Adjuvant Treatment for Breast Cancer Patients Using Individualized Neoantigen Peptide Vaccination—A Retrospective Observation
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. DNA Sequencing and Bioinformatic Analyses
2.3. Vaccine Formulation and Administration
2.4. Immune Monitoring
3. Results
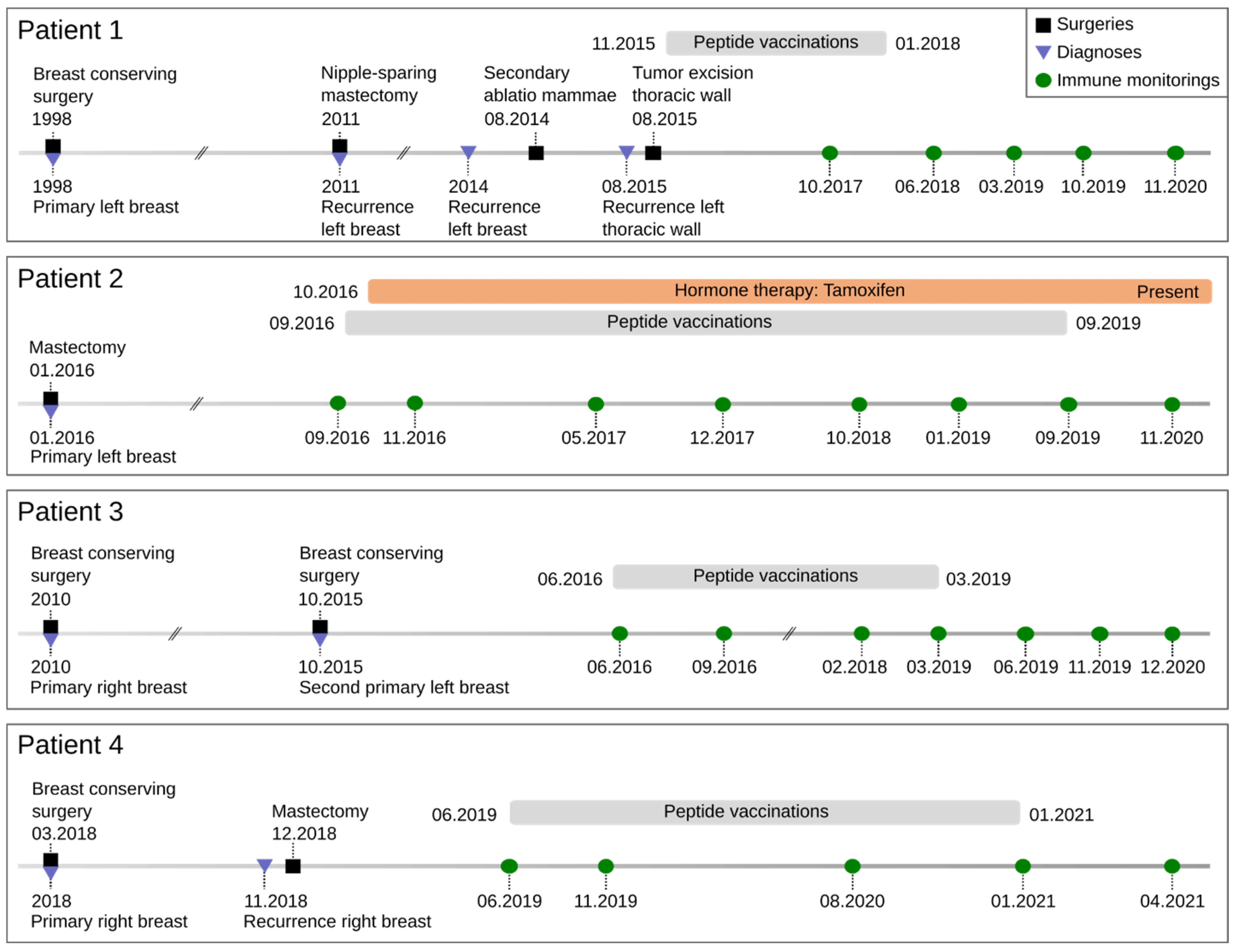
3.1. Demographic and Clinical Characteristics of the Patient Cohort
3.2. Vaccines
3.3. Safety
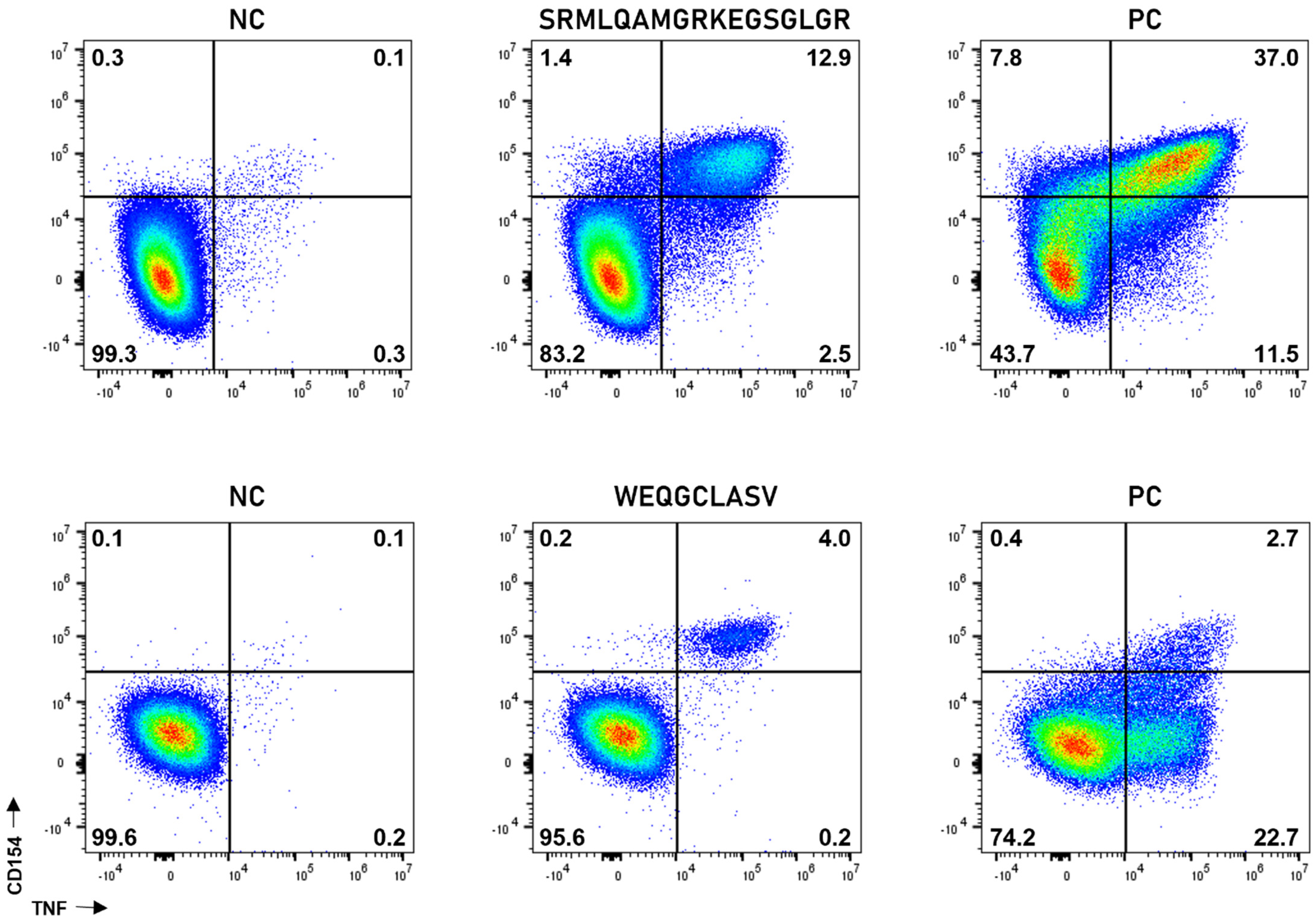
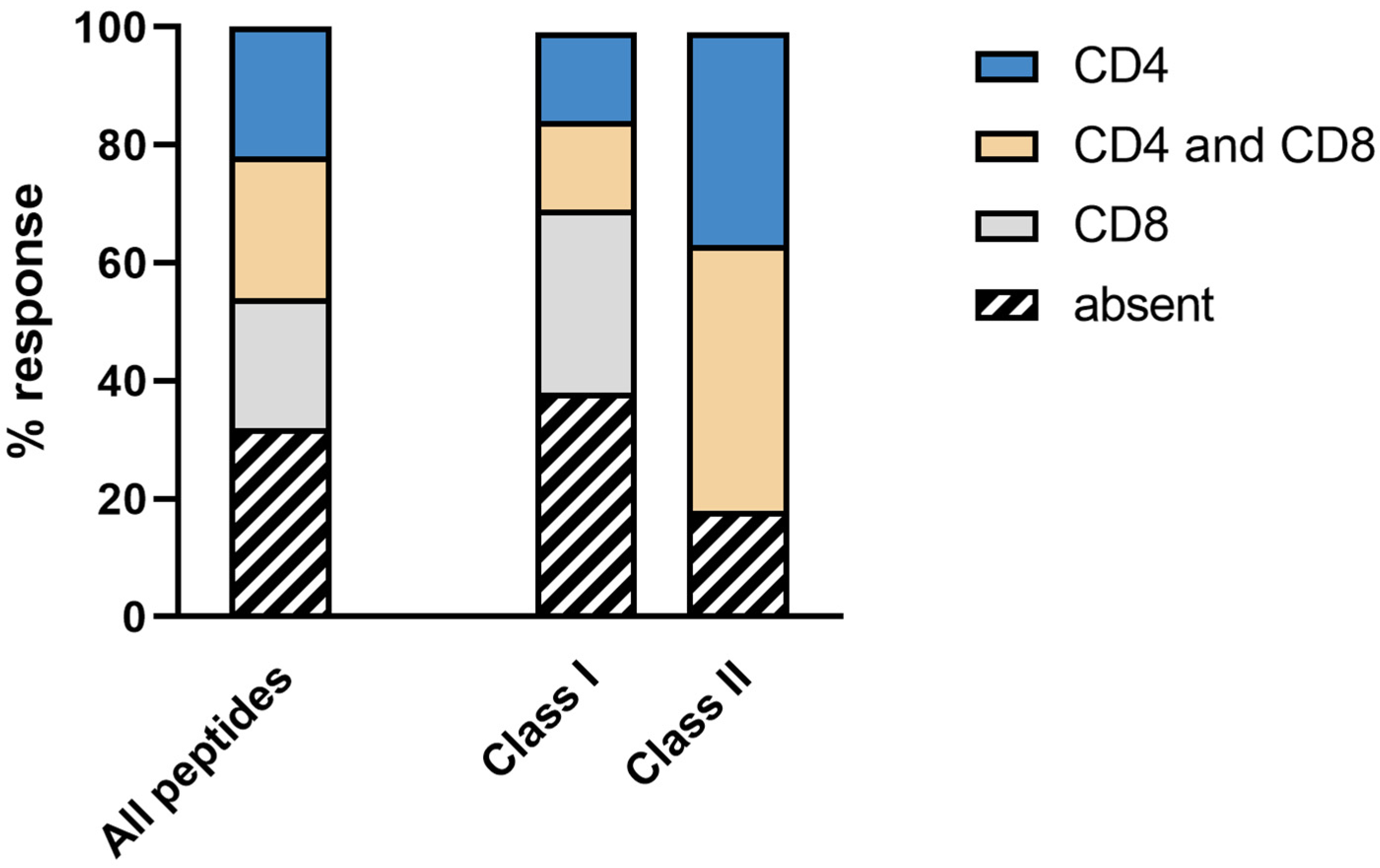
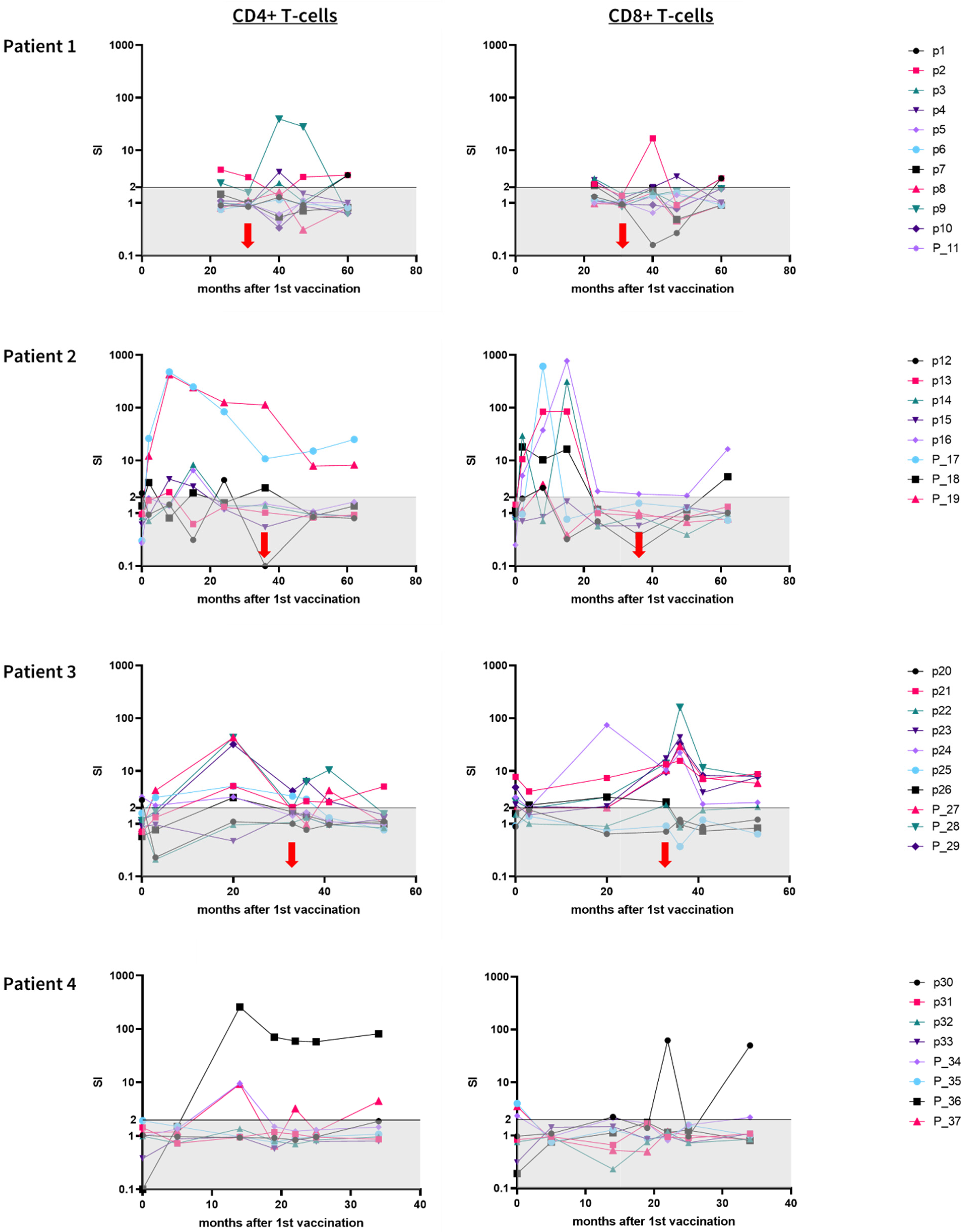
3.4. Immunogenicity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cullinane, C.M.; Byrne, J.; Akmenkalne, L.; O’Leary, D.P.; Connors, A.M.; Corrigan, M.A.; Redmond, H.P.; Kelly, L.; O’Sullivan, M.J. The LOCalizer Radiofrequency Identification System: An Effective New Technology for Localizing Non-Palpable Breast Lesions for Surgery. Surg. Innov. 2021, 28, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Skowronek, J.; Chicheł, A. Brachytherapy in breast cancer: An effective alternative. Prz. Menopauzalny Menopause Rev. 2014, 13, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, M.; Lee, A.; Gao, S.; Wang, D.; Barry, P.N.; Diaz, R.; Bagadiya, N.R.; Park, H.S.; Yu, J.B.; Wilson, L.D.; et al. Is Proton Therapy a “Pro” for Breast Cancer? A Comparison of Proton vs. Non-proton Radiotherapy Using the National Cancer Database. Front. Oncol. 2018, 8, 678. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.W.S.; Wu, M.; Cho, W.C.S.; To, K.K.W. Recent Advances in the Treatment of Breast Cancer. Front. Oncol. 2018, 8, 227. [Google Scholar] [CrossRef] [PubMed]
- PDQ Adult Treatment Editorial Board. Breast Cancer Treatment (Adult) (PDQ®): Patient Version. 6 October 2022. In PDQ Cancer Information Summaries [Internet]; National Cancer Institute: Bethesda, MD, USA, 2022. [Google Scholar] [PubMed]
- Blows, F.M.; Driver, K.E.; Schmidt, M.K.; Broeks, A.; Van Leeuwen, F.E.; Wesseling, J.; Cheang, M.C.U.; Gelmon, K.; Nielsen, T.O.; Blomqvist, C.; et al. Subtyping of Breast Cancer by Immunohistochemistry to Investigate a Relationship between Subtype and Short and Long Term Survival: A Collaborative Analysis of Data for 10,159 Cases from 12 Studies. PLoS Med. 2010, 7, e1000279. [Google Scholar] [CrossRef]
- Geurts, Y.M.; Witteveen, A.; Bretveld, R.; Poortmans, P.M.; Sonke, G.; Strobbe, L.J.A.; Siesling, S. Patterns and predictors of first and subsequent recurrence in women with early breast cancer. Breast Cancer Res. Treat. 2017, 165, 709–720. [Google Scholar] [CrossRef]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Iwasaki, A.; Omer, S.B. Why and How Vaccines Work. Cell 2020, 183, 290–295. [Google Scholar] [CrossRef]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2019, 120, 3210–3229. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, M.; Qin, Y.; Gao, W.; Tao, L.; Su, W.; Zhong, J. Neoantigen: A New Breakthrough in Tumor Immunotherapy. Front. Immunol. 2021, 12, 672356. [Google Scholar] [CrossRef] [PubMed]
- Slingluff, C.L. The present and future of peptide vaccines for cancer: Single or multiple, long or short, alone or in combination? Cancer J. 2011, 17, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Ellrott, K.; Bailey, M.H.; Saksena, G.; Covington, K.R.; Kandoth, C.; Stewart, C.; Hess, J.; Ma, S.; Chiotti, K.E.; McLellan, M.; et al. Scalable Open Science Approach for Mutation Calling of Tumor Exomes Using Multiple Genomic Pipelines. Cell Syst. 2018, 6, 271–281.e7. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, T.A.; Tolaney, S.M. Tumor mutational burden as a predictor of immunotherapy response in breast cancer. Oncotarget 2021, 12, 394–400. [Google Scholar] [CrossRef]
- Matsushita, H.; Vesely, M.D.; Koboldt, D.C.; Rickert, C.G.; Uppaluri, R.; Magrini, V.J.; Arthur, C.D.; White, J.M.; Chen, Y.S.; Shea, L.K.; et al. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 2012, 482, 400–404. [Google Scholar] [CrossRef]
- Delamarre, L.; Mellman, I.; Yadav, M. Cancer immunotherapy. Neo approaches to cancer vaccines. Science 2015, 348, 760–761. [Google Scholar] [CrossRef]
- Gouttefangeas, C.; Rammensee, H.-G. Personalized cancer vaccines: Adjuvants are important, too. Cancer Immunol. Immunother. 2018, 67, 1911–1918. [Google Scholar] [CrossRef]
- Hollingsworth, R.E.; Jansen, K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines 2019, 4, 7. [Google Scholar] [CrossRef]
- Varga, Z.; Lebeau, A.; Bu, H.; Hartmann, A.; Penault-Llorca, F.; Guerini-Rocco, E.; Schraml, P.; Symmans, F.; Stoehr, R.; Teng, X.; et al. An international reproducibility study validating quantitative determination of ERBB2, ESR1, PGR, and MKI67 mRNA in breast cancer using MammaTyper®. Breast Cancer Res. 2017, 19, 55. [Google Scholar] [CrossRef]
- Sonntag, K.; Hashimoto, H.; Eyrich, M.; Menzel, M.; Schubach, M.; Döcker, D.; Battke, F.; Courage, C.; Lambertz, H.; Handgretinger, R.; et al. Immune monitoring and TCR sequencing of CD4 T cells in a long term responsive patient with metastasized pancreatic ductal carcinoma treated with individualized, neoepitope-derived multipeptide vaccines: A case report. J. Transl. Med. 2018, 16, 23. [Google Scholar] [CrossRef]
- Shastri, N.; Schwab, S.; Serwold, T. Producing Nature’s Gene-Chips: The Generation of Peptides for Display by MHC Class I Molecules. Annu. Rev. Immunol. 2002, 20, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Blumendeller, C.; Boehme, J.; Frick, M.; Schulze, M.; Rinckleb, A.; Kyzirakos, C.; Kayser, S.; Kopp, M.; Kelkenberg, S.; Pieper, N.; et al. Use of plasma ctDNA as a potential biomarker for longitudinal monitoring of a patient with metastatic high-risk upper tract urothelial carcinoma receiving pembrolizumab and personalized neoepitope-derived multipeptide vaccinations: A case report. J. Immunother. Cancer 2021, 9, e001406. [Google Scholar] [CrossRef] [PubMed]
- Szolek, A.; Schubert, B.; Mohr, C.; Sturm, M.; Feldhahn, M.; Kohlbacher, O. OptiType: Precision HLA typing from next-generation sequencing data. Bioinformatics 2014, 30, 3310–3316. [Google Scholar] [CrossRef] [PubMed]
- Zelba, H.; Worbs, D.; Harter, J.; Pieper, N.; Kyzirakos-Feger, C.; Kayser, S.; Seibold, M.; Bartsch, O.; Ködding, J.; Biskup, S. A Highly Specific Assay for the Detection of SARS-CoV-2–Reactive CD4+ and CD8+ T Cells in COVID-19 Patients. J. Immunol. 2021, 206, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.-P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef] [PubMed]
- McNeel, D.G.; Becker, J.T.; Eickhoff, J.C.; Johnson, L.E.; Bradley, E.; Pohlkamp, I.; Staab, M.J.; Liu, G.; Wilding, G.; Olson, B.M. Real-Time Immune Monitoring to Guide Plasmid DNA Vaccination Schedule Targeting Prostatic Acid Phosphatase in Patients with Castration-Resistant Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 3692–3704. [Google Scholar] [CrossRef]
- Carreno, B.M.; Magrini, V.; Becker-Hapak, M.; Kaabinejadian, S.; Hundal, J.; Petti, A.A.; Ly, A.; Lie, W.-R.; Hildebrand, W.H.; Mardis, E.R.; et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015, 348, 803–808. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu-Lieskovan, S.; Chmielowski, B.; Govindan, R.; Naing, A.; Bhardwaj, N.; Margolin, K.; Awad, M.M.; Hellmann, M.D.; Lin, J.J.; et al. A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients with Advanced Melanoma, Non-small Cell Lung Cancer, or Bladder Cancer. Cell 2020, 183, 347–362.e24. [Google Scholar] [CrossRef]
- Walter, S.; Weinschenk, T.; Stenzl, A.; Zdrojowy, R.; Pluzanska, A.; Szczylik, C.; Staehler, M.; Brugger, W.; Dietrich, P.-Y.; Mendrzyk, R.; et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat. Med. 2012, 18, 1254–1261. [Google Scholar] [CrossRef]
- Mittendorf, E.A.; Lu, B.; Melisko, M.; Hiller, J.P.; Bondarenko, I.; Brunt, A.M.; Sergii, G.; Petrakova, K.; Peoples, G.E. Efficacy and Safety Analysis of Nelipepimut-S Vaccine to Prevent Breast Cancer Recurrence: A Randomized, Multicenter, Phase III Clinical Trial. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 4248–4254. [Google Scholar] [CrossRef] [PubMed]
- Bartelink, H.; Horiot, J.-C.; Poortmans, P.; Struikmans, H.; Bogaert, W.V.D.; Barillot, I.; Fourquet, A.; Borger, J.; Jager, J.; Hoogenraad, W.; et al. Recurrence Rates after Treatment of Breast Cancer with Standard Radiotherapy with or without Additional Radiation. N. Engl. J. Med. 2001, 345, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Brewster, A.M.; Hortobagyi, G.N.; Broglio, K.R.; Kau, S.-W.; Santa-Maria, C.A.; Arun, B.; Buzdar, A.U.; Booser, D.J.; Valero, V.; Bondy, M.; et al. Residual Risk of Breast Cancer Recurrence 5 Years after Adjuvant Therapy. J. Natl. Cancer Inst. 2008, 100, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelba, H.; McQueeney, A.; Rabsteyn, A.; Bartsch, O.; Kyzirakos, C.; Kayser, S.; Harter, J.; Latzer, P.; Hadaschik, D.; Battke, F.; et al. Adjuvant Treatment for Breast Cancer Patients Using Individualized Neoantigen Peptide Vaccination—A Retrospective Observation. Vaccines 2022, 10, 1882. https://doi.org/10.3390/vaccines10111882
Zelba H, McQueeney A, Rabsteyn A, Bartsch O, Kyzirakos C, Kayser S, Harter J, Latzer P, Hadaschik D, Battke F, et al. Adjuvant Treatment for Breast Cancer Patients Using Individualized Neoantigen Peptide Vaccination—A Retrospective Observation. Vaccines. 2022; 10(11):1882. https://doi.org/10.3390/vaccines10111882
Chicago/Turabian StyleZelba, Henning, Alex McQueeney, Armin Rabsteyn, Oliver Bartsch, Christina Kyzirakos, Simone Kayser, Johannes Harter, Pauline Latzer, Dirk Hadaschik, Florian Battke, and et al. 2022. "Adjuvant Treatment for Breast Cancer Patients Using Individualized Neoantigen Peptide Vaccination—A Retrospective Observation" Vaccines 10, no. 11: 1882. https://doi.org/10.3390/vaccines10111882
APA StyleZelba, H., McQueeney, A., Rabsteyn, A., Bartsch, O., Kyzirakos, C., Kayser, S., Harter, J., Latzer, P., Hadaschik, D., Battke, F., Hartkopf, A. D., & Biskup, S. (2022). Adjuvant Treatment for Breast Cancer Patients Using Individualized Neoantigen Peptide Vaccination—A Retrospective Observation. Vaccines, 10(11), 1882. https://doi.org/10.3390/vaccines10111882





