1. Introduction
Streptococcus pneumoniae or pneumococcus is a pathogen responsible for diseases of varying severity that are one of the major causes of mortality and morbidity worldwide. Pneumococcal infections affect people of all ages, but children under two years of age and adults 65 years of age and over are at the highest risk.
Based on the polysaccharide characteristics of the capsule with which it is coated, over 90 globally widespread serotypes are classified, of which about 40 are capable of generating infections in humans. However, only a limited number of them cause severe infections, called invasive pneumococcal infections, in various age groups of the world population. The distribution of invasive
S. pneumoniae serotypes changes according to different geographical areas. In 2000, the World Health Organization (WHO) [
1] detected 14.5 million cases of invasive infections globally. In the USA, in 2019, the Center for Disease Control (CDC) [
2] estimated that about 150,000 hospitalisations were caused by pneumococcal disease. In Italy, in 2019 and 2020 (the latter is the year in which data are influenced by the effects of the COVID-19 pandemic on the healthcare services), through a surveillance system active since 2007, 1679 and 499 cases of invasive pneumococcal disease (IPD) were identified, respectively, with an incidence (number of new cases among the population) of 2.81/0.84 per 100,000 inhabitants, respectively. It should be considered that IPD cases are reported in the surveillance database by hospitals or regional health authorities on a voluntary basis [
3]. Since the starting of the surveillance, the Italian regions have shown a different propensity to report cases and to submit the pneumococcal isolates to serotyping; therefore, a certain level of under-reporting of IPD cases cannot be excluded.
Today, in Europe and the USA, the incidence of invasive infections is about 100 cases per 100,000 inhabitants. Generally, in temperate climates the incidence of pneumococcal infections peaks in winter months [
4,
5,
6,
7,
8].
Transmission of
S. pneumoniae occurs from person to person through direct contact or by inhaling respiratory droplets produced by talking, coughing, and sneezing. It is estimated that 20 to 40% of children and 5 to 10% of adults are asymptomatic carriers of the bacterium in the nasopharynx [
9], the only known reservoir in humans. The incubation period is uncertain, but is assumed to be about 1–3 days. It is most likely to spread in indoor social settings, such as nursing homes, long-term care facilities, hospital wards, prisons, military bases, universities or schools, homeless dormitories, and kindergartens [
10].
Pneumococcal disease can include many different types of infections. Most pneumococcal infections are mild; however, some of them can be fatal or cause long-term problems [
1]. Invasive pneumococcal disease is the term used for the most severe pneumococcal infections, particularly bacteraemia, sepsis, and meningitis.
The risk of developing a pneumococcal infection is higher in the following cases: chronic diseases (such as heart and lung disorders, diabetes, and liver diseases); alcohol-addiction; disorders that weaken the immune system, such as HIV infection; drugs that suppress the immune system, such as corticosteroids or chemotherapy drugs; absence of a functioning spleen; sickle cell anaemia; stay in a long-term care facility; and smoking [
10].
Currently, the National Vaccine Prevention Plan (PNPV) strongly recommends pneumococcal vaccination in the first year of life (for those born from 2012 onwards), envisaging three doses and a coverage target greater than or equal to 95% in newborns. As for the adult population, vaccination is recommended in people 65 years of age, providing for two doses (a first dose of 13-valent conjugate vaccine and a second dose of 23-valent polysaccharide vaccine, at least 2 months apart) and a coverage target of at least 75% of the people aged 65 years.
Conjugate vaccines have ensured significant protection against pneumococcal infection with effects extending to all age groups through the induction of herd immunity. At the same time, the limited serotype coverage of conjugate vaccines led to a phenomenon of partial replacement, i.e., substitution of part of the circulating serotypes. Therefore, though the use of pneumococcal conjugate vaccines (PCVs) has greatly reduced PCV vaccine-type disease, there continues to be a clinical and economic burden of pneumococcal disease.
While vaccination coverage in the paediatric population has been stable around the coverage target for more than a decade, vaccination coverage data in adults are not systematically and uniformly collected. The available data are derived from local or regional studies, and show that coverage is suboptimal [
11,
12].
In 2022, based on the results of a large programme of Phase 1, 2, and 3 clinical trials, European Medicine Agency (EMA) also approved a 20-valent, single-dose polysaccharide pneumococcal conjugate vaccine administered intramuscularly, indicated for protecting adults aged 18 years and over against 20 serotypes responsible for most cases of invasive disease. It is an evolution of the 13-valent vaccine as it contains seven additional capsular polysaccharides associated with invasive pneumococcal disease with high mortality rates and antibiotic resistance. The results of Phase 3 studies in children are expected [
13].
The availability of a new vaccine, and the knowledge gap on cost-effectiveness of its adoption, makes it appropriate to produce a first (to our knowledge) assessment of its cost-effectiveness in the Italian adult population, which is the primary goal of this analysis.
2. Materials and Methods
The 20-valent pneumococcal conjugate vaccine (PCV20) cost-effectiveness in adults was evaluated by adopting the National Health Service (NHS) perspective and adapting a cost-effectiveness model developed for Pfizer Inc. by Policy Analysis Inc. (Brookline, MA, USA) to the Italian context.
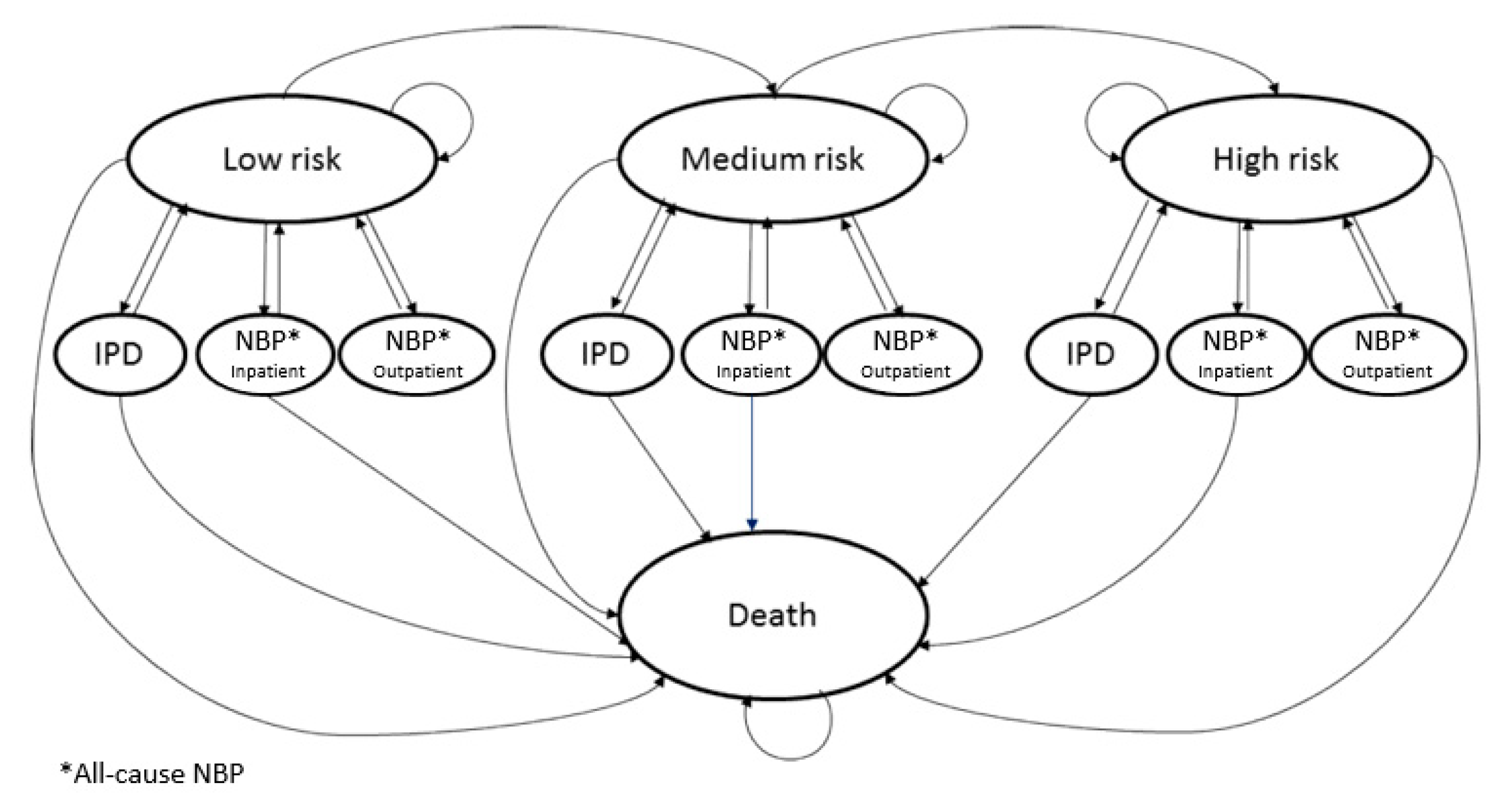
The model works for individual age cohorts of the population selected for vaccination, using a Markovian structure. The population can also be disaggregated by risk level.
The
Figure 1 summarises the states considered in the Markov model.
In adapting the model to the Italian context, following the current PNPV guidelines, the effects of vaccination with PCV20 were compared with the vaccination strategy envisaging the administration of 13-valent pneumococcal conjugate vaccine (PCV13). For completing the analysis, comparisons were also made with 15-valent pneumococcal conjugate vaccine (PCV15). The PCV15 was recently approved by the European Commission (EC) for active immunization for the prevention of invasive disease and pneumonia caused by Streptococcus pneumoniae in individuals 18 years of age and older.
As far as age groups are concerned, the analyses were conducted envisaging vaccination of the cohorts of subjects 65 to 74 years of age.
Some information is also provided on the impact of vaccination of the 65 years of age cohort alone. These estimates provide an indication of the economic and financial burden actually incurred by the NHS.
A lifetime horizon was adopted in the simulations; costs and (health) consequences were discounted at a 3% discount rate per year.
As far as costs are concerned, in adapting the model to the Italian context, direct health costs were taken into account from the NHS perspective.
The efficiency evaluations of the model were expressed both in cost per life year gained (cost-effectiveness) and per QALY gained (cost utility).
2.1. Population
The model is populated with the adult population (yearly average) inferred from Italian National Institute of Statistics (Istat) statistics. In the absence of Italian evidence regarding the Italian distribution by risk levels, the composition (by age group) surveyed by the Center for Disease Control and Prevention (CDC) in the USA [
14] was used. Risk levels are defined as follows [
15]:
Low risk: immunocompetent people without chronic clinical conditions;
Medium risk: immunocompetent people with at least 1 chronic clinical condition (such as cardiovascular, liver, pulmonary disease, diabetes, asthma, smoking, and alcohol abuse);
High risk: immunocompromised people due to chronic renal failure or neoplasm (including leukaemia, lymphoma, and solid tumours diagnosed less than 3 years ago).
Due to the absence of information on transition probabilities among risk level for the Italian population, in the simulations it was assumed that there is no switching between risk levels.
2.2. Past Vaccination Coverage
For the sake of simplicity, the model assumes that none of the adults in the cohort considered have previously received PCV13: the hypothesis is also supported by the relatively short time elapsed since the start of vaccinations in children (7-valent pneumococcal conjugate vaccine was approved in 2005 and then replaced by PCV13 in 2010).
2.3. Incidence of Disease
The model distinguishes between invasive pneumococcal disease (IPD), disaggregating meningitis from the other forms of bacteraemia, and the other types of nonbacteremic pneumonia (NBP), disaggregated into hospitalised and non-hospitalised ones. In the model, disease incidences are used for determining transition to the above-mentioned states (
Table 1).
As to IPD, reference was made to the surveys of the Istituto Superiore di Sanità (ISS) [
3]; the 2019 survey was used since the 2020 data were influenced by the pandemic emergency.
With regard to NBP, reference was made to the evidence inferable from the Ministry of Health [
16] surveys on hospital activity (flow of hospital discharge forms (SDO) year 2019).
In particular, all hospitalisations (ordinary ones and outpatients in day hospital settings) with ICD-IX diagnoses from 480 to 486 were extracted, detecting an overall incidence of 751.0 ordinary cases per 100,000 inhabitants aged over 64 years. The incidences detected (also by age group) were then corrected by risk level on the basis of the information provided, for the years 2017–2018, by the CDC [
17]. Literature evidence [
18,
19,
20] was used to estimate the share of non-hospitalised NBP cases.
2.4. Composition of Disease Cases by Serotype
With regard to the attribution of disease cases to the different serotypes, for invasive pneumococcal diseases (IPDs) reference was made to the data collected (year 2019, 2020) by the ISS [
3]; for pneumococcal CAP (P-NBP) evidence from the PUMA study [
21] was used, considering a proportion of P-NBP of 15.1% for the population aged 65–74 years (
Table 2).
2.5. Mortality
As for the general population mortality, reference was made to the biometric tables published by Istat.
Mortality for IPD (separately for meningitis and other types of bacteraemia) was inferred from literature [
22].
Mortality related to hospitalised NBP cases (by age) was processed using the hospital discharge forms (SDO) published by the Ministry of Health (for the year 2019) [
16]. Mortality for non-hospitalised cases (that in Italy are largely underestimated) reasonably is very low; unfortunately no evidence was founded for Italy, consequently it was prudentially assumed to be one-tenth of the mortality rate for the corresponding hospitalised cases.
In the absence of evidence on mortality by risk level for the Italian population, it was prudentially assumed to be constant (
Table 3).
2.6. Quality of Life (Utility)
With specific reference to the quality of life (QoL), reference was made to the work of Sisk et al. [
23] as cited by Boccalini et al. [
22]: QoL comes from an elaboration on the USA population Activities of Daily Living (ADL) resulting from the National Health Interview Survey (1990), applying a multi-attribute utility scaling technique.
Disutilities applied to cases of IPD and NBP were derived from the work of Boccalini et al. [
22] that analysed the economic impact of pneumococcal vaccination strategies specifically in the Italian adults (
Table 4). Their approach may underestimate the QALYs gap since it considers only the disutilities generated during the hospitalisation period. Other studies adopted a more extensive approach: for example, Mangen et al. [
24] quantified in 0.13 the QALY difference (on an annual basis) in two cohorts, diseased and non diseased subjects, based on hospitalisation with suspected community-acquired pneumonia.
2.7. Healthcare Costs
The prices of the vaccines adopted are equal to the present (EUR 2022) maximum cost of transfer to the NHS (i.e., the price paid by the Italian public NHS to manufacturer).
The cost of administration can vary at regional level, due to the federal organization of the Italian NHS the published tariff by the Emilia Romagna Region [
25] was used, as the region is a reference at the national level (
Table 5).
Regarding hospitalisation costs (
Table 6), the average fees processed (by age class) on the basis of the Diagnosis Related Groups (DRGs) associated with ordinary hospitalisations (SDO, Ministry of Health) were assumed; for non-hospitalised NBP cases, in absence of an estimate of the consultation in primary care (Italian general practitioners are paid on a capitation basis), a value equal to the value of the fees applied to the DRGs associated with outpatients in day hospital settings was assumed.
2.8. Vaccine Efficacy
With specific reference to the efficacy of vaccines against IPD and NBP (
Table 7), for the low/medium risk population, the reference used is the evidence produced by the Community-Acquired Pneumonia Immunization Trial in Adults (CAPiTA) [
26,
27].
For the high-risk population, vaccine efficacy was assumed to be 80% of the corresponding values for the low/medium risk population based on evidence from the work of Klugman et al. [
28].
The efficacy of conjugate vaccines is assumed to be constant for the first 5 years and is then reduced by 5% per year for the following 5 years (up to year 10), by 10% between years 11 and 15. It is finally nullified after year 16.
2.9. Sensitivity Analysis
A one-way deterministic and a probabilistic sensitivity analysis was performed.
Simulations were elaborated on the principal variables affecting results; specifically:
For the one-way sensitivity analysis a variation of ±25% was assumed, except disease disutilities, assumed to vary in the range between 0.01 (Boccalini et al. [
22]) and 0.13 (Mangen et al. [
24]).
For the probabilistic analysis a Beta distribution was assumed for disease incidence parameter, mortality, and effectiveness of vaccines, while a Gamma distribution was used for medical costs.
3. Results
A total of 13.5% of the Italian population, i.e., 6,795,374, are aged between 65 and 74 years, of whom 48% are assumed to have a low risk, 40.6% a medium risk, and the remaining 11.4% a high risk (see definition and caveat expressed in paragraph 2.1).
Assuming complete (100%) vaccination of cohorts aged 65–74 years, in the (lifetime) comparison between PCV20 and PCV13, the former is dominant (lower incremental cost for a better health outcome).
A reduction in disease events is estimated: −1208 deaths; −1171 cases of bacteraemia (excluding meningitis); −227 cases of meningitis; −9845 hospitalised NBP cases; and −21,058 non-hospitalised NBP cases.
Overall, in the Italian population, a total benefit gain of 6581.6 in terms of life years and of 4734.0 in terms of QALY is estimated.
On the cost side, against an increase in costs due to vaccinations equal to EUR 40.568 million, other direct health costs are reduced by EUR 48.032 million, with a net savings of EUR +7.464 million (
Table 8).
In the case of vaccination of the 65 years of age cohort alone, as envisaged by the PNPV, the cost-effectiveness of PCV20 vs. PCV13 is confirmed, with an Incremental Cost-Effectiveness Ratio (ICER) of EUR 196 per life year gained and EUR 268 per QALY gained.
On the cost side, the increase in costs due to vaccinations is EUR 4.946 million and the other direct health costs are reduced by EUR 4.817 million, with an incremental net cost of EUR +0.129 million.
Assuming vaccination of the cohorts aged 65–74 years, the comparison between PCV20 and PCV15 results in an ICER of EUR 66 per life year gained and EUR 91 per QALY gained.
The estimated reduction in disease events is −1009 deaths; −940 cases of bacteraemia (excluding meningitis); −183 meningitis; −8350 hospitalised NBP cases; −17,858 non-hospitalised NBP cases, with an overall gain of 5536.7 life years and 3984.7 QALYs.
On the cost side, against an increase in costs due to vaccinations equal to EUR 40.568 million, the other direct health costs decreased by EUR 40.205 million, with an incremental net cost of EUR +0.364 million.
Sensitivity Analysis
Table 9 reports the Incremental cost per QALY gained resulting from the one-way sensitivity analysis.
The simulations demonstrate that results are quite robust: PCV20 remains dominant vs. PCV13 and PCV15 in most of the simulations, and in the remaining is cost-effective assuming a very low willingness to pay.
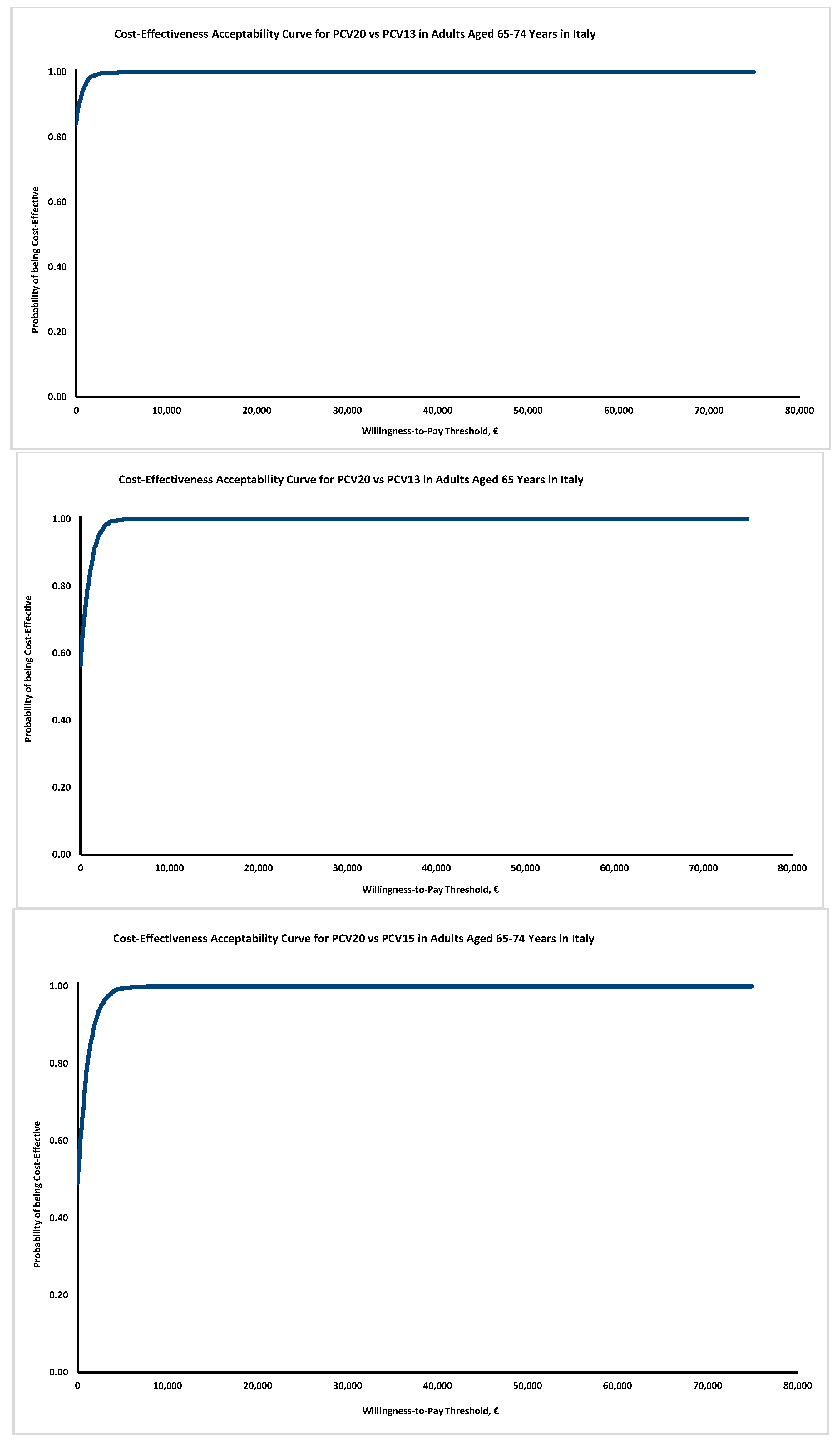
The probabilistic sensitivity analysis (
Figure 2) confirms the results’ robustness: although in Italy no explicit thresholds are available, the probability of PCV20 to be cost-effective also at a very modest threshold of EUR 5000 exceeds 90% in all the simulations proposed.
4. Discussion
In 2020, EUR 106.5 million were spent for pneumococcal vaccination (children and adults), of which 93.5% was for PCV13, 2.4% was for PCV10 (used for children), and 4.2% was for 23-valent pneumococcal polysaccharide vaccine (PPV23) [
29].
Assuming a cost for PCV20 of EUR 55.97 per dose to be borne by the NHS, the changeover of vaccinations from PCV13 to PCV20, for the 65–74 years of age cohorts (not considering the other subjects at risk), implies a financial burden for the NHS (in other terms an increase in NHS costs due to the immunization with PCV20 instead of PCV13) equal to EUR 40.568 million (including administration costs, which are an ongoing expense and would be consistent regardless of which PCV was administered).
Although the other direct health costs are reduced by about EUR 48.032 million, particularly due to the reduction in hospitalisations due to IPD and NBP, thus providing a net savings of EUR 7.464 million in the long term, this is a strategy that is difficult to implement in the NHS [
30]; in fact, the financial burden it would generate in the short term is expected to be not sustainable: nonetheless, our results assess the significant dimension of the improvement in health outcomes that can be potentially obtained with an increase in the investment in immunisation.
Against the increase in burdens on the NHS, the health effects are indeed appreciable: deaths attributable to S. pneumoniae are reduced by 1208 (−8.7 per 100,000 inhabitants aged over 64 years); the cases of bacteraemia, excluding meningitis are reduced by 1171 (−8.4 per 100,000 inhabitants aged over 64 years). The cases of meningitis are reduced by 227 (−1.6 per 100,000 inhabitants aged over 64 years); hospitalised NBP cases are reduced by 9845 (−71 per 100,000 inhabitants aged over 64 years) and non-hospitalised NBP cases are reduced by 21,058 (−151.9 per 100,000 inhabitants aged over 64 years).
The reduction in deaths results in an overall gain in life years equal to 6581.6, and a gain in quality of life due to the reduction in hospitalisation-related disutilities, equal to 4734 QALYs. The results are likely to be conservative due to the assumptions made in relation to the disease disutilities in the base case.
Overall, PCV20 is dominant over PCV13, having lower costs and better health outcomes, thus demonstrating the social efficiency of the investment. Results are very robust as indicated by one-way and probabilistic sensitivity analysis.
Limiting immunisation to the 65 years of age cohort only, as envisaged by the PNPV (but not considering the other subjects at risk), the switch from PCV13 to PCV20 implies a cost for the National Health Service equal to EUR 4.946 million (i.e., 4.6% of the current spending on pneumococcal vaccines). The other direct health costs are reduced by EUR 4.817 million, particularly due to the decrease in IPD and NBP hospitalisations, thus bringing the net burden to EUR +0.129 million: an amount that is largely sustainable, being equal to 1.2 per thousand of the current spending on pneumococcal vaccines.
We can therefore conclude that the switch from PCV13 to PCV20 vaccination is certainly a sustainable investment, as well as efficient for the NHS, assuming modest willingness-to-pay thresholds compared with those normally used at regulatory level in Italy.
Moreover, with respect to the use of PCV15, assuming for it a price equal to that of PCV13, the results obtained are confirmed: the ICER of PCV20 compared with PCV15 is EUR 66 per life year gained and EUR 91 per QALY gained. PCV20 is therefore cost-effective even assuming a very low social willingness to pay. The financial differential of burden on the NHS remains unchanged with respect to PCV13, since the same price was assumed for PCV15 as for PCV13.
The importance of pneumococcal vaccination in adults has been the subject of numerous analyses, and is now an established fact, as can be seen from the Italian PNPV, which recommends it for adults aged 65 years, as well as for individuals at risk. The current PNPV recommends the use of PCV13, followed by vaccination with the PPV23 after at least two months.
Unfortunately, very limited evidence is available on the cost-effectiveness of the new PCV20. The present paper, to our knowledge, is one of the very first attempt to produce an assessment of the economic impact derived by a strategy of substitution of PCV13 immunization with PCV20.
The model suffers from a number of limitations linked, in particular, to the lack of data for the Italian population: first and foremost, the limitation of adopting a risk composition of the population deduced from US data, as well as levels of utility (and disutility) inferred from literature, not specific to the Italian population.
The production of the cited figures should be a priority in terms of public health planning: in the meanwhile, the strategy used in the paper for “missing” data was to adopt a conservative approach.
Moreover, in the absence of disaggregated data on vaccination in adults and children, it appears evident that in Italian practice, the use of PPV23 after PCV13 in adults is substantially a marginal choice. Given its lack of use in actual Italian practice, PPV23 was not considered in the analysis.
Finally, the financial impact of the assessments made is underestimated due to the fact that the needs of the subjects at risk are not taken into account. However, the clinical effects are also proportionally underestimated so that it can be assumed that the results obtained in terms of ICER remain valid even in the real context of the implementation of the vaccination strategy.
5. Conclusions
The Italian National Vaccine Prevention Plan recommends pneumococcal vaccination for adults aged 65 years, as well as for individuals at risk.
Although the coverage achieved in adults is not known, it is assumed that it is not yet optimal.
The availability of the 20-valent conjugate vaccine provides an opportunity to prevent additional pneumococcal disease. The cost-effectiveness (cost utility) model, adapted to the Italian context used to assess the switch to PCV20 (from PCV13 and/or PCV15) confirms the significance of the achievable health benefits. The vaccination strategy according to the PNPV (vaccination of the cohort aged 65 years) is not only efficient (cost-effective) by adopting a low threshold of (social) willingness to pay, but is also largely sustainable, with a limited impact on the current national budget for vaccines.
Author Contributions
Conceptualization, B.P., G.I., A.O., F.S., R.D.V., D.d.; methodology, B.P., G.I., A.O., F.S., R.D.V., D.d.; software, B.P., F.S., D.d.; validation, B.P., G.I., A.O., F.S., R.D.V., D.d.; formal analysis, B.P., G.I., A.O., F.S., R.D.V., D.d.; investigation, B.P., G.I., A.O., F.S., R.D.V., D.d.; resources, B.P., D.d.; data curation, B.P., F.S., D.d.; writing—original draft preparation, B.P., F.S., D.d.; writing—review and editing, B.P., F.S., D.d.; visualization, B.P., G.I., A.O., F.S., R.D.V., D.d.; supervision, G.I., A.O., F.S.; project administration, B.P., D.d.; funding acquisition, B.P., D.d. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Pfizer Italia: the funders collaborate in the design of the study, in the collection, analyses, or interpretation of data.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The model presented in this study are available on reasonable request from the corresponding author.
Conflicts of Interest
B.P. received payments or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from the following commercial sources: Allergan, Amgen, Astellas, Eli Lilly, Janssen Cilag, Nestlé HS, Novartis, Novo Nordisk, Pfizer, Servier, Takeda, and Teva; in addition she received consulting fees from UCB. G.I. received funding for scientific advisory boards, travel, and speaker honoraria from Pfizer, GSK, MSD, Sanofi Pasteur and Seqirus. A.O. received funding for scientific advisory boards, travel, and speaker honoraria from Pfizer, MSD, Sanofi Pasteur and Seqirus. F.S. received payments or honoraria for scientific advisory boards lectures, presentations, speaker bureaus, manuscript writing, or educational events from Amgen, Biogen Idec, Biomarine, Boehringer-Ingelheim, Celgene, Daiichy Sankyo, Eli Lilly, Genzyme, Janssen Cilag, Gore, Lundbeck, MSD Italia, Nestlè Health Science, Novartis, Novo Nordisk, Obi, Pfizer, Roche, Sanofi, Servier, Takeda, and Teva. R.D.V. is a Pfizer employee. D.d. received payments or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from the following commercial sources: Allergan, Amgen, Astellas, Eli Lilly, Janssen Cilag, Nestle’ HS, Novartis, Novo Nordisk, Pfizer, Servier, Takeda, and Teva.
References
- O’Brien, K.L.; Wolfson, L.J.; Watt, J.P.; Henkle, E.; Deloria-Knoll, M.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T. Hib and Pneumococcal Global Burden of Disease Study Team, Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet 2009, 374, 893–902. [Google Scholar]
- Pneumococcal Disease. Available online: https://www.cdc.gov/pneumococcal/about/index.html (accessed on 28 June 2022).
- Istituto Superiore di Sanità-Dip. to Malattie Infettive. Sorveglianza delle Malattie Batteriche Invasive in Italia; Rapporto 2020; ISS: Roma, Italy, November 2021. [Google Scholar]
- Camilli, C.R.; D’Ambrosio, F.; Del Grosso, M.; Pimentel de Araujo, F.; Caporali, M.G.; Del Manso, M.; Gherardi, G.; D’Ancona, F.; Pantosti, A.; Pneumococcal Surveillance Group. Impact of pneumococcal conjugate vaccine (PCV7 and PCV13) on pneumococcal invasive diseases in Italian children and insight into evolution of pneumococcal population structure. Vaccine 2017, 35, 4587–4593. [Google Scholar]
- Sierotipi di Pneumococco in Italia. Available online: https://www.epicentro.iss.it/infettive/SierotipiPneumococco (accessed on 28 June 2022).
- Factsheet about Pneumococcal Disease. Available online: https://www.ecdc.europa.eu/en/pneumococcal-disease/facts (accessed on 28 June 2022).
- Moriondo, M.; Nieddu, F.; Pecile, P.; Zoppi, F.; Bondi, T.; Ricci, S.; Ghiori, F.; Iacopelli, J.; Liccioli, G.; Azzari, C. Le infezioni pneumococciche dell’adulto in Italia e le possibilità di prevenzione. Quale ruolo per i nuovi vaccini coniugati? Riv. Soc. Ital. Med. Gen. 2012, 2, 62–65. [Google Scholar]
- da Pneumococco, I. Msd Manuals. Available online: https://www.msdmanuals.com/it-it/professionale/malattie-infettive/cocchi-gram-positivi/infezioni-da-pneumococco (accessed on 28 June 2022).
- Pneumococcal Disease. Available online: https://www.cdc.gov/vaccines/pubs/pinkbook/pneumo.html#streptococcus-pneumoniae (accessed on 28 June 2022).
- Cherazard, R.; Epstein, M.; Doan, T.L.; Salim, T.; Bharti, S. Antimicrobial Resistant Streptococcus pneumoniae: Prevalence, Mechanisms, and Clinical Implications. Am. J. Ther. 2017, 24, e361–e369. [Google Scholar]
- Orsi, A.; Ansaldi, F.; Trucchi, C.; Rosselli, R.; Icardi, G. Pneumococcus and the elderly in Italy: A summary of available evidence regarding carriage, clinical burden of lower respiratory tract infections and on-field effectiveness of PCV13 vaccination. Int. J. Mol. Sci. 2016, 17, 1140. [Google Scholar]
- Prato, R.; Fortunato, F.; Cappelli, M.G.; Chironna, M.; Martinelli, D. Effectiveness of the 13-valent pneumococcal conjugate vaccine against adult pneumonia in Italy: A case-control study in a 2-year prospective cohort. BMJ Open 2018, 8, e019034. [Google Scholar]
- Apexxnar Epar Product Information. Available online: https://www.ema.europa.eu/en/documents/product-information/apexxnar-epar-product-information_it.pdf (accessed on 28 June 2022).
- Center for Disease Control and Prevention. National Health Interview Survey; Prevention CfDCa; Center for Disease Control and Prevention: Atlanta, GA, USA, 2018.
- Zimmerman, R.K.; Lauderdale, D.S.; Tan, S.M.; Wagener, D.K. Prevalence of high-risk indications for influenza vaccine varies by age, race, and income. Vaccine 2010, 28, 6470–6477. [Google Scholar]
- della Salute, M. Schede di Dimissione Ospedaliera; ISS: Roma, Italy, 2020. [Google Scholar]
- Center for Disease Control and Prevention. Active Bacterial Core Surveillance (ABCs); Report Emerging Infections Program Network Streptococcus pneumoniae 2018; Center for Disease Control and Prevention: Atlanta, GA, USA, 2020.
- Nelson, J.C.; Jackson, M.; Yu, O.; Whitney, C.G.; Bounds, L.; Bittner, R.; Zavitkovsky, A.; Jackson, L.A. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine 2008, 26, 4947–4954. [Google Scholar]
- Weycker, D.; Farkouh, R.A.; Strutton, D.R.; Edelsberg, J.; Sato, R.; Jackson, L.A. Rates and costs of invasive pneumococcal disease and pneumonia in persons with underlying medical conditions. BMC Health Serv. Res. 2016, 16, 182. [Google Scholar]
- Astengo, M.; Paganino, C.; Amicizia, D.; Trucchi, C.; Tassinari, F.; Sticchi, C.; Sticchi, L.; Orsi, A.; Icardi, G.; Piazza, M.F.; et al. Economic burden of pneumococcal disease in individuals aged 15 years and older in the Liguria region of Italy. Vaccines 2021, 9, 1380. [Google Scholar]
- Orsi, A.; Domnich, A.; Mosca, S.; Ogliastro, M.; Sticchi, L.; Prato, R.; Martinelli, D.; Fortunato, F.; Vitale, F.; Tramuto, F.; et al. Prevalence of pneumococcal serotypes in community-acquired pneumonia among older adults in Italy—A multicenter cohort study. Clin. Microbiol. Infect. 2022. forthcoming. [Google Scholar]
- Boccalini, S.; Bechini, A.; Levi, M.; Tiscione, E.; Gasparini, R.; Bonanni, P. Cost-effectiveness of new adult pneumococcal vaccination strategies in Italy. Hum. Vaccines Immunother. 2013, 9, 699–706. [Google Scholar]
- Sisk, J.E.; Whang, W.; Butler, J.C.; Sneller, V.-P.; Whitney, C.G. Cost-Effectiveness of Vaccination against Invasive Pneumococcal Disease among People 50 through 64 Years of Age: Role of Comorbid Conditions and Race. Ann. Intern. Med. 2003, 138, 960–968. [Google Scholar]
- Mangen, M.-J.J.; Huijts, S.M.; Bonten, M.J.M.; de Wit, G.A. The impact of community-acquired pneumonia on the health-related quality-of-life in elderly. BMC Infect. Dis. 2017, 17, 208. [Google Scholar]
- Servizio Sanitario Regione Emilia Romagna. Tariffario Vaccinazioni; AUSL Imola: Imola, Italy, 2019. [Google Scholar]
- Bonten, M.J.M.; Huijts, S.M.; Bolkenbaas, M.; Webber, C.; Patterson, S.; Gault, S.; van Werkhoven, C.H.; van Deursen, A.M.; Sanders, E.A.; Verheij, T.J.; et al. Polysaccharide Conjugate Vaccine against Pneumococcal Pneumonia in Adults. NEJM 2015, 372, 1114–1125. [Google Scholar]
- Mangen, M.-J.J.; Rozenbaum, M.H.; Huijts, S.M.; van Werkhoven, C.H.; Postma, D.F.; Atwood, M.; van Deursen, A.M.; van der Ende, A.; Grobbee, D.E.; Sanders, E.A.; et al. Cost-effectiveness of adult pneumococcal conjugate vaccination in the Netherlands. Eur. Respir. J. 2015, 46, 1407–1416. [Google Scholar]
- Klugman, K.P.; Madhi, S.A.; Huebner, R.E.; Kohberger, R.; Mbelle, N.; Pierce, N. A Trial of a 9-Valent Pneumococcal Conjugate Vaccine in Children with and Those without HIV Infection. NEJM 2003, 349, 1341–1348. [Google Scholar]
- AIFA. OSMED Interattivo. Available online: https://www.aifa.gov.it/osmed-interattivo (accessed on 28 June 2022).
- Boccalini, S.; Bechini, A.; Gasparini, R.; Panatto, P.; Amicizia, D.; Bonanni, P. Economic studies applied to vaccines against invasive diseases: An updated budget. Hum. Vaccines Immunother. 2017, 13, 417–422. [Google Scholar]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).