed effects model across two vaccination periods. PLoS ONE 2022, 17, e0266958. [Google Scholar] [CrossRef]
- Vulpis, E.; Giulimondi, F.; Digiacomo, L.; Zingoni, A.; Safavi-Sohi, R.; Sharifi, S.; Caracciolo, G.; Mahmoudi, M. The Possible Role of Sex as an Important Factor in Development and Administration of Lipid Nanomedicine-Based COVID-19 Vaccine. Mol. Pharm. 2021, 18, 2448–2453. [Google Scholar] [CrossRef]
- Jensen, A.; Stromme, M.; Moyassari, S.; Chadha, A.S.; Tartaglia, M.C.; Szoeke, C.; Ferretti, M.T. COVID-19 vaccines: Considering sex differences in efficacy and safety. Contemp. Clin. Trials. 2022, 115, 106700. [Google Scholar] [CrossRef] [PubMed]
- Fischinger, S.; Boudreau, C.M.; Butler, A.L.; Streeck, H.; Alter, G. Sex differences in vaccine-induced humoral immunity. Semin. Immunopathol. 2019, 41, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Ciarambino, T.; Para, O.; Giordano, M. Immune system and COVID-19 by sex differences and age. Womens Health 2021, 17, 1–6. [Google Scholar] [CrossRef] [PubMed]
| Age Groups | Number (%) | 3rd BNT162b2 Vaccine Dose | Gender (n = 173) | COVID-19 Infection (N = 48) | BMI kg/m2 (N = 167) | Time of COVID-19 Infection Regarding the Vaccination (N = 48) | Regular Medication (n = 167) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptoms | ||||||||||||||||
| YES | No | Female | Male | Yes | No | 18.4–28.4 | 28.4–38.4 | 38.4–48.4 | 48.4–58.4 | Before 1st Dose | Between 1st and 2d Dose | Between 2d and 3d Dose | Yes | No | ||
| <21 | 7 (0.6) | 6 (5.8) | 1 (1.4) | 5 (4.6) | 2 (3) | - | - | 1 (0.6) | - | - | - | 1 (2.3) | - | 1 (1.1) | - | |
| 21–30 | 32 (19.2) | 13 (12.5) | 17 (25) | 21 (19.6) | 11 (16.7) | 8 (17.8) | 2 (66.7) | 25 (15) | 5 (11.6) | 2 (50) | 10 (23.3) | - | 1 (33.3) | 10 (11.5) | 22 (27.5) | |
| 31–40 | 30 (18) | 15 (14.4) | 14 (20.6) | 18 (16.8) | 12 (18.2) | 10 (22.2) | 1 (33.3) | 23 (14) | 5 (11.6) | 1 (25) | 1 (100) | 9 (20.9) | 2 (100) | 1 (33.3) | 12 (13.8) | 18 (22.5) |
| 41–50 | 40 (23.9) | 27 (25.9) | 13 (19.1) | 23 (21.5) | 17 (25.8) | 11 (24.4) | - | 29 (17.4) | 11 (25.6) | - | - | 11 (25.6) | - | 20 (22.9) | 20 (25) | |
| 51–60 | 30 (17.9) | 22 (21.2) | 9 (13.4) | 19 (17.8) | 11 (16.7) | 7 (15.5) | - | 19 (11.4) | 10 (23.3) | 1 (25) | - | 7 (16.3) | - | 14 (16.1) | 16 (20) | |
| 61–70 | 24 (14.4) | 16 (15.4) | 9 (13.4) | 15 (14) | 9 (13.6) | 5 (11.1) | - | 15 (9) | 9 (20.9) | - | - | 5 (11.6) | - | 1 (33.3) | 21 (24.1) | 3 (3.8) |
| >71 | 11 (5.9) | 5 (4.8) | 6 (8.8) | 6 (5.6) | 4 (6.1) | 1 (2.2) | - | 8 (4.8) | 2 (4.7) | - | - | 1 (2.3) | - | 9 (10.3) | 1 (1.25) | |
| Total | 173 | 104 (60.5) | 68 (39.5) | 107 (61.8) | 66 (38.2) | 45 (93.8) | 3 (6.3) | 120 (71.9) | 43 (25.7) | 4 (2.4) | 1 (0.6) | 43 (89.6) | 2 (4.2) | 3 (6.3) | 87 (52.1) | 80 (47.9) |
| Symptom | No. * | Percentage ** |
|---|---|---|
| Fatigue | 36 | 87.8 |
| Anosmia | 28 | 68.3 |
| Headache | 28 | 68.3 |
| Fever | 23 | 56.1 |
| Anorexia | 22 | 53.7 |
| Myalgia | 18 | 43.9 |
| Dry cough | 17 | 41.5 |
| Rhinorrhea | 16 | 39 |
| Arthralgia | 15 | 36.6 |
| Backache | 13 | 31.7 |
| Diarrhea | 7 | 17.1 |
| Nausea | 7 | 17.1 |
| Productive cough | 4 | 9.8 |
| Nausea | 3 | 7.3 |
| Vomiting | 3 | 7.3 |
| Ageusia | 2 | 4.9 |
| Other *** | 8 | 19.2 |
| Gender | Number (%) | 3rd BNT162b2 Vaccine Dose (N = 172) | Contact with COVID-19 Positives | COVID-19 Infection (N = 170) | COVID-19 Symptoms (N = 48) | ||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | No | Yes | No | Yes | No | Yes | ||
| Female | 107 (61.8) | 35 (51.5) | 71 (68.3) | 78 (64.5) | 25 (54.3) | 78 (63.9) | 27 (56.3) | 2 (66.7) | 25 (55.6) |
| Male | 66 (38.2) | 33 (48.5) | 33 (31.7) | 43 (35.5) | 21 (49.7) | 44 (37.1) | 21 (43.8) | 1 (33.3) | 20 (44.4) |
| Total | 173 | 68 (9.5) | 104 (60.5) | 121 (72.5) | 46 (27.5) | 122 (71.8) | 48 (28.2) | 3 (6.7) | 45 (93.8) |
| Occupation | |||||||||
| Nurse | 31 (18.2) | 7 (10.4) | 24 (23.3) | 7 (10.4) | 24 (23.3) | 16 (13.2) | 13 (28.3) | 19 (16.1) | 10 (20.8) |
| Doctor | 24 (14.1) | 6 (8.9) | 18 (17.5) | 6 (8.9) | 18 (17.5) | 13 (11.1) | 11 (23.9) | 19 (16.1) | 5 (10.4) |
| Administrative/office staff | 25 (14.7) | 13 (19.4) | 11 (10.7) | 13 (19.4) | 10 | 22 (18.6) | 2 (4.2) | 18 (15.3) | 6 (12.5) |
| Laboratory staff | 12 (7.1) | 6 (8.9) | 6 (5.8) | 6 (8.9) | 6 (5.8) | 9 (7.7) | 3 (6.5) | 9 (7.6) | 3 (6.3) |
| Medical assistant | 10 (5.9) | 5 (7.5) | 5 (4.9) | 5 (7.5) | 5 (4.9) | 8 (6.8) | 2 (4.3) | 10 (8.4) | - |
| Medical specialist | 10 (5.9) | 4 (5.9) | 6 (5.8) | 4 (5.9) | 6 (5.8) | 7 (5.9) | 3 (6.5) | 5 (4.2) | 5 (10.4) |
| Head of Departemnt | 8 (4.7) | 4 (5.9) | 4 (3.9) | 4 (5.9) | 4 (3.9) | 7 (5.9) | 1 (2.2) | 7 (5.9) | 1 (2.1) |
| Sanitar | 6 (3.5) | 2 (2.9) | 4 (3.9) | 2 (2.9) | 4 (3.9) | 2 (1.7) | 4 (8.7) | 4 (3.4) | 2 (4.2) |
| Professor/Lecturer | 5 (2.9) | 3 (4.5) | 2 (1.9) | 3 (4.5) | 2 (1.9) | 4 (3.4) | 1 (2.1) | 4 (3.4) | 1 (2.1) |
| IT | 4 (2.4) | 1 (1.5) | 3 (2.9) | 1 (1.5) | 3 (2.9) | 4 (3.4) | - | 2 (1.7) | 2 (4.2) |
| Midwife | 3 (1.8) | 1 (1.5) | 2 (1.9) | 1 (1.5) | 2 (1.9) | 3 (2.6) | 2 (1.7) | 1 (2.1) | |
| Pharmacist | 3 (1.8) | 1 (1.5) | 2 (1.9) | 1 (1.5) | 2 (1.9) | 2 (1.7) | 1 (2.1) | 2 (1.7) | 1 (2.1) |
| Dentist | 2 (1.2) | - | 2 (1.9) | - | 2 (1.9) | 2 (1.7) | 1 (9.1) | 1 (2.1) | |
| Physiotherapist | 2 (1.2) | - | 2 (1.9) | - | 2 (1.9) | - | 2 (4.2) | - | 2 (4.2) |
| Researcher | 2 (1.2) | - | 2 (1.9) | - | 2 (1.9) | 2 (1.7) | 2 (1.7) | - | |
| Student | 2 (1.2) | 2 (2.9) | - | 2 (2.9) | - | 2 (1.7) | 2 (1.7) | - | |
| Psychologist | 1 (0.6) | 1 (1.5) | - | 1 (1.5) | - | 1 (0.9) | 1 (9.1) | - | |
| Nutritionist | 1 (0.6) | - | 1 (0.9) | - | 1 (0.9) | 1 | - | 1 (2.1) | |
| Other | 20 (11.8) | 11 (16.4) | 9 (8.7) | 11 (16.4) | 8 (7.8) | 14 (11.9) | 4 | 11 (9.3) | 7 (14.6) |
| Total | 170 | 67 | 103 | 67 | 102 | 118 | 48 | 118 | 48 |
| Comorbidities | Number (%) | Contact with COVID-19 Positives | COVID-19 Infection-Symptoms | Post-Full Vaccination Symptoms * | 3rd BNT162b2 Vaccine Dose | ||||
|---|---|---|---|---|---|---|---|---|---|
| No-No | Yes-Yes | Yes-No | Yes | No | Post-Vaccination Symptoms | ||||
| Obesity (BMI 30–39 kg/m2) | 22 (19.8) | 5 (25) | 15 (20.3) | 7 (20) | - | 4 (22.2) | 12 (16.4) | 10 (26.3) | 5 (31.3) |
| Allergy | 18 (16.2) | 3 (15) | 12 (16.2) | 6 (17.4) | - | 7 (38.9) | 13 (17.8) | 5 (13.2) | 4 (25) |
| Gastrointestinal diseases | 12 (10.8) | 4 (20) | 7 (9.5) | 5 (14.3) | 1 (50) | 2 (11.1) | 8 (10.9) | 4 (10.5) | 1 (6.3) |
| Blood factors diseases | 10 (9) | 3 (15) | 9 (12.2) | 1 (2.9) | - | - | 4 (5.5) | 6 (15.8) | 1 (6.3) |
| Hypercholerterolemia | 8 (7.2) | 1 (5) | 7 (9.5) | - | - | 1 (5.6) | 7 (9.6) | 1 (2.6) | 1 (6.3) |
| Neurological/Psychological diseases | 7 (6.3) | 2 (10) | 5 (6.8) | 2 (5.7) | - | - | 7 (9.6) | - | 1 (6.3) |
| Chronic heart disease | 6 (5.4) | - | 5 (6.8) | - | 1 (50) | - | 3 (4.1) | 3 (7.9) | - |
| Palmunary diseases | 3 (2.7) | - | 3 (4.1) | - | - | - | 1 (1.4) | 2 (5.3) | - |
| Surgery | 3 (2.7) | - | 3 (4.1) | - | - | - | 1 (1.4) | 2 (5.3) | - |
| Immunosupression/immunodeficiency | 2 (1.8) | - | 2 (2.7) | - | - | - | 2 (2.8) | - | - |
| Asthma | 2 (1.8) | - | 2 (2.7) | - | - | - | 1 (1.4) | 1 (2.6) | - |
| Osteo disorders | 2 (1.8) | - | 1 (1.4) | 1 (2.9) | - | 1 (5.6) | 2 (2.8) | 2 (12.5) | |
| Prostate hypertrophy | 2 (1.8) | - | - | 2 (5.7) | - | - | 1 (1.4) | 1 (2.6) | - |
| Skin disease | 2 (1.8) | 1 (5) | 1 (1.4) | 1 (2.9) | - | 1 (5.6) | 1 (1.4) | 1 (2.6) | - |
| Multiple sclerosis | 1 (0.9) | - | 1 (1.4) | - | - | - | 1 (1.4) | - | - |
| Other | 11 (9.9) | 1 (5) | - | 11 (31.4) | - | 2 (11.1) | 9 (12.3) | 2 (5.3) | 1 (6.3) |
| Total | 111 | 20 (18) | 74 (66.7) | 35 (31.5) | 2 (1.8) | 18 (20) | 73 (65.8) | 38 (34.2) | 16 (21.9) |
| Anti-Spike IgG Concentration (ng/mL) | Age Groups | Gender (N = 173) | COVID Infection-Symptoms (N = 165) | Booster Dose Vaccination (N = 172) | Symptoms after Vaccination-COVID-19 Infection (N = 162) | |||||||||||||||
| <21 | 21–30 | 31–40 | 41–50 | 51–60 | 61–70 | >71–80 | Total | F | M | No-No | Yes-Yes | Yes-No | No | Yes | Yes-Yes | Yes-No | No-Yes | No-No | ||
| <1000 | - | - | - | - | 1 (3.3) | - | - | 1 (0.6) | 1 (0.9) | - | - | 1 (2.2) | - | - | 1 (9.6) | - | - | - | - | |
| 1000–6000 | - | 1 (3.1) | 1 (3.3) | 6 (15) | 5 (16.7) | 3 (12.5) | 2 (11.1) | 18 (10.4) | 15 (14) | 3 (4.5) | 16 (13.7) | 2 (4.4) | - | 6 (8.8) | 12 (11.5) | - | 8 (13.6) | 1 (7.7) | 7 (11.5) | |
| 6000–11,000 | 1 (14.3) | 5 (15.6) | 7 (23.3) | 3 (7.5) | 8 (26.7) | 8 (33.3) | 1 (9.1) | 33 (19.1) | 21 (19.6) | 12 (18.2) | 25 (21.4) | 6 (13.3) | - | 13 (19.1) | 20 (19.2) | 4 (13.8) | 12 (20.3 | 1 (7.7) | 15 (24.6) | |
| 11,000–16,000 | 3 (42.9) | 5 (15.6) | 8 (26.7) | 14 (35) | 9 (30) | 3 (12.5) | 4 (36.4) | 46 (26.6) | 29 (27.1) | 17 (25.8) | 37 (31.6) | 6 (13.3) | 1 (33.3) | 21 (30.9) | 24 (23.1) | 2 (6.9) | 18 (30.5) | 4 (30.8) | 18 (29.5) | |
| 16,000–21,000 | 3 (42.9) | 19 (59.4) | 13 (43.3) | 15 (37.5) | 7 (23.3) | 10 (41.7) | 5 (45.5) | 70 (40.5) | 39 (34.4) | 31 (46.9) | 36 (30.7) | 28 (62.2) | 2 (66.7) | 28 (41.2) | 42 (40.4) | 22 (75.9) | 18 (30.5) | 7 (53.8) | 20 (32.8) | |
| 21,000–26,000 | - | 2 (6.3) | 1 (3.3) | 2 (5) | - | - | - | 5 (2.9) | 2 (1.9) | 3 (4.5) | 3 (2.6) | 2 (4.4) | - | - | 5 (4.8) | 1 (3.4) | 3 (5.1) | - | 1 (1.6) | |
| Total | 7 (4) | 32 (18.5) | 30 (17.3) | 40 (23.1) | 30 (17.3) | 24 (13.9) | 11 (5.9) | 173 | 107 (61.8) | 66 (38.2) | 117 (70.9) | 45 (27.3) | 3 (1.8) | 68 (39.5) | 104 (60.5) | 29 (17.9) | 59 (36.4) | 13 (8) | 61 (37.7) | |
| Anti-Spike IgM Concentration (ng/mL) | <1000 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1000–2000 | 4 (57.1) | 8 (25) | 4 (13.3) | 17 (42.5) | 14 (46.7) | 10 (41.7) | 3 (27.3) | 60 (34.7) | 38 (35.5) | 22 (33.3) | 38 (32.5) | 16 (35.6) | - | 20 (29.4) | 40 (38.5) | 10 (34.5) | 20 (32.9) | 3 (23.1) | 20 (3.3) | |
| 2000–3000 | 3 (42.9) | 13 (40.6) | 15 (50) | 15 (37.5) | 14 (46.7) | 7 (29.2) | 2 (11.1) | 70 (40.5) | 41 (38.3) | 28 (42.4) | 50 (42.7) | 15 (33.3) | 2 (66.7) | 30 (44.1) | 38 (36.5) | 10 (34.5) | 25 (42.4) | 6 (46.2) | 24 (39.3) | |
| 3000–4000 | - | 7 (21.8) | 7 (23.3) | 5 (12.5) | 1 (3.3) | 6 (25) | 1 (9.1) | 27 (15.6) | 17 (15.9) | 10 (15.2) | 19 (16.2) | 8 (17.8) | - | 12 (17.6) | 15 (14.4) | 5 (17.2) | 8 (13.6) | 3 (23.1) | 11 (18) | |
| 4000–5000 | - | 3 (9.4) | 3 (10) | 2 (5) | 1 (3.3) | 1 (4.2) | 2 (11.1) | 12 (6.9) | 6 (5.6) | 6 (9.1) | 7 (5.9) | 4 (8.9) | - | 4 (5.9) | 8 (7.7) | 3 (10.3) | 3 (5.1) | 1 (7.7) | 5 (8.2) | |
| 5000–6000 | - | 1 (3.1) | 1 (3.3) | 1 (2.5) | - | - | 1 (9.1) | 4 (2.3) | 4 (3.7) | - | 2 (1.7) | 2.2 | 1 (33.3) | 2 (2.9) | 2 (1.9) | 1 (3.4) | 3 (5.1) | - | - | |
| 12,000–13,000 | - | - | - | - | - | - | 1 (9.1) | 1 (0.6) | 1 (0.9) | - | 1 (0.9) | - | - | 1 (1.5) | - | - | - | - | 1 (1.6) | |
| Total | 7 (4) | 32 (18.5) | 30 (17.3) | 40 (23.1) | 30 (17.3) | 24 (13.9) | 11 (5.9) | 173 | 107 (61.8) | 66 (38.2) | 117 (69.4) | 45 (24.3) | 3 (1.7) | 68 (39.5) | 104 (60.5) | 29 (17.9) | 59 (36.4) | 13 (8) | 61 (37.7) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golshani, M.; Svobodová, L.M.; Štěpánek, L.; Zeman, J.; Nytrová, P.; Posová, H.; Petrásková, P.; Novotná, O.; Nováková, M.; Černý, V.; et al. SARS-CoV-2 Specific Humoral Immune Responses after BNT162b2 Vaccination in Hospital Healthcare Workers. Vaccines 2022, 10, 2038. https://doi.org/10.3390/vaccines10122038
Golshani M, Svobodová LM, Štěpánek L, Zeman J, Nytrová P, Posová H, Petrásková P, Novotná O, Nováková M, Černý V, et al. SARS-CoV-2 Specific Humoral Immune Responses after BNT162b2 Vaccination in Hospital Healthcare Workers. Vaccines. 2022; 10(12):2038. https://doi.org/10.3390/vaccines10122038
Chicago/Turabian StyleGolshani, Maryam, Ludmila Maffei Svobodová, Lubomír Štěpánek, Jan Zeman, Petra Nytrová, Helena Posová, Petra Petrásková, Olga Novotná, Michaela Nováková, Viktor Černý, and et al. 2022. "SARS-CoV-2 Specific Humoral Immune Responses after BNT162b2 Vaccination in Hospital Healthcare Workers" Vaccines 10, no. 12: 2038. https://doi.org/10.3390/vaccines10122038
APA StyleGolshani, M., Svobodová, L. M., Štěpánek, L., Zeman, J., Nytrová, P., Posová, H., Petrásková, P., Novotná, O., Nováková, M., Černý, V., Beneš, J., Kolářová, L., Vokurka, M., & Hrdý, J. (2022). SARS-CoV-2 Specific Humoral Immune Responses after BNT162b2 Vaccination in Hospital Healthcare Workers. Vaccines, 10(12), 2038. https://doi.org/10.3390/vaccines10122038






