Prevalence and Risk Factors of Adverse Effects and Allergic Reactions after COVID-19 Vaccines in a Mexican Population: An Analytical Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Sources
2.2. Study Setting
2.3. Participants and Procedures
2.4. Measures, Variables, and Data Collection
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Naming the Coronavirus Disease (COVID-19) and the Virus that Causes It; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Chan, W.F.; Yuan, S.; Kok, K.; Wang To, K.; Chu, H.; Yang, J.; Xing, F.; BNurs, J.L.; Yip, C.C.-Y.; Poon, R.W.-S.; et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Z.; Ge, J. Scientific research progress of COVID-19/SARS-CoV-2 in the first five months. J. Cell Mol. Med. 2020, 24, 6558–6570. [Google Scholar] [CrossRef]
- Chilamakuri, R.; Agarwal, S. COVID-19: Characteristics and Therapeutics. Cells 2021, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Lurie, N.; Saville, M.; Hatchett, R.; Halton, J. Developing COVID-19 vaccines at pandemic speed. N. Engl. J. Med. 2020, 382, 1969–1973. [Google Scholar] [CrossRef]
- Qamar, N.; Rukh, G.; Khan, S. Vaccines for COVID-19: An insight on their effectiveness and adverse effects. J. Med. Vir. 2022, 94, 3554–3560. [Google Scholar] [CrossRef]
- Barouch, D.H. COVID-19 Vaccines—Immunity, Variants, Boosters. N. Engl. J. Med. 2022, 387, 1011–1020. [Google Scholar] [CrossRef]
- Hahn, W.; Wiley, Z. COVID-19 Vaccines. Infect. Dis. Clin. N. Am. 2022, 36, 481–494. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kanneganti, T.D. Innate immunity: The first line of defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef]
- Cirillo, N. Reported orofacial adverse effects of COVID-19 vaccines: The knowns and the unknowns. J. Oral Pathol. Med. 2021, 50, 424–427. [Google Scholar] [CrossRef]
- Aga Khames, Q.A.; Alkhaffaf, W.H.; Hatem, T.H.; Nassir, K.F.; Batineh, Y.; Dahham, A.; Shaban, D.; Aga Khames, L.; Agha, M.; Traqchi, M. Safety of COVID-19 vaccines. J. Med. Virol. 2021, 93, 6588–6594. [Google Scholar] [CrossRef] [PubMed]
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: An updated practice parameter. Ann. Allergy Asthma Immunol. 2010, 105, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Hee Chung, E. Vaccine allergies. Clin. Exp. Vaccine Res. 2014, 3, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Fathy, R.; McMahon, D.; Freeman, E. COVID-19 vaccines and the skin. The landscape of cutaneous vaccine reactions worldwide. Dermatol. Clin. 2021, 39, 653–673. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristic and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar] [CrossRef]
- Trougakos, P.I.; Terpos, E.; Alexopoulos, H.; Politou, M.; Paraskevis, D.; Scorilas, A.; Kastritis, E.; Andreakos, E.; Dimopoulos, M. Adverse effects of COVID-19 mRNA vaccines: The spike hypothesis. Trends Mol. Med. 2022, 28, 542–554. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Wu, B.-J.; Su, M.-C.; Lin, Y.-H.; Chiang, S.-C.; Wu, J.-C.; Chen, T.-J.; Chen, Y.-C. RiskFactors and Incidence Rates of Self-Reported Short-Term Adverse Events of COVID-19 Vaccine Booster Dose. Vaccines 2022, 10, 1115. [Google Scholar] [CrossRef]
- Camacho-Moll, M.E.; Salinas-Martínez, A.M.; Tovar-Cisneros, B.; García-Onofre, J.I.; Navarrete-Floriano, G.; Bermúdez-de León, M. Sec. Family Medicine and Primary Care. Front. Public Health 2022, 10, 1–12. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Strobe Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2017, 370, 1453–1457. [Google Scholar] [CrossRef]
- Secretaría de Salud. Estrategia Nacional de Vacunación.2022. COVID-19 Comunicado Técnico. 04 de Julio de 2022. 2022. Available online: https://www.gob.mx/salud/documentos/informe-tecnico-diario-covid19-2022 (accessed on 17 October 2022).
- Klein, N.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for adverse events after COVID-19 mRNA vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Bagher, A.M.; Binmahfouz, L.S.; Eid, B.G.; Almukadi, H.; Badr-Eldin, S.M.; El-Hamamsy, M.; Mohammedsaleh, Z.M.; Saleh, F.M.; Almuhayawi, M.S.; et al. The Adverse Reactions of Pfizer BioNTech COVID-19 Vaccine Booster Dose are Mild and Similar to the Second Dose Responses: A Retrospective Cross-Sectional Study. Int. J. Gen. Med. 2022, 15, 6821–6836. [Google Scholar] [CrossRef]
- Ruiz-Quiñones, J.A.; Narváez-Osorio, V.M.; Ulín-Tejeda, O.A.; Flores-Barrientos, O.I.; Suárez-Méndez, S.; Baeza-Flores, G.D.C. Side effects of the Pfizer BioNTech vaccine in health workers of a hospital in the southeast of Mexico. J. Infect. Dev. Ctries. 2022, 16, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Dighriri, I.M.; Alhusayni, K.M.; Mobarki, A.Y.; Aljerary, I.S.; Alqurashi, K.A.; Aljuaid, F.A.; Alamri, K.A.; Mutwalli, A.A.; Maashi, N.A.; Aljohani, A.M.; et al. Pfizer-BioNTech COVID-19 Vaccine (BNT162b2) Side Effects: A Systematic Review. Cureus 2022, 14, e23526. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Imamura, H.; Condon, S.; Boroughs, K.; Nilsson, S.C.; Anderson, T.; Caterson, I.D. Pfizer/BioNtech BNT162b2: Adverse events and insights from an Australian mass vaccination clinic for COVID-19. Intern. Med. J. 2022, 52, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111, Erratum in: Lancet 2021, 397, 98. [Google Scholar] [CrossRef]
- Paudel, S.; Poudel, B.; Gautam, S.; Sharma, P.; Uranw, S.; Sharma, S.K. ChAdOx1 nCoV-19 vaccine and its self-reported adverse events: A cross-sectional study from Western Nepal. J. Glob. Health Rep. 2021, 5, e2021069. [Google Scholar] [CrossRef]
- Alghamdi, A.N.; Alotaibi, M.I.; Alqahtani, A.S.; Al Aboud, D.; Abdel-Moneim, A.S. BNT162b2 and ChAdOx1 SARS-CoV-2 Post-vaccination Side-Effects Among Saudi Vaccinees. Front. Med. 2021, 8, 760047. [Google Scholar] [CrossRef]
- Al-Qazaz, H.K.; Al-Obaidy, L.M.; Attash, H.M. COVID-19 vaccination, do women suffer from more side effects than men? A retrospective cross-sectional study. Pharm. Pract. 2022, 20, 2678. [Google Scholar] [CrossRef]
- Green, M.S.; Peer, V.; Magid, A.; Hagani, N.; Anis, E.; Nitzan, D. Gender Differences in Adverse Events Following the Pfizer-BioNTech COVID-19 Vaccine. Vaccines 2022, 10, 233. [Google Scholar] [CrossRef]
- Xiong, X.; Yuan, J.; Li, M.; Jiang, B.; Lu, Z.K. Age and Gender Disparities in Adverse Events Following COVID-19 Vaccination: Real-World Evidence Based on Big Data for Risk Management. Front. Med. 2021, 8, 14. [Google Scholar] [CrossRef]
- Kadali, R.A.K.; Janagama, R.; Peruru, S.; Malayala, S.V. Side effects of BNT162b2 mRNA COVID-19 vaccine: A randomized, cross-sectional study with detailed self-reported symptoms from healthcare workers. Int. J. Infect. Dis. 2021, 106, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Lee, Y.W.; Lim, S.Y.; Lee, J.H.; Lim, J.S.; Lee, S.; Park, S.; Kim, S.K.; Lim, Y.J.; Kim, E.O.; et al. Adverse Reactions Following the First Dose of ChAdOx1 nCoV-19 Vaccine and BNT162b2 Vaccine for Healthcare Workers in South Korea. J. Korean Med. Sci. 2021, 36, e115. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Pokorná, A.; Mekhemar, M.; Conrad, J.; Klugarová, J.; Koščík, M.; Klugar, M.; Attia, S. Safety of ChAdOx1 nCoV-19 Vaccine: Independent Evidence from Two EU States. Vaccines 2021, 9, 673. [Google Scholar] [CrossRef] [PubMed]
- Yesuf, E.A.; Riad, A.; Sofi-Mahmudi, A.; Sudhakar, M.; Mekonnen, A.; Endalkachew, S.; Mama, F.; Muhidin, S.; Ayele, B.; Yahya, M.; et al. Self-reported side effects of the Oxford AstraZeneca COVID-19 vaccine among healthcare workers in Ethiopia, Africa: A cross-sectional study. Front. Public Health 2022, 10, 7794. [Google Scholar] [CrossRef] [PubMed]
| Sex | Males, n = 141 (31.80%) | Females, n = 302 (68.2%) | All, n = 443 (100%) | 100% | |
|---|---|---|---|---|---|
| Age # | 26.4 (±12.06) | 25.72 (±10.92) | 25.93 (±11.29) | ||
| n (%) | n (%) | n (%) | p Value | ||
| Comorbidities | 39 (37.2) | 66 (62.8) | 105 (23.7) | 0.1 | 0.7 (0.46 to 1.15) |
| Hypertension | 6 (50) | 6 (50) | 12 (2.7) | 0.1 | 0.4 (0.14 to 1.44) |
| Diabetes | 3 (37.5) | 5 (62.5) | 8 (1.8) | 0.4 | 0.7 (0.18 to 3.28) |
| Overweight or obesity | 17 (33.3) | 36 (66.6) | 54 (12.1) | 0.5 | 0.9 (0.53 to 1.84) |
| Thyroidal disorders | 0 (0) | 8 (100) | 8 (1.8) | <0.05 * | 1 (1 to 1.04) |
| Cancer | 1 (100) | 0 (0) | 1 (0.2) | 0.3 | 0.9 (0.97 to 1) |
| Rheumatological | 0 (0) | 1 (100) | 1 (0.2) | 0.4 | 1 (0.99 to 1.01) |
| Atopic disease | 23 (41.1) | 33 (58.9) | 56 (12.6) | 0.07 | 0.6 (0.35 to 1.12) |
| COVID-19 posterior to vaccination | 50 (35.5) | 91 (64.5) | 141 (31.8) | 0.2 | 0.7 (0.51 to 1.19) |
| Allergic reactions | 8 (14.3) | 48 (85.7) | 56 (12.6) | <0.001 ** | 3.1 (1.44 to 6.83) |
| Adverse effects | 83 (34.3) | 159 (65.7) | 242 (54.6) | 0.1 | 0.7 (0.51 to 1.16) |
| Vaccine Doses | No Allergic Reactions, n (%) | Allergic Reactions, n (%) | Fisher’s Test p Value | Odds Ratio (CI 95%) |
|---|---|---|---|---|
| Pfizer-BioNTech | 578 (45.4) | 66 (5.2) | 0.003 ** | 1.6 (1.18 to 2.3) |
| Other vaccines | 528 (41.5) | 100 (7.8) | ||
| Oxford-AstraZeneca | 441 (34.6) | 92 (7.2) | 0.002 ** | 1.87 (1.35 to 2.6) |
| Other vaccines | 665 (52.3) | 74 (5.8) | ||
| CanSino | 59 (4) | 5 (0.4) | 0.4 | 0.7 (0.27 to 1.64) |
| Other vaccines | 1047 (82.9) | 161 (12.6) | ||
| Moderna | 15(1) | 1 (0.07) | 0.7 | 2.2 (0.38 to 24.15) |
| Other vaccines | 1091 (86.3) | 165 (13) | ||
| Johnson and Johnson | 1 (0.07) | 0 (0) | 1 | Not enough subjects |
| Other vaccines | 1105 (86.9) | 166 (13.05) | ||
| Sinovac | 11 (0.086) | 1 (0.07) | 1 | 1.6 (0.29 to 18.01) |
| Other vaccines | 1095 (86) | 165 (13) | ||
| Sputnik V | 1 (0.07) | 1 (0.07) | 0.2 | 0.14 (0 to 2.8) |
| Other vaccines | 1105 (87) | 165 (13) |
| Vaccines | Total Doses That Caused Allergic Reactions | Chest Pain | Dyspnea | Hives | Anaphylaxis | Angioedema | Required Medical Attention |
|---|---|---|---|---|---|---|---|
| Pfizer-BioNTech | 66 | 40 (60.6%) | 35 (53%) | 8 (12.12%) | 4 (6%) | 1 (1.5%) | 0 |
| Oxford-AstraZeneca | 92 | 75 (81.5%) | 60 (65.2%) | 8 (8.7%) | 5 (5.4%) | 6 (6.5%) | 3 (3.3%) |
| CanSino | 5 | 5 (100%) | 4 (80%) | 3 (60%) | 0 | 1 (100%) | 0 |
| Moderna | 1 | 1 (100%) | 0 | 1 (100%) | 0 | 1 (100%) | 0 |
| Johnson and Johnson | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sinovac | 1 | 1 (100%) | 1 (100%) | 1 (100%) | 0 | 0 | 0 |
| Sputnik V | 1 | 1 (100%) | 1 (100%) | 0 | 0 | 0 | 0 |
| All vaccines | 166 | 123 (74.1%) | 101 (60.8%) | 18 (10.8%) | 9 (5.4%) | 9 (5.4%) | 3 (1.8%) |
| All Vaccines | Pfizer-BioNTech | AstraZeneca | CanSino | Moderna | Johnson and Johnson | Sinovac | Sputnik V | |
|---|---|---|---|---|---|---|---|---|
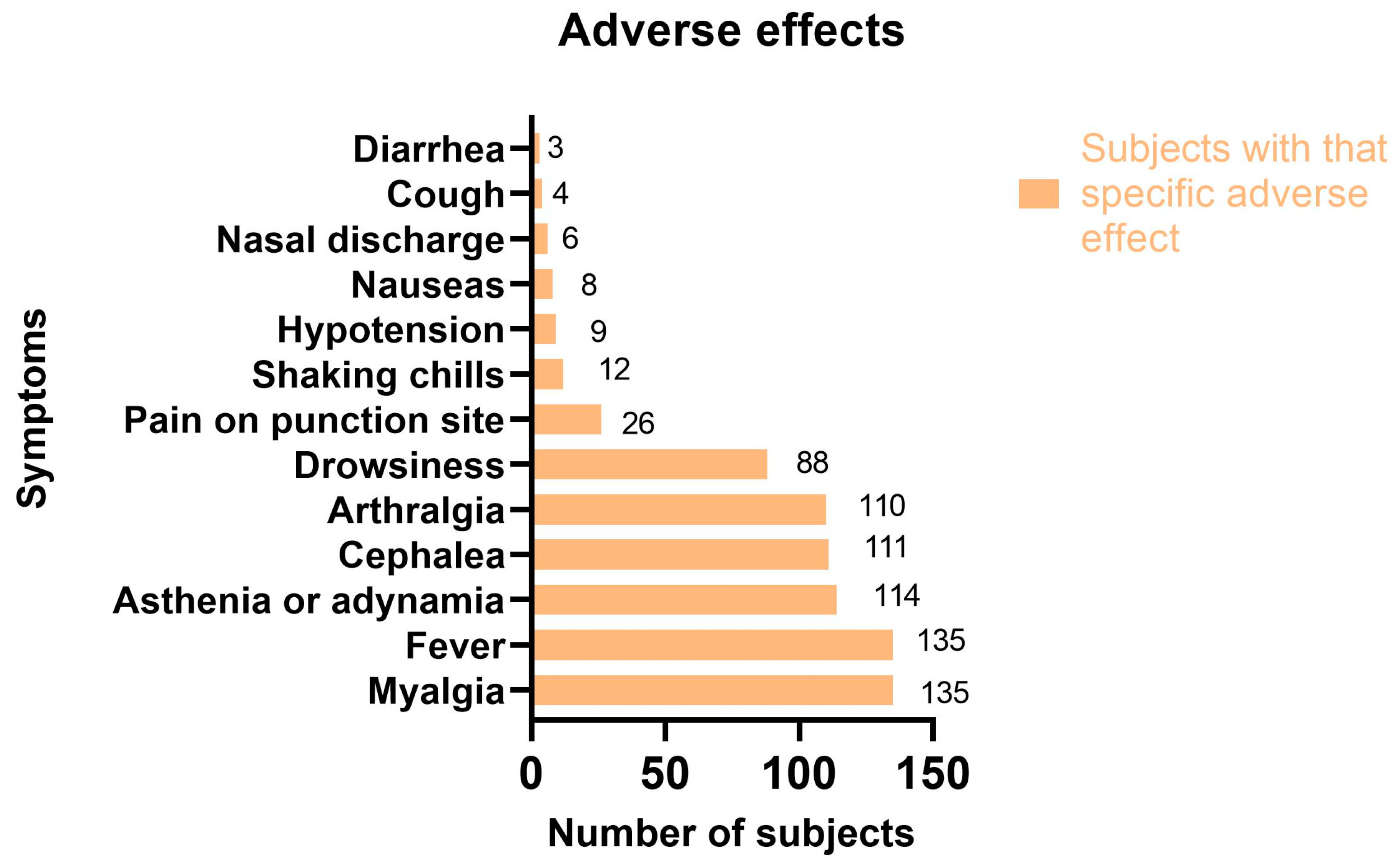
| Total adverse effects (% of all doses) | 727 | 329 (45.25%) | 334 (45.94%) | 50 (6.8%) | 12 (1.65%) | 0 | 1 (0.13%) | 1 (0.13%) |
| Myalgia | 658 (90.5%) | 300 (91.1%) | 306 (91.6%) | 42 (84%) | 8 (66.6%) | 0 | 1 (100%) | 1 (100%) |
| Fever | 623 (85.7%) | 278 (84.5%) | 300 (89.8%) | 40 (80%) | 4 (33.3%) | 0 | 1 (100%) | 0 |
| Asthenia or adynamia | 180 (24.7) | 78 (23.7%) | 60 (17.9%) | 34 (68%) | 6 (50%) | 0 | 1 (100%) | 1 (100%) |
| Cephalea | 71 (9.7%) | 20 (6%) | 35 (10.4%) | 10 (20%) | 4 (33.3%) | 0 | 1 (100%) | 1 (100%) |
| Arthralgia | 120 (16.5%) | 47 (14.2%) | 50 (14.9%) | 18 (36%) | 4 (33.3%) | 0 | 1 (100%) | 0 |
| Drowsiness | 119 (16.3%) | 60 (18.2%) | 45 (13.4%) | 12 (24%) | 1 (8.3%) | 0 | 1 (100%) | 0 |
| Pain at puncture site | 142 (19.5%) | 57 (17.3%) | 65 (19.4%) | 15 (30%) | 3 (25%) | 0 | 1 (100%) | 1 (100%) |
| Shaking chills | 116 (15.9%) | 52 (15.8%) | 63 (18.8%) | 1 (2%) | 0 | 0 | 0 | 0 |
| Hypotension | 11 (1.5%) | 5 (1.5%) | 4 (1.2%) | 2 (4%) | 0 | 0 | 0 | 0 |
| Nauseas | 10 (1.3% | 6 (1.8%) | 2 (0.6%) | 2 (4%) | 0 | 0 | 0 | 0 |
| Nasal discharge | 9 (1.2%) | 4 (1.2%) | 3 (0.9%) | 0 | 2 (16.6%) | 0 | 0 | 0 |
| Cough | 128 (1.6%) | 3 (0.9%) | 3 (0.9%) | 4 (8%) | 2 (0.6%) | 0 | 0 | 0 |
| Diarrhea | 4 (0.5%) | 2 (0.6%) | 2 (0.6%) | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Granados Villalpando, J.M.; Romero Tapia, S.d.J.; Baeza Flores, G.d.C.; Ble Castillo, J.L.; Juarez Rojop, I.E.; Lopez Junco, F.I.; Olvera Hernández, V.; Quiroz Gomez, S.; Ruiz Quiñones, J.A.; Guzmán Priego, C.G. Prevalence and Risk Factors of Adverse Effects and Allergic Reactions after COVID-19 Vaccines in a Mexican Population: An Analytical Cross-Sectional Study. Vaccines 2022, 10, 2012. https://doi.org/10.3390/vaccines10122012
Granados Villalpando JM, Romero Tapia SdJ, Baeza Flores GdC, Ble Castillo JL, Juarez Rojop IE, Lopez Junco FI, Olvera Hernández V, Quiroz Gomez S, Ruiz Quiñones JA, Guzmán Priego CG. Prevalence and Risk Factors of Adverse Effects and Allergic Reactions after COVID-19 Vaccines in a Mexican Population: An Analytical Cross-Sectional Study. Vaccines. 2022; 10(12):2012. https://doi.org/10.3390/vaccines10122012
Chicago/Turabian StyleGranados Villalpando, Jesús Maximiliano, Sergio de Jesus Romero Tapia, Guadalupe del Carmen Baeza Flores, Jorge Luis Ble Castillo, Isela Esther Juarez Rojop, Frida Isabel Lopez Junco, Viridiana Olvera Hernández, Sergio Quiroz Gomez, Jesús Arturo Ruiz Quiñones, and Crystell Guadalupe Guzmán Priego. 2022. "Prevalence and Risk Factors of Adverse Effects and Allergic Reactions after COVID-19 Vaccines in a Mexican Population: An Analytical Cross-Sectional Study" Vaccines 10, no. 12: 2012. https://doi.org/10.3390/vaccines10122012
APA StyleGranados Villalpando, J. M., Romero Tapia, S. d. J., Baeza Flores, G. d. C., Ble Castillo, J. L., Juarez Rojop, I. E., Lopez Junco, F. I., Olvera Hernández, V., Quiroz Gomez, S., Ruiz Quiñones, J. A., & Guzmán Priego, C. G. (2022). Prevalence and Risk Factors of Adverse Effects and Allergic Reactions after COVID-19 Vaccines in a Mexican Population: An Analytical Cross-Sectional Study. Vaccines, 10(12), 2012. https://doi.org/10.3390/vaccines10122012







