Abstract
Extracellular vesicles (EVs) produced by various immune cells, including B and T cells, macrophages, dendritic cells (DCs), natural killer (NK) cells, and mast cells, mediate intercellular communication and have attracted much attention owing to the novel delivery system of molecules in vivo. DCs are among the most active exosome-secreting cells of the immune system. EVs produced by cancer cells contain cancer antigens; therefore, the development of vaccine therapy that does not require the identification of cancer antigens using cancer-cell-derived EVs may have significant clinical implications. In this review, we summarise the molecular mechanisms underlying EV-based immune responses and their therapeutic effects on tumour vaccination.
1. Introduction
Extracellular vesicles (EVs) are lipid bilayer structures secreted by living cells and are classified into either exosomes, microvesicles (MVs), or apoptotic bodies, based on the intracellular production mechanism and size (Figure 1) [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]. Apoptotic bodies are released from apoptotic cells, whereas exosomes and MVs are released from healthy cells. Exosomes are endosomal membrane-derived vesicles, approximately 50–150 nm in size, formed during endocytosis and secreted by almost all types of cells, and are present in large numbers in body fluids such as blood, urine, cerebrospinal fluid, tears, and saliva. Their main constituents are lipids, proteins, and nucleic acids, including microRNAs (miRNAs), messenger RNA (mRNA), and DNA derived from secretory cells transferred to other cells [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]. Exosomes are involved in various physiological activities, such as immune regulation, neurodegeneration, and cancer development, as well as in the onset of disease mediated by intercellular communication involving the uptake of EVs into recipient cells [,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,,]. Hence, preventive, diagnostic, and therapeutic strategies that target or use exosomes are likely to be effective and have significant potential in a clinical setting. Exosomes contain multivesicular body-related proteins, such as apoptosis-linked gene 2-interacting protein X (Alix); tumour susceptibility gene 101 (TSG101); the endosomal sorting complex required for transport complex (in late endosomes); heat shock proteins, such as HSP70 and HSP90; proteins involved in intracellular transport, such as Rab GTPase; and transmembrane protein family tetraspanins, such as CD9, CD61, and CD81, in addition to endosome membrane-derived lipids, such as cholesterol and sphingomyelin, whose expression levels differ based on the cell type from which they are secreted [,,,,]. Exosomes are classified based on size; ~35 nm particles are referred to as exomeres, 60–80 nm particles as small exosomes, and 90–120 nm particles as large exosomes [,,,,,,,], all of which exhibit different expression patterns for proteins, lipids, nucleic acids, and N-glycans.
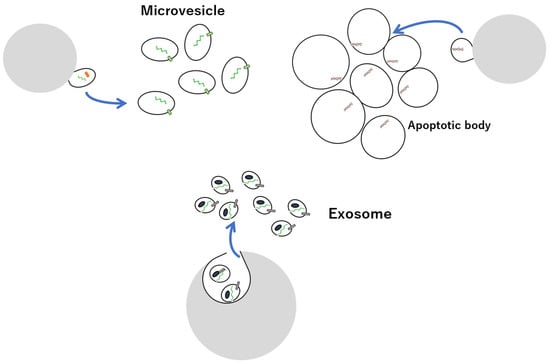
Figure 1.
Overview of EVs exosomes, microvesicles, and apoptotic bodies.
Conversely, MVs are vesicles with a wide range of sizes (100–1000 nm) and bud directly from the cell membrane into the outside of the cell [,]. Although MVs production mechanisms differ, many MVs are similar to exosomes, rendering them indistinguishable. Apoptotic bodies, micrometres in size, are particles in which apoptotic cells are displayed from the membrane and can be separated via low-speed centrifugation, hence are distinguishable from MVs and exosomes [,,,].
EVs are produced by various immune cells, including B and T cells, macrophages, dendritic cells (DC), natural killer (NK) cells, mast cells, and thymocytes [,,,,,] and are rich in proteins with immune functions, such as antigen-presenting molecules (major histocompatibility complex (MHC) class I, MHC class II, CD1), adhesion molecules (CD11b, intercellular adhesion molecule 1 (ICAM-1)), and co-stimulatory proteins (CD86) [,,,]. Additionally, exosomes are involved in releasing intracellular components to the outside of cells as waste products, and through their loaded immune-related molecules, they have various immune functions, such as antigen presentation, including the priming of early T cells, differentiation of mature T cells, development of effector functions, and regulation of immune-related cells [,,,,].
Vaccine therapy is based on antigen presentation attributed to heightened immune function of EVs produced by DCs and antigen-presenting cells (APCs) [,,,,]. Meanwhile, EVs produced by cancer cells contain cancer antigens; hence, the development of vaccine therapy that requires no identification of cancer antigens using cancer-cell-derived EVs is possible. EVs produced from mesenchymal stem cells (MSCs) have anti-inflammatory and tissue-regeneration properties, which may facilitate the development of immunotherapy [,,,]. Moreover, the development of novel immunotherapies that utilise EV characteristics, such as easy uptake by phagocytic cells or release from cells near target tissues, is underway [,]. In this review, we summarise EV-based vaccine therapies and novel immunotherapies.
2. EV-Based Vaccine Therapy for Cancer
2.1. Immune Response via APCs with Functions of Chemokine
Cancer antigens and neoantigens can induce strong immune responses. When a cancer vaccine is administered, it is first taken up by APCs, including DCs that migrate to the lymph node, thereby activating antigen-specific T cells leading to the initiation of an immune response [,,]. After undergoing positive and negative regulation, tumour-specific T cells exit the lymph node and migrate to the tumour site to eliminate cancer cells, although this process is strongly influenced by the immune environment created by the tumour [,,]. In the cancer immune cycle, cancer antigens are released from cancer cells that have experienced cell death and are taken up by DCs [,]. These DCs mature, migrate to the lymph nodes, and present the captured cancer antigen to the MHCI molecules, thereby activating T cells [,,,], which in turn migrate and infiltrate the tumour tissue to recognise and eliminate tumour cells. The interaction between T cell-expressed integrin alpha L (LFA1) and ICAM-1 on the vascular endothelium initiates T cell infiltration into tumours that are suppressed by vascular endothelial growth factor A (VEGF-A), which is produced by cancer cells, further releasing new cancer antigens and continuing the cancer immune cycle [,,]. In this process, the loss of beta-2 microglobulin, which is involved in the MHCI antigen presentation pathway in cancer cells, allows cancer cells to evade recognition by T cells [,,]. Cancer cells in which β-catenin signalling is activated have low chemokine (C-C motif) ligand 4 production and suppress the accumulation of conventional DC (cDC1), which produces chemokine (C-X-C motif) ligand 10 (CXCL10) in the tumour, thereby hindering T cell infiltration [,,]. The deletion of the phosphatase and tensin homologue (Pten) in cancer cells suppresses T cell infiltration into the tumour site [,,]. In this process, interferon-γ (IFN-γ), produced by T and NK cells, suppresses tumour cell proliferation and enhances MHCI antigen presentation. Additionally, the M1 type of tumour-associated macrophages (TAMs) promotes anti-tumour immunity [,,]. When cancer cells undergo immunogenic cell death, such as necrosis, high mobility group box 1, released at the same time as the antigen, acts on Toll-like receptors TLR2 and TLR4 on DCs to induce their maturation, leading to the efficient induction of anti-tumour immunity [,,]. Among the DCs subsets within tumours, having a large amount of cDC1 is beneficial, since they take up cancer antigens from tumours and migrate to lymph nodes, in which chemokine expressions, such as CXCL9, 10, and CCL5, in the tumour and intratumoural environment induce stronger T cell migration and cross-prime CD8+ T cells [,,]. Furthermore, NK cells in tumour tissues produce chemokines, such as CXCL1, CCL5, and FMS-like tyrosine kinase 3 ligands, which affect cDC1 []. The depletion of NK cells strongly suppresses the potentiation of immune checkpoint blockage action by interleukin (IL)-18, which regulates innate immunity, enhances the anti-tumour effect of immune checkpoint blockage through the induction of characteristic NK cells, which accumulate in the peritoneal cavity during early treatment prior to CD8+ T cells, and expresses CXCL1, whose depletion decreases the recruitment of CD103+CXCR1+ cDCs to the peritoneum [,,].
During antigen presentation by DCs, the expression of chemokine (C-C motif) receptor 7 (CCR7) on DCs induces migration to lymph nodes, where the chemokines CXCL13, CCL19, and CCL21 play a central role in adaptive immunity by exerting their effects via the actions of CXCL13 or CCL19 and CCL21 on receptors such as CXCR5 and CCR7, respectively [,,,]. These chemokines have two important functions in lymphocytes. First, interactions between CCL21, produced in the epithelial cells of lymph nodes, and CCR7, expressed on the surface of lymphocytes, activate integrins and induce adhesive responses. As a result, lymphocytes change shape and enter the lymph nodes through intercellular spaces that constitute high endothelial venules. Second, in the parenchyma of the lymph node, stromal cells secrete CCL19 and CCL21 around T cells, thereby promoting the accumulation of T cells that strongly express CCR7 [,]. In contrast, CXCL13 expressed in the B cell region attracts B cells expressing CXCR5. These T and B cells in turn migrate along networks shaped by stromal cells, forming a 3D microenvironment within a lymph node, such as follicular DCs in B cell follicles and reticular fibroblasts in T cell areas.
In addition, the CD40/CD40L immune checkpoint pathway, which rescues tumour cells from apoptosis, prolongs survival and enhances the proliferation, activation, or maturation of APCs, including primarily macrophages and DCs, to produce IL-12 or IL-18 to stimulate NK cells. These, in turn, produce IFN-γ and express co-stimulatory molecules, such as CD80/CD86, ICAM-1, and CD44, which are required for the non-energetic full activation of T cells following T cell receptor stimulation [,].
Furthermore, regulatory T cells in lymph nodes interact with cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), CD80, and CD86 on DCs to suppress T cell priming [,]. Anti-tumour immune responses are suppressed when cancer cells undergo tolerance-inducing cell death, such as apoptosis, during the release of cancer antigens []. Furthermore, cancer-associated fibroblasts (CAFs) create an immunosuppressive environment by remodelling the extracellular matrix of the tumour microenvironment [,,]. In many cancer patients, one or more of these steps in the cycle are impaired, thereby preventing the induction of effective cancer immune responses.
2.2. EV Application of Vaccine Therapy
Therapy using EVs derived from cancer cells has gained popularity since it contains cancer antigens and induces a cancer antigen-specific immune response, which provides an anti-tumour effect []. Although inducing anti-tumour immunity using cancer-cell-derived EVs has been reported, the effect remains to be verified. Insufficient dynamic control of EVs and the low delivery efficiency to APCs, along with insufficient antigen presentation efficiency due to the effect of immunostimulatory agents, adjuvants, or low delivery efficiency to APCs remain major challenges. Pharmacokinetics analysis has shown that intravenously administered EVs derived from cancer cells accumulated in some tissues, such as the liver, spleen, and lungs. In the liver and spleen, it is taken up by macrophages and by vascular endothelial cells in the lungs [,]. Consequently, they are quick to disappear from the blood. Conversely, topical administration, which is frequently used in vaccine administration, showed that EVs are rapidly taken up by macrophages and other cells. Therefore, for the development of vaccine therapy using cancer-cell-derived EVs, it is necessary to control their kinetics, that is, continuous antigen delivery to DCs by imparting retention of EV at the administration site, thereby conferring tropism to DCs and controlling the intracellular dynamics of engulfed DC [,,,,]. APCs, including DCs, are easy to use in large particles compared with other cell types.
Additionally, the disappearance of locally administered particles from the administration site is delayed as the size increases. This increase in size caused by EV formation aggregates may be used to confer APC tropism and retention at the administration site. The uptake of EV aggregates by DCs, in which EVs are linked by complementary strands of DNA, is increased by 2-fold, while the uptake into other cell types is reduced by approximately half. Furthermore, retention at the administration site following subcutaneous administration in mice has been shown to significantly increase due to aggregate formation. In mouse solid tumour models, EV aggregates are shown to induce anti-tumour immunity more efficiently than EVs and markedly delay tumour growth [,,]. Therefore, EV aggregation is a useful kinetic control method to obtain the kinetics required for vaccine therapy.
3. Antigen Presentation through EVs Derived from Cancer Cells
DCs are among the most active exosome-secreting cells in the immune system, and they take up foreign substances such as bacteria that invade the body into phagosomes, fragment them into antigenic peptides, and activate T cells by placing them on MHC class II molecules, where are they presented on the cell surface [,]. In contrast, intracellular antigenic peptides are presented by MHC class I molecules; however, exosomes secreted by DCs contain both MHC classes I and II. By binding to antigen-specific T cell receptors of CD8+ cytotoxic T cells and CD4+ helper T cells, T cells can be activated away from DCs (Figure 2). However, since the expression of co-stimulatory molecules, which are important for T cell activation, is lower on the exosome surface than DCs surface, the activation is as low as 5–10% compared with cases where DCs are activated by direct contact with T cells. Furthermore, by carrying antigen peptide/MHC complexes, exosomes not only play a direct antigen-presenting function but deliver exosomes into other APCs. It is also possible to indirectly promote antigen presentation by passing antigens to MHC molecules within cells [,,]. When antigens are presented by DCs that have endocytosed EVs derived from cancer cells, they are presented to MHC class II by degradation of the antigenic protein by endosomes, and to the MHC class I by degradation of the antigenic protein in the cytoplasm. In contrast, exosomes derived from phagocytic cells, such as macrophages, contain antigens derived from phagocytosed bacteria; it is possible to promote the efficient activation of T cells by transferring the antigen to DCs with stronger antigen-presenting ability via exosomes. When considering cancer therapy, the induction of cell-mediated immunity is desirable, where antigen presentation to MHC class I is important for this purpose. The escape of EVs from endosomes is useful for delivering endocytosed proteins to the cytoplasm, hence modifying cancer-cell-derived EVs with GALA peptide, a 30-amino-acid synthetic peptide with a glutamic acid–alanine–leucine–alanine (EALA) repeat, exerts a membrane-disrupting ability in a low-pH environment. Morishita et al. effectively illustrated the possibility to destroy lipid membranes at low pH, escape endosomes in engulfed DCs, and efficiently present cancer-cell-derived antigens to MHC class I on DCs [,,]. Thus, GALA modification promotes the transport of exosome inclusions into the cytoplasm and enhances the MHC class I presentation of cancer antigens based on the control of intracellular dynamics. Therefore, since cancer-cell-derived exosomes contain cancer antigens and can induce anti-tumour immune responses, they can be used as novel cancer vaccine formulations without the identification of cancer antigens. Furthermore, DC directivity can be conferred by a DC-directing ligand. To transfer CD40L to the surface of cancer-cell-derived EVs, a CD40L-LA (Lactadherin) fusion protein in which LA has an affinity for phosphatidylserine, a lipid present in the EV membrane, was used. Liu et al. revealed that CD40L-modified EVs show an increased ability to activate DCs than unmodified EVs [].
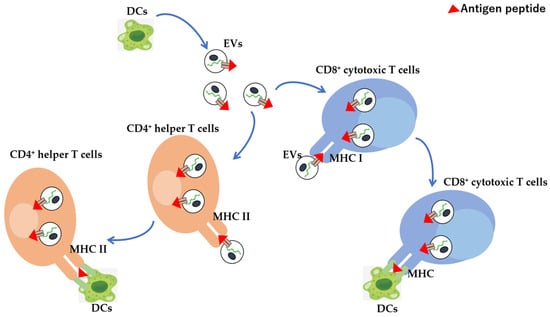
Figure 2.
EV-mediated antigen presentation to CD4+ helper or CD8+ cytotoxic T cells from dendritic cells. DC-derived EVs, which can migrate to tumours or the spleen directly or indirectly present antigens to CD4+ helper and CD8+ cytotoxic T cells via MHC molecules, thereby inducing immune responses. Red triangle: antigen peptide.
4. Antigen-Specific Immune Response in Cancer
The immune response that eliminates foreign substances such as bacteria and viruses does not attack host cells. This mechanism, referred to as “autoimmune tolerance”, is induced by eliminating harmful cells such as cancer cells and unnecessary cells in the body by phagocytes, such as macrophages and DCs [,]. This process prevents dead cells from releasing harmful substances and helps the surrounding tissues to maintain normal function.
However, if the elimination of dead cells is delayed, or the types of cells that eat dead cells change, immune tolerance breaks down. Cancer vaccines for cancer immunotherapy are composed of cancer antigens and adjuvants, which induce antigen-specific cytotoxic T cells through APC presentation and promote the exclusion of cancer cells.
When DCs that differentiate in vitro are loaded with tumour antigens and reinjected into the patient, they present antigens to naïve T cells. CD8+ naïve T cells are activated to differentiate into cytotoxic T cells, which results in antigen-specific antitumour activity. Conversely, CD4+ naïve T cells differentiate into helper T cells to assist cytotoxic T cells, which present antigens circulating in the bloodstream and improve disease conditions and the suppression of disease progression, even in patients with distant metastases. Further, some anti-tumour effects of DCs are retained in memory T cells. The activation of DCs that have taken up cancer antigens is important for the induction of cancer antigen-specific immune responses. Therefore, developing a method to deliver cancer-cell-derived EVs and adjuvants to the same DCs will have significant therapeutic implications. The modification of cancer-cell-derived exosomes containing cancer antigens with an adjuvant induces potent anti-tumour immunity through the simultaneous delivery of cancer antigens and adjuvants to DCs. Morishita et al. reported that cancer-cell-derived EVs were modified with an SAV-LA fusion protein between biotin-binding protein streptavidin (SAV) and LA, reacting with a biotin derivative of CpG DNA, an immunostimulatory nucleic acid []. This nucleic acid has attracted attention as an adjuvant consisting of unmethylated cytosine and guanine (CpG), frequently present in the genomic DNA (CpG-DNA) of bacteria and viruses for host defence in mammalian innate immunity, in which two sequences, K type and D type, are particularly effective for CpG-DNA. The K type mainly induces the proliferation of B cells and production of cytokines such as IL-6, while the D type induces the production of the type I interferon from plasmacytoid DCs. The mechanism underlying immune activation involves the binding of CpG-DNA to TLR9 in the endosome, which induces the nuclear factor kappa B (NF-κB) pathway through the adaptor molecule myeloid differentiation primary response gene 88, thereby inducing the production of various cytokines [,,].
When CpG DNA-modified EV was added, uptake into DCs increased compared to the addition of CpG DNA alone. Therefore, CpG DNA modification of EVs enables the simultaneous delivery of cancer antigens and adjuvant CpG DNA to the same cell []. Additionally, DCs supplemented with CpG DNA-modified EVs showed a higher cytokine production than those with CpG DNA or EVs alone. Furthermore, in an in vivo experimental system using tumour-bearing mouse models, CpG DNA-modified EVs can induce cancer antigen-specific cellular and humoral immune responses and significantly suppress tumour growth and metastasis to the lungs, thereby prolonging survival [,,]. Therefore, the CpG DNA modification of cancer-cell-derived EVs enables the simultaneous delivery of cancer antigen and adjuvants to the same DCs and the induction of anti-tumour immunity.
5. DC-Derived EVs as Prospective Vaccines
DCs, which serve as a link between innate and adaptive immunity, are involved in the initiation and suppression of immune responses. Antigen presentation to naïve cytotoxic and helper T cells is performed through MHC classes I and II molecules, respectively. Molecules contained in DC-derived EVs are MHC class I/II and CD86 proteins, which are capable of mediating antigen presentation to CD8+ and CD4+ T cells and the subsequent proliferation of T cells [].
CD1a, b, c, or d proteins are involved in the presentation of lipid antigens, while ICAM-1 plays an important role in regulating DC-T cell communication, where ICAM-1 can either promote the uptake of DC-derived EVs by target DCs or the interaction of T cells with DCs that retain DC-derived EVs on their outer surface as ligands for Mac1 integrin (CD11b/CD18) and lymphocyte function-associated antigen 1 (LFA1, CD11a/CD18). Additionally, DC-derived EVs are rich in tetraspanins, including CD9, CD37, CD53, CD63, CD81, and CD82, which regulate DC interactions. Thus, DC-derived EVs can induce cellular and humoral immunity by antigen presentation via DCs that have incorporated them. A clinical trial for vaccine therapy using DC-derived EVs has been conducted, showing that although its safety has been confirmed, its therapeutic effect is limited. Ovalbumin (OVA) has been previously used as a model antigen; EVs were recovered from DCs spiked with OVA, along with LPS and IFN-γ as activators of DCs, where it carried OVA, in addition to MHC class I, antigen presentation, and co-stimulatory molecules such as CD86 [,,]. As DC-derived EVs have a strong ability to activate immune cells, the addition of macrophages and DCs further exacerbates the activation of these cells. These DC-derived EV-loaded DCs can present antigens to T cells, while the DC-derived EVs can directly present antigens to T cells. Furthermore, the administration of DC-derived EVs to tumour-bearing model mice, established by transplanting OVA-expressing cancer cells, induced cellular and humoral immunity specific to the loaded OVA antigen thereby displaying anti-tumour effects. Thus, EVs recovered from activated DCs have properties that contribute to vaccine therapy, while the optimisation of the activation state of DCs may produce EVs with enhanced activity.
6. Application of MSC-Derived EVs in Modulating the Immune Response
MSCs are fibroblast-like progenitor cells recovered from the bone marrow, adipose tissue, and umbilical cord. They have adipogenic, chondrogenic, and osteogenic differentiation potential [,,,,,,,]. MSCs do not express human leukocyte antigen (HLA) class II antigens; therefore, they are not only less immunogenic in allogeneic transplantation but are also capable of suppressing the function of various immune cells. To date, clinical trials have been conducted using MSCs as novel therapeutic agents to treat various diseases, including acute diseases, such as ischaemic stroke and myocardial infarction. MSCs are also intended anti-inflammatory therapy for diseases caused by uncontrolled inflammatory reactions, such as acute graft-versus-host disease (GvHD) and Crohn’s disease [,,,].
Initially, MSCs were characterised by their cell differentiation potential and their direct interaction with immune cells. However, it has become clear that the immunoregulatory ability of MSCs is based on paracrine action; EVs containing marker proteins, such as CD9, CD81, and ALIX, at approximately 100 nm from MSC culture supernatant have therapeutic effects on disease model mice. Furthermore, in a clinical trial where EVs recovered from steroid-refractory GvHD patients, increasing the dose of MSC-derived EVs resulted in a long-term reduction in GvHD symptoms and reduced steroid dose. The addition of MSC-derived EVs to patient-derived peripheral blood cells suppressed the secretion of the inflammatory cytokines IL-1β, tumour necrosis factor-alpha (TNFα), and IFNγ []. Moreover, MSC-derived EVs comprised anti-inflammatory cytokines, transforming growth factor-β (TGF-β), IL-10, and HLA-G, further improving GvHD symptoms and chronic kidney disease. Notably, the levels of TGF-β and IL-10 in peripheral blood were significantly elevated relatively early following administration of MSC-derived EVs, and their levels remained high even after one year. Conversely, the inflammatory cytokine TNFα was significantly suppressed by the administration of MSC-derived EVs.
In addition to GvHD, MSC therapy may be clinically applied to other diseases such as severe heart failure and type I diabetes [,,,]. The administration of MSCs improves heart failure in the disease model mice, and adiponectin produced by adipocytes increases the exosomes produced by MSCs, thereby promoting their therapeutic effects. Furthermore, in a mouse model that developed diabetes by inhibiting the binding of Programmed Cell Death Protein 1/Programmed Cell Death Ligand 1 (PD-L1), immune cell infiltration into the space between pancreatic insulin-producing cells was observed using immune checkpoint inhibitors. In particular, there was a marked increase in cytotoxic macrophages that destroyed pancreatic insulin-producing cells. The administration of human adipose tissue-derived MSCs to these mice suppressed the infiltration of immune cells and the onset of diabetes. Furthermore, following the administration of MSCs, a marked increase in MSC-derived exosomes in the blood of mice was observed, suggesting that humoral factors such as exosomes may be involved in the suppression of diabetes development. The infiltration of immune cells, such as cytotoxic macrophages, was also observed in human islets following the administration of immune checkpoint inhibitors. Thus, humoral factors, such as exosomes produced by human adipose tissue-derived MSCs, suppress the onset of type 1 diabetes induced by immune checkpoint inhibition.
7. EV-Based Immunotherapy
7.1. Immune-Related Molecules Loaded into Exosomes
EVs not only contain protein antigens but also secretory cell-derived mRNAs and non-coding RNAs, especially miRNAs, and their functions have attracted much attention. These RNAs are protected by the lipid bilayer membrane of exosomes, and thus are not degraded by RNases and remain stable in the blood and body fluids. While there are RNAs detected in both exosomes and their secreting cells, there are others that can only be detected in either, suggesting the existence of a mechanism to selectively incorporate specific RNAs into exosomes taken up by target cells fused with the endosomal membrane. This releases trapped RNA into the target cell cytoplasm. In this study, the released mRNAs are translated into proteins, miRNAs suppress the translation of target genes, while exosomes regulate gene expression in target cells. Immune cells that encounter a foreign object horizontally transmit their activation state to cells that have not yet encountered it through RNA. In addition, various immune-related molecules are loaded into exosomes, which in turn regulate the immune responses. For example, exosomes derived from cytotoxic T cells and NK cells carry TNF family proteins, such as Fas ligand, TRAIL, and CD40 ligand, and induce apoptosis in target cells []. Similarly, some cancer cells release exosomes loaded with the Fas ligand and TRAIL, which induce apoptosis in immune cells, thereby escaping immune attack. The TNF family proteins are produced in a membrane-bound form and cleaved by a membrane-type metalloprotease to become soluble. Apoptosis-inducing activity is mainly mediated by the membrane form, while the activity of the soluble form is weak. Moreover, TNF family proteins on exosomes are stable without being cleaved by membrane metalloproteases and have strong apoptosis-inducing activity by forming trimers through the membrane. The exosome-mediated transport of TNF family proteins is involved in the development of various inflammatory and autoimmune diseases. For example, exosomes released from synovial fibroblasts of patients with rheumatoid arthritis accumulate high concentrations of membrane-bound TNF-α, exacerbating the pathology of rheumatoid arthritis [,]. Further, cancer-cell-derived exosomes have various immunosuppressive effects in addition to inducing apoptosis in immune cells. For example, exosomes suppress the expression of the NKG2D receptor in NK cells, which is involved in the recognition mechanism of cancer cells and reduces cancer cytotoxicity [,,,].
In contrast, when cancer-cell-derived exosomes are taken up by monocytes, the effects of TGF-β and prostaglandin E2 contained in the exosomes induce the differentiation of monocytes into bone-marrow-derived immunosuppressive cells, which release various immunosuppressive molecules such as IL-10, thereby promoting the inactivation of immunocompetent cells and the induction of regulatory T cells to suppress anti-tumour immunity []. Through these mechanisms, cancer cells may suppress the functions of the immune cells that attack them and promote cancer progression.
7.2. EV-Mediated Cytokine Enhancement and Tumour Progression
Cytokines are paracrine factors involved in the immune system where EV-mediated cytokine enhancement has been reported. For example, IFN-γ bound to its receptor on the EV surface stimulates target cells more efficiently than IFN-γ alone. Additionally, for the antagonism of CD47, a “Not Eat Me” signal is overexpressed on the surface of most tumours by a signal-regulatory protein alpha. This protein is expressed on phagocytic cells that interact with CD47-bearing EVs, inhibiting CD47 on cancer cells and thereby increasing cancer cell phagocytosis and inducing anti-tumour T cell responses [,,,]. Additionally, EVs have been shown to evade immune clearance better than artificial liposomes, likely due to the expression of CD47 on exosomal membranes. Furthermore, CD47 is overexpressed in tumours where its expression is associated with poor progression-free survival and inversely correlated with macrophage infiltration in tumour tissues loaded on exosomes, thereby promoting tumour immune evasion. The inhibition of exosome secretion or uptake via the knockdown of RAB27A, a regulator of exosome secretion, reduces the expression of CD47 in tumour cells, promotes phagocytosis by M1 macrophages in tumour tissues, and suppresses tumour progression []. siRNA-loaded exosomes are selectively taken up by pancreatic tumour cells, which is facilitated by enhanced micropinocytosis. This reduces oncogenic KRAS signalling and suppresses tumour growth in multiple mouse models of pancreatic cancer []. Therefore, increasing cancer cell phagocytosis by antagonising CD47 signalling with EVs may be an effective approach for cancer immunotherapy. Furthermore, anti-PD-L1 therapy using platelets and platelet-derived EVs has also been reported, where the PD-L1 monoclonal antibody is bound to the surface of platelets [,]. When these platelets are intravenously administered, they become activated and release EVs into tumour tissue. In contrast, anti-PD-L1-bound platelets are activated in the tumour microenvironment and release anti-PD-L1-bound EVs, which inhibit immune checkpoints in tumour tissue, thereby increasing the number of infiltrating CD8+ and CD4+ T cells within the tumour. In addition, the number of CD4+ Foxp3+ T cells was reduced, while the proliferation of CD8+ and CD4+ effector T cells within the tumour was enhanced. Furthermore, the administration of anti-PD-L1 antibody-conjugated platelets suppressed cancer growth and metastasis in tumour-bearing mice and significantly prolonged their survival. This suggests that platelet-secreted EVs are effective delivery carriers that inhibit immune checkpoint molecules. In addition, based on the susceptibility of EVs to macrophage uptake, anti-inflammatory therapy has been reported by loading EVs with the NF-κB binding domain (NBD), an inhibitory peptide of NF-κB, which is a transcriptional factor that promotes inflammation and delivers EVs to macrophages [,,]. For this strategy, a fusion protein is prepared in which Gag, an EV inner-membrane tropic protein, is fused with NBD. The addition of Gag-NBD-loaded EVs to LPS-stimulated macrophages significantly suppressed macrophage inflammatory responses, that is, inflammatory cytokine production, nitric oxide (NO) synthase induction, and subsequent NO production [,]. Therefore, EVs-mediated immune responses are essential factors for tumour progression.
8. Conclusions
EVs derived from cancer cells contain cancer antigens, and their administration induces a cancer antigen-specific immune response, thereby exerting an anti-tumour effect. Therapeutic studies using mouse solid tumour models have shown that EV aggregates can induce anti-tumour immunity more efficiently than EVs alone and markedly delay tumour growth. When cancer-cell-derived exosomes are taken up by monocytes, the TGF-β and prostaglandin E2 contained in the exosomes induce the differentiation of monocytes into bone-marrow-derived immunosuppressive cells, which in turn release various immunosuppressive molecules, such as IL-10, thereby promoting the inactivation of immunocompetent cells and the induction of regulatory T cells to suppress anti-tumour immunity. Understanding the molecular mechanisms in which EVs-mediated immunosuppression will help to develop a novel therapeutic strategy against cancers.
Author Contributions
Y.M. and R.Y. drafted the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Fukuda Foundation for Medical Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Shefer, A.; Yalovaya, A.; Tamkovich, S. Exosomes in Breast Cancer: Involvement in Tumor Dissemination and Prospects for Liquid Biopsy. Int. J. Mol. Sci. 2022, 23, 8845. [Google Scholar] [CrossRef]
- Gul, B.; Syed, F.; Khan, S.; Iqbal, A.; Ahmad, I. Characterization of extracellular vesicles by flow cytometry: Challenges and promises. Micron 2022, 161, 103341. [Google Scholar] [CrossRef]
- Hermann, D.M.; Xin, W.; Bähr, M.; Giebel, B.; Doeppner, T.R. Emerging roles of extracellular vesicle-associated non-coding RNAs in hypoxia: Insights from cancer, myocardial infarction and ischemic stroke. Theranostics 2022, 12, 5776–5802. [Google Scholar] [CrossRef]
- Khan, F.H.; Reza, M.J.; Shao, Y.F.; Perwez, A.; Zahra, H.; Dowlati, A.; Abbas, A. Role of exosomes in lung cancer: A comprehensive insight from immunomodulation to theragnostic applications. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188776. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Yoshioka, Y.; Sakamoto, S.; Ichikawa, T.; Ochiya, T. Extracellular vesicles in bone homeostasis: Key roles of physiological and pathological conditions. J. Bone Miner. Metab. 2022; in press. [Google Scholar] [CrossRef]
- De Sousa, K.P.; Rossi, I.; Abdullahi, M.; Ramirez, M.I.; Stratton, D.; Inal, J.M. Isolation and characterization of extracellular vesicles and future directions in diagnosis and therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2022, 27, e1835. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.Y.; Kee, L.T.; Al-Masawa, M.E.; Lee, Q.H.; Subramaniam, T.; Kok, D.; Ng, M.H.; Law, J.X. Scalable Production of Extracellular Vesicles and Its Therapeutic Values: A Review. Int. J. Mol. Sci. 2022, 23, 7986. [Google Scholar] [CrossRef]
- Procyk, G.; Bilicki, D.; Balsam, P.; Lodziński, P.; Grabowski, M.; Gąsecka, A. Extracellular Vesicles in Atrial Fibrillation-State of the Art. Int. J. Mol. Sci. 2022, 23, 7591. [Google Scholar] [CrossRef]
- Ramos-Zaldívar, H.M.; Polakovicova, I.; Salas-Huenuleo, E.; Corvalán, A.H.; Kogan, M.J.; Yefi, C.P.; Andia, M.E. Extracellular vesicles through the blood-brain barrier: A review. Fluids Barriers CNS 2022, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Luo, H.; Lee, J.W. Role of extracellular vesicles in lung diseases. Chin. Med. J. 2022; in press. [Google Scholar] [CrossRef]
- Arifin, D.R.; Witwer, K.W.; Bulte, J.W.M. Non-Invasive imaging of extracellular vesicles: Quo vaditis in vivo? J. Extracell. Vesicles 2022, 11, e12241. [Google Scholar] [CrossRef]
- Zeng, Y.; Qiu, Y.; Jiang, W.; Shen, J.; Yao, X.; He, X.; Li, L.; Fu, B.; Liu, X. Biological Features of Extracellular Vesicles and Challenges. Front. Cell Dev. Biol. 2022, 10, 816698. [Google Scholar] [CrossRef] [PubMed]
- Kee, L.T.; Ng, C.Y.; Al-Masawa, M.E.; Foo, J.B.; How, C.W.; Ng, M.H.; Law, J.X. Extracellular Vesicles in Facial Aesthetics: A Review. Int. J. Mol. Sci. 2022, 23, 6742. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Yashiro, R. Therapeutic Strategy of Mesenchymal-Stem-Cell-Derived Extracellular Vesicles as Regenerative Medicine. Int. J. Mol. Sci. 2022, 23, 6480. [Google Scholar] [CrossRef]
- Vinaiphat, A.; Sze, S.K. Proteomics for comprehensive characterization of extracellular vesicles in neurodegenerative disease. Exp. Neurol. 2022, 355, 114149. [Google Scholar] [CrossRef]
- Han, C.; Qin, G. Reporter Systems for Assessments of Extracellular Vesicle Transfer. Front. Cardiovasc. Med. 2022, 9, 922420. [Google Scholar] [CrossRef]
- Suades, R.; Greco, M.F.; Padró, T.; Badimon, L. Extracellular Vesicles as Drivers of Immunoinflammation in Atherothrombosis. Cells 2022, 11, 1845. [Google Scholar] [CrossRef]
- Trisko, J.; Fleck, J.; Kau, S.; Oesterreicher, J.; Holnthoner, W. Lymphatic and Blood Endothelial Extracellular Vesicles: A Story Yet to Be Written. Life 2022, 12, 654. [Google Scholar] [CrossRef]
- Anusha, R.; Priya, S. Dietary Exosome-Like Nanoparticles: An Updated Review on Their Pharmacological and Drug Delivery Applications. Mol. Nutr. Food Res. 2022, 66, e2200142. [Google Scholar] [CrossRef]
- Vafaei, S.; Mansoori, M.; Hashemi, F.; Basiri, M. Exosome Odyssey to Original Line in Dental Regeneration. J. Oral. Biosci. 2022, 64, 271–278. [Google Scholar] [CrossRef]
- Ginini, L.; Billan, S.; Fridman, E.; Gil, Z. Insight into Extracellular Vesicle-Cell Communication: From Cell Recognition to Intracellular Fate. Cells 2022, 11, 1375. [Google Scholar] [CrossRef]
- Yang, J.; Shin, T.S.; Kim, J.S.; Jee, Y.K.; Kim, Y.K. A new horizon of precision medicine: Combination of the microbiome and extracellular vesicles. Exp. Mol. Med. 2022, 54, 466–482. [Google Scholar] [CrossRef]
- Wu, Q.; Duan, W.Z.; Chen, J.B.; Zhao, X.P.; Li, X.J.; Liu, Y.Y.; Ma, Q.Y.; Xue, Z.; Chen, J.X. Extracellular Vesicles: Emerging Roles in Developing Therapeutic Approach and Delivery Tool of Chinese Herbal Medicine for the Treatment of Depressive Disorder. Front. Pharmacol. 2022, 13, 843412. [Google Scholar] [CrossRef]
- Bağcı, C.; Sever-Bahcekapili, M.; Belder, N.; Bennett, A.P.S.; Erdener, Ş.E.; Dalkara, T. Overview of extracellular vesicle characterization techniques and introduction to combined reflectance and fluorescence confocal microscopy to distinguish extracellular vesicle subpopulations. Neurophotonics 2022, 9, 021903. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; Hochreiter, B.; Schmid, J.A. Extracellular Vesicles Linking Inflammation, Cancer and Thrombotic Risks. Front. Cell Dev. Biol. 2022, 10, 859863. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Tang, F.; Li, J.; Yu, H.; Wu, M.; Wu, Y.; Zeng, H.; Hou, K.; Zhang, Q. Tumor-derived exosomes: The emerging orchestrators in melanoma. Biomed. Pharmacother. 2022, 149, 112832. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.; Liang, Y.; Cai, H.; Wang, F.; Chen, X.; Yin, Q.; Wang, K.; Wang, Y. Emerging function and clinical significance of extracellular vesicle noncoding RNAs in lung cancer. Mol. Ther. Oncolytics 2022, 24, 814–833. [Google Scholar] [CrossRef] [PubMed]
- Mun, D.; Oh, S.; Kim, Y. Perspectives on Bovine Milk-Derived Extracellular Vesicles for Therapeutic Applications in Gut Health. Food Sci. Anim. Resour. 2022, 42, 197–209. [Google Scholar] [CrossRef]
- Nafar, S.; Nouri, N.; Alipour, M.; Fallahi, J.; Zare, F.; Tabei, S.M.B. Exosome as a target for cancer treatment. J. Investig. Med. 2022, 70, 1212–1218. [Google Scholar] [CrossRef]
- Hua, Y.; Chang, X.; Fang, L.; Wang, Z. Subgroups of Extracellular Vesicles: Can They Be Defined by "Labels?". DNA Cell Biol. 2022, 41, 249–256. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Extracellular Vesicles and Thrombogenicity in Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 1774. [Google Scholar] [CrossRef]
- Soler-Botija, C.; Monguió-Tortajada, M.; Munizaga-Larroudé, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S. Mechanisms governing the therapeutic effect of mesenchymal stromal cell-derived extracellular vesicles: A scoping review of preclinical evidence. Biomed. Pharmacother. 2022, 147, 112683. [Google Scholar] [CrossRef]
- Araujo-Abad, S.; Saceda, M.; de Juan Romero, C. Biomedical application of small extracellular vesicles in cancer treatment. Adv. Drug Deliv. Rev. 2022, 182, 114117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.W.; Zheng, W.; Xiang, Z.; Ye, C.S.; Yin, Q.Q.; Wang, S.H.; Xu, C.A.; Wu, W.H.; Hui, T.C.; Wu, Q.Q.; et al. Clinical implications of exosome-derived noncoding RNAs in liver. Lab. Investig. 2022, 102, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Wang, X.; Du, J.; Han, Q.; Zhang, D.; Zhu, H. A systematic review and Meta-analysis of urinary extracellular vesicles proteome in diabetic nephropathy. Front. Endocrinol. 2022, 13, 866252. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y. Extracellular vesicles in idiopathic pulmonary fibrosis: Pathogenesis and therapeutics. Inflamm. Regen. 2022, 42, 23. [Google Scholar] [CrossRef]
- Műzes, G.; Sipos, F. Mesenchymal Stem Cell-Derived Secretome: A Potential Therapeutic Option for Autoimmune and Immune-Mediated Inflammatory Diseases. Cells 2022, 11, 2300. [Google Scholar] [CrossRef]
- Liu, G.; Yin, X.M. The Role of Extracellular Vesicles in Liver Pathogenesis. Am. J. Pathol. 2022, 192, 1358–1367. [Google Scholar] [CrossRef]
- Georgatzakou, H.T.; Fortis, S.P.; Papageorgiou, E.G.; Antonelou, M.H.; Kriebardis, A.G. Blood Cell-Derived Microvesicles in Hematological Diseases and beyond. Biomolecules 2022, 12, 803. [Google Scholar] [CrossRef]
- Abbaszadeh, H.; Ghorbani, F.; Abbaspour-Aghdam, S.; Kamrani, A.; Valizadeh, H.; Nadiri, M.; Sadeghi, A.; Shamsasenjan, K.; Jadidi-Niaragh, F.; Roshangar, L.; et al. Chronic obstructive pulmonary disease and asthma: Mesenchymal stem cells and their extracellular vesicles as potential therapeutic tools. Stem Cell Res. Ther. 2022, 13, 262. [Google Scholar] [CrossRef]
- Wei, W.; Pan, Y.; Yang, X.; Chen, Z.; Heng, Y.; Yang, B.; Pu, M.; Zuo, J.; Lai, Z.; Tang, Y.; et al. The Emerging Role of the Interaction of Extracellular Vesicle and Autophagy-Novel Insights into Neurological Disorders. J. Inflamm. Res. 2022, 15, 3395–3407. [Google Scholar] [CrossRef]
- Frommeyer, T.C.; Gilbert, M.M.; Brittain, G.V.; Wu, T.; Nguyen, T.Q.; Rohan, C.A.; Travers, J.B. UVB-Induced Microvesicle Particle Release and Its Effects on the Cutaneous Microenvironment. Front. Immunol. 2022, 13, 880850. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Yan, Y.; Tan, Y. Role of Exosomes in Chronic Liver Disease Development and Their Potential Clinical Applications. J. Immunol. Res. 2022, 2022, 1695802. [Google Scholar] [CrossRef] [PubMed]
- Neri, T.; Celi, A.; Tinè, M.; Bernardinello, N.; Cosio, M.G.; Saetta, M.; Nieri, D.; Bazzan, E. The Emerging Role of Extracellular Vesicles Detected in Different Biological Fluids in COPD. Int. J. Mol. Sci. 2022, 23, 5136. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, W.; Wang, Y.; Wang, H.; Liu, S. Extracellular vesicle-mediated crosstalk between pancreatic cancer and stromal cells in the tumor microenvironment. J. Nanobiotechnol. 2022, 20, 208. [Google Scholar] [CrossRef]
- Shang, X.; Fang, Y.; Xin, W.; You, H. The Application of Extracellular Vesicles Mediated miRNAs in Osteoarthritis: Current Knowledge and Perspective. J. Inflamm. Res. 2022, 15, 2583–2599. [Google Scholar] [CrossRef]
- Zhao, Z.; Guo, N.; Chen, W.; Wang, Z. Leveraging Extracellular Non-coding RNAs to Diagnose and Treat Heart Diseases. J. Cardiovasc. Transl. Res. 2022, 15, 456–468. [Google Scholar] [CrossRef]
- Gomez, N.; James, V.; Onion, D.; Fairclough, L.C. Extracellular vesicles and chronic obstructive pulmonary disease (COPD): A systematic review. Respir. Res. 2022, 23, 82. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Wang, J.; Yi, H.; Song, Y. Extracellular Vesicles in the Pathogenesis, Treatment, and Diagnosis of Spinal Cord Injury: A Mini-Review. Curr. Stem Cell Res. Ther. 2022, 17, 317–327. [Google Scholar] [CrossRef]
- Muhammad, S.A.; Abbas, A.Y.; Imam, M.U.; Saidu, Y.; Bilbis, L.S. Efficacy of stem cell secretome in the treatment of traumatic brain injury: A systematic review and meta-analysis of preclinical studies. Mol. Neurobiol. 2022, 59, 2894–2909. [Google Scholar] [CrossRef]
- Gabisonia, K.; Khan, M.; Recchia, F.A. Extracellular vesicle-mediated bidirectional communication between heart and other organs. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H769–H784. [Google Scholar] [CrossRef] [PubMed]
- Piening, L.M.; Wachs, R.A. Matrix Bound Nanovesicles: What are they and what do they do? Cells Tissues Organs, 2022; in press. [Google Scholar] [CrossRef]
- Sabaratnam, R.; Wojtaszewski, J.F.P.; Højlund, K. Factors mediating exercise-induced organ crosstalk. Acta. Physiol. 2022, 234, e13766. [Google Scholar] [CrossRef] [PubMed]
- Al-Koussa, H.; AlZaim, I.; El-Sabban, M.E. Pathophysiology of Coagulation and Emerging Roles for Extracellular Vesicles in Coagulation Cascades and Disorders. J. Clin. Med. 2022, 11, 4932. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, L.; Shi, K.; Zhang, K.; Zheng, C.; Jin, Y. Extracellular Vesicles for Immunomodulation in Tissue Regeneration. Tissue Eng. Part. C Methods. 2022, 28, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Marki, A.; Ley, K. The expanding family of neutrophil-derived extracellular vesicles. Immunol. Rev. 2022; in press. [Google Scholar] [CrossRef]
- Yari, H.; Mikhailova, M.V.; Mardasi, M.; Jafarzadehgharehziaaddin, M.; Shahrokh, S.; Thangavelu, L.; Ahmadi, H.; Shomali, N.; Yaghoubi, Y.; Zamani, M.; et al. Emerging role of mesenchymal stromal cells (MSCs)-derived exosome in neurodegeneration-associated conditions: A groundbreaking cell-free approach. Stem Cell Res. Ther. 2022, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Pischiutta, F.; Caruso, E.; Cavaleiro, H.; Salgado, A.J.; Loane, D.J.; Zanier, E.R. Mesenchymal stromal cell secretome for traumatic brain injury: Focus on immunomodulatory action. Exp. Neurol. 2022, 357, 114199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, Z.; Gao, B.; Zhang, L. Exosome mediated biological functions within skeletal microenvironment. Front. Bioeng. Biotechnol. 2022, 10, 953916. [Google Scholar] [CrossRef]
- Gangadaran, P.; Rajendran, R.L.; Kwack, M.H.; Jeyaraman, M.; Hong, C.M.; Sung, Y.K.; Ahn, B.C. Application of Cell-Derived Extracellular Vesicles and Engineered Nanovesicles for Hair Growth: From Mechanisms to Therapeutics. Front. Cell Dev. Biol. 2022, 10, 963278. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Luo, B. Effects of Exosomal Viral Components on the Tumor Microenvironment. Cancers 2022, 14, 3552. [Google Scholar] [CrossRef]
- Imanbekova, M.; Suarasan, S.; Lu, Y.; Jurchuk, S.; Wachsmann-Hogiu, S. Recent advances in optical label-free characterization of extracellular vesicles. Nanophotonics 2022, 11, 2827–2863. [Google Scholar] [CrossRef]
- Yu, H.; Huang, Y.; Yang, L. Research progress in the use of mesenchymal stem cells and their derived exosomes in the treatment of osteoarthritis. Ageing Res. Rev. 2022, 80, 101684. [Google Scholar] [CrossRef]
- Song, Q.; Yu, H.; Han, J.; Lv, J.; Lv, Q.; Yang, H. Exosomes in urological diseases—Biological functions and clinical applications. Cancer Lett. 2022, 544, 215809. [Google Scholar] [CrossRef]
- Dhar, R.; Mallik, S.; Devi, A. Exosomal microRNAs (exoMIRs): Micromolecules with macro impact in oral cancer. 3 Biotech 2022, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Paskeh, M.D.A.; Entezari, M.; Mirzaei, S.; Zabolian, A.; Saleki, H.; Naghdi, M.J.; Sabet, S.; Khoshbakht, M.A.; Hashemi, M.; Hushmandi, K.; et al. Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J. Hematol. Oncol. 2022, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Golan-Gerstl, R.; Reif, S. Extracellular vesicles in human milk. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yu, Y.; Zhu, G.; Zeng, L.; Xu, S.; Cheng, H.; Ouyang, Z.; Chen, J.; Pathak, J.L.; Wu, L.; et al. The Emerging Role of Plant-Derived Exosomes-Like Nanoparticles in Immune Regulation and Periodontitis Treatment. Front. Immunol. 2022, 13, 896745. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Ma, J.; Zhou, Y.; Lu, R. Focusing on Future Applications and Current Challenges of Plant Derived Extracellular Vesicles. Pharmaceuticals 2022, 15, 708. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Wang, R. Roles of Exosome Genomic DNA in Colorectal Cancer. Front. Pharmacol. 2022, 13, 923232. [Google Scholar] [CrossRef] [PubMed]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Cifola, I.; Caratelli, S.; Sconocchia, G.; D’Agnano, I.; Cenciarelli, C. Glioma extracellular vesicles for precision medicine: Prognostic and theragnostic application. Discov. Oncol. 2022, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, A.N.; Suresh, D.; Kaur, S.; Kumar, D.P. The promise of small particles: Extracellular vesicles as biomarkers in liver pathology. J. Physiol. 2022; in press. [Google Scholar] [CrossRef]
- Xia, J.; Miao, Y.; Wang, X.; Huang, X.; Dai, J. Recent progress of dendritic cell-derived exosomes (Dex) as an anti-cancer nanovaccine. Biomed. Pharmacother. 2022, 152, 113250. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Amin Mahdian, S.M.; Ebrahimi, M.S.; Taghizadieh, M.; Vosough, M.; Sadri Nahand, J.; Hosseindoost, S.; Vousooghi, N.; Javar, H.A.; Larijani, B.; et al. Microfluidics for detection of exosomes and microRNAs in cancer: State of the art. Mol. Ther. Nucleic Acids 2022, 28, 758–791. [Google Scholar] [CrossRef]
- Chouaib, B.; Cuisinier, F.; Collart-Dutilleul, P.Y. Dental stem cell-conditioned medium for tissue regeneration: Optimization of production and storage. World J. Stem Cells 2022, 14, 287–302. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, M.; Peng, M. Progress of exosome research in systemic lupus erythematosus. Cytokine X 2022, 4, 100066. [Google Scholar] [CrossRef]
- Liu, H.; Liang, J.; Ye, X.; Huang, M.; Ma, L.; Xie, X.; Liu, D.; Cao, H.; Simal-Gandara, J.; Rengasamy, K.R.R.; et al. The potential role of extracellular vesicles in bioactive compound-based therapy: A review of recent developments. Crit. Rev. Food Sci. Nutr. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cao, H.; Guo, J.; Yuan, Y.; Ni, G. Effects of BMSC-Derived EVs on Bone Metabolism. Pharmaceutics 2022, 14, 1012. [Google Scholar] [CrossRef]
- Gao, H.; Zhang, L.; Wang, Z.; Yan, K.; Zhao, L.; Xiao, W. Research Progress on Transorgan Regulation of the Cardiovascular and Motor System through Cardiogenic Exosomes. Int. J. Mol. Sci. 2022, 23, 5765. [Google Scholar] [CrossRef]
- Lee, C.; Han, J.; Jung, Y. Pathological Contribution of Extracellular Vesicles and Their MicroRNAs to Progression of Chronic Liver Disease. Biology 2022, 11, 637. [Google Scholar] [CrossRef]
- Kang, F.; Jiang, F.; Ouyang, L.; Wu, S.; Fu, C.; Liu, Y.; Li, Z.; Tian, Y.; Cao, X.; Wang, X.; et al. Potential Biological Roles of Exosomal Long Non-Coding RNAs in Gastrointestinal Cancer. Front. Cell Dev. Biol. 2022, 10, 886191. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.R.; Khan, N.L.A.; Godakumara, K.; Dissanayake, K.; Piibor, J.; Muhandiram, S.; Eapen, S.; Heath, P.R.; Fazeli, A. The role of extracellular vesicles in endometrial receptivity and their potential in reproductive therapeutics and diagnosis. Reprod. Biol. 2022, 22, 100645. [Google Scholar] [CrossRef] [PubMed]
- Malekian, F.; Shamsian, A.; Kodam, S.P.; Ullah, M. Exosome engineering for efficient and targeted drug delivery: Current status and future perspective. J. Physiol. 2022; in press. [Google Scholar] [CrossRef]
- Fang, Y.; Dai, X. Emerging Roles of Extracellular Non-Coding RNAs in Vascular Diseases. J. Cardiovasc. Transl. Res. 2022, 15, 492–499. [Google Scholar] [CrossRef]
- Bazzoni, R.; Tanasi, I.; Turazzi, N.; Krampera, M. Update on the Role and Utility of Extracellular Vesicles in Hematological Malignancies. Stem Cells 2022, 40, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Sun, Y.; Wan, C.; Dai, X.; Wu, S.; Lo, P.C.; Huang, J.; Lovell, J.F.; Jin, H.; Yang, K. Microparticles: Biogenesis, characteristics and intervention therapy for cancers in preclinical and clinical research. J. Nanobiotechnol. 2022, 20, 189. [Google Scholar] [CrossRef]
- Allegra, A.; Petrarca, C.; Di Gioacchino, M.; Casciaro, M.; Musolino, C.; Gangemi, S. Exosome-Mediated Therapeutic Strategies for Management of Solid and Hematological Malignancies. Cells 2022, 11, 1128. [Google Scholar] [CrossRef]
- Sykaras, A.G.; Christofidis, K.; Politi, E.; Theocharis, S. Exosomes on Endometrial Cancer: A Biomarkers Treasure Trove? Cancers 2022, 14, 1733. [Google Scholar] [CrossRef]
- Huang, Z.; Keramat, S.; Izadirad, M.; Chen, Z.S.; Soukhtanloo, M. The Potential Role of Exosomes in the Treatment of Brain Tumors, Recent Updates and Advances. Front. Oncol. 2022, 12, 869929. [Google Scholar] [CrossRef]
- Zhang, P.; Rasheed, M.; Liang, J.; Wang, C.; Feng, L.; Chen, Z. Emerging Potential of Exosomal Non-coding RNA in Parkinson’s Disease: A Review. Front. Aging Neurosci. 2022, 14, 819836. [Google Scholar] [CrossRef]
- Liu, K.; Gao, X.; Kang, B.; Liu, Y.; Wang, D.; Wang, Y. The Role of Tumor Stem Cell Exosomes in Cancer Invasion and Metastasis. Front. Oncol. 2022, 12, 836548. [Google Scholar] [CrossRef]
- Xu, K.; Jin, Y.; Li, Y.; Huang, Y.; Zhao, R. Recent Progress of Exosome Isolation and Peptide Recognition-Guided Strategies for Exosome Research. Front. Chem. 2022, 10, 844124. [Google Scholar] [CrossRef] [PubMed]
- Huldani, H.; Abdalkareem Jasim, S.; Olegovich Bokov, D.; Abdelbasset, W.K.; Nader Shalaby, M.; Thangavelu, L.; Margiana, R.; Qasim, M.T. Application of extracellular vesicles derived from mesenchymal stem cells as potential therapeutic tools in autoimmune and rheumatic diseases. Int. Immunopharmacol. 2022, 106, 108634. [Google Scholar] [CrossRef]
- Letafati, A.; Najafi, S.; Mottahedi, M.; Karimzadeh, M.; Shahini, A.; Garousi, S.; Abbasi-Kolli, M.; Sadri Nahand, J.; Tamehri Zadeh, S.S.; Hamblin, M.R.; et al. MicroRNA let-7 and viral infections: Focus on mechanisms of action. Cell Mol. Biol. Lett. 2022, 27, 14. [Google Scholar] [CrossRef]
- Yang, L.; Patel, K.D.; Rathnam, C.; Thangam, R.; Hou, Y.; Kang, H.; Lee, K.B. Harnessing the Therapeutic Potential of Extracellular Vesicles for Biomedical Applications Using Multifunctional Magnetic Nanomaterials. Small 2022, 18, e2104783. [Google Scholar] [CrossRef]
- Widjaja, G.; Jalil, A.T.; Budi, H.S.; Abdelbasset, W.K.; Efendi, S.; Suksatan, W.; Rita, R.S.; Satria, A.P.; Aravindhan, S.; Saleh, M.M.; et al. Mesenchymal stromal/stem cells and their exosomes application in the treatment of intervertebral disc disease: A promising frontier. Int. Immunopharmacol. 2022, 105, 108537. [Google Scholar] [CrossRef] [PubMed]
- Whittle, K.; Kao, S.; Clarke, S.; Grau, G.E.R.; Hosseini-Beheshti, E. Exploring the role of extracellular vesicles and their protein cargo in lung cancer metastasis: A review. Crit. Rev. Oncol. Hematol. 2022, 171, 103603. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, U. Exosomes in cardiovascular diseases: A blessing or a sin for the mankind. Mol. Cell Biochem. 2022, 477, 833–847. [Google Scholar] [CrossRef]
- Hussain, S.; Fatima, A.; Fan, X.X.; Malik, S.I. REVIEW-The Biological importance of cells secreted Exosomes. Pak. J. Pharm. Sci. 2021, 34, 2273–2279. [Google Scholar]
- Zhang, H.; Xing, J.; Dai, Z.; Wang, D.; Tang, D. Exosomes: The key of sophisticated cell-cell communication and targeted metastasis in pancreatic cancer. Cell Commun. Signal 2022, 20, 9. [Google Scholar] [CrossRef]
- Alghamdi, M.; Alamry, S.A.; Bahlas, S.M.; Uversky, V.N.; Redwan, E.M. Circulating extracellular vesicles and rheumatoid arthritis: A proteomic analysis. Cell Mol. Life Sci. 2021, 79, 25. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.; Liu, Y.; Li, J.; Wu, C.; Tang, H. Exosomal Non-Coding RNAs: New Insights into the Biology of Hepatocellular Carcinoma. Curr. Oncol. 2022, 29, 5383–5406. [Google Scholar] [CrossRef]
- Guo, X.; Sui, R.; Piao, H. Tumor-derived small extracellular vesicles: Potential roles and mechanism in glioma. J. Nanobiotechnol. 2022, 20, 383. [Google Scholar] [CrossRef]
- Suresh, P.S.; Thankachan, S.; Venkatesh, T. Landscape of Clinically Relevant Exosomal tRNA-Derived Non-coding RNAs. Mol. Biotechnol. 2022; in press. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Morrow, A.L.; Newburg, D.S. Human Milk Extracellular Vesicles: A Biological System with Clinical Implications. Cells 2022, 11, 2345. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamamoto, Y.; Ochiya, T. Extracellular vesicle-mediated immunoregulation in cancer. Int. J. Hematol. 2022; in press. [Google Scholar] [CrossRef]
- Barone, A.; d’Avanzo, N.; Cristiano, M.C.; Paolino, D.; Fresta, M. Macrophage-Derived Extracellular Vesicles: A Promising Tool for Personalized Cancer Therapy. Biomedicines 2022, 10, 1252. [Google Scholar] [CrossRef]
- Chen, K.; Li, Y.; Xu, L.; Qian, Y.; Liu, N.; Zhou, C.; Liu, J.; Zhou, L.; Xu, Z.; Jia, R.; et al. Comprehensive insight into endothelial progenitor cell-derived extracellular vesicles as a promising candidate for disease treatment. Stem Cell Res. Ther. 2022, 13, 238. [Google Scholar] [CrossRef]
- Carnino, J.M.; Lee, H. Extracellular vesicles in respiratory disease. Adv. Clin. Chem. 2022, 108, 105–127. [Google Scholar] [CrossRef]
- Kim, S.B. Function and therapeutic development of exosomes for cancer therapy. Arch. Pharm. Res. 2022, 45, 295–308. [Google Scholar] [CrossRef]
- Li, F.; Kang, X.; Xin, W.; Li, X. The Emerging Role of Extracellular Vesicle Derived From Neurons/Neurogliocytes in Central Nervous System Diseases: Novel Insights into Ischemic Stroke. Front. Pharmacol. 2022, 13, 890698. [Google Scholar] [CrossRef]
- Rother, N.; Yanginlar, C.; Pieterse, E.; Hilbrands, L.; van der Vlag, J. Microparticles in Autoimmunity: Cause or Consequence of Disease? Front. Immunol. 2022, 13, 822995. [Google Scholar] [CrossRef]
- Jing, W.; Wang, H.; Zhan, L.; Yan, W. Extracellular Vesicles, New Players in Sepsis and Acute Respiratory Distress Syndrome. Front. Cell Infect. Microbiol. 2022, 12, 853840. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, H.; Zhang, M.; He, Y.; Li, X.; Xu, Y.; Liu, X. Hypoxia Induced Changes of Exosome Cargo and Subsequent Biological Effects. Front. Immunol. 2022, 13, 824188. [Google Scholar] [CrossRef]
- Hosseinikhah, S.M.; Gheybi, F.; Moosavian, S.A.; Shahbazi, M.A.; Jaafari, M.R.; Sillanpää, M.; Kesharwani, P.; Alavizadeh, S.H.; Sahebkar, A. Role of exosomes in tumour growth, chemoresistance and immunity: State-of-the-art. J. Drug Target, 2022; in press. [Google Scholar] [CrossRef]
- Boilard, E.; Bellio, M. Platelet extracellular vesicles and the secretory interactome join forces in health and disease. Immunol. Rev. 2022; in press. [Google Scholar] [CrossRef]
- Di Bella, M.A. Overview and Update on Extracellular Vesicles: Considerations on Exosomes and Their Application in Modern Medicine. Biology 2022, 11, 804. [Google Scholar] [CrossRef]
- Quesnel, A.; Broughton, A.; Karagiannis, G.S.; Filippou, P.S. Message in the bottle: Regulation of the tumor microenvironment via exosome-driven proteolysis. Cancer Metastasis Rev. 2022; in press. [Google Scholar] [CrossRef]
- Kumari, M.; Anji, A. Small but Mighty-Exosomes, Novel Intercellular Messengers in Neurodegeneration. Biology 2022, 11, 413. [Google Scholar] [CrossRef]
- Al Halawani, A.; Mithieux, S.M.; Yeo, G.C.; Hosseini-Beheshti, E.; Weiss, A.S. Extracellular Vesicles: Interplay with the Extracellular Matrix and Modulated Cell Responses. Int. J. Mol. Sci. 2022, 23, 3389. [Google Scholar] [CrossRef]
- Guo, Y.; Zhai, Y.; Wu, L.; Wang, Y.; Wu, P.; Xiong, L. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Pleiotropic Impacts on Breast Cancer Occurrence, Development, and Therapy. Int. J. Mol. Sci. 2022, 23, 2927. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, C.; Jin, F.; Han, G.; Cui, C. Therapeutic effect of extracellular vesicles from different cell sources in traumatic brain injury. Tissue Cell 2022, 76, 101772. [Google Scholar] [CrossRef]
- Keshtkar, S.; Kaviani, M.; Soleimanian, S.; Azarpira, N.; Asvar, Z.; Pakbaz, S. Stem Cell-Derived Exosome as Potential Therapeutics for Microbial Diseases. Front. Microbiol. 2022, 12, 786111. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, J.H. The emerging roles of extracellular vesicles as intercellular messengers in liver physiology and pathology. Clin. Mol. Hepatol. 2022; in press. [Google Scholar] [CrossRef]
- Dehkordi, N.R.; Dehkordi, N.R.; Farjoo, M.H. Therapeutic properties of stem cell-derived exosomes in ischemic heart disease. Eur. J. Pharmacol. 2022, 920, 174839. [Google Scholar] [CrossRef]
- Buffolo, F.; Monticone, S.; Camussi, G.; Aikawa, E. Role of Extracellular Vesicles in the Pathogenesis of Vascular Damage. Hypertension 2022, 79, 863–873. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Qin, Y.; Zhao, S.; Zheng, J.C. Exosome: A novel neurotransmission modulator or non-canonical neurotransmitter? Ageing Res. Rev. 2022, 74, 101558. [Google Scholar] [CrossRef]
- Li, C.C.; Hsu, W.F.; Wo, A.M. Exosomes-Potential for Blood-Based Marker in Alzheimer’s Disease. Acta. Neurol. Taiwan. 2022; in press. [Google Scholar]
- Zhang, W.; Xing, J.; Liu, T.; Zhang, J.; Dai, Z.; Zhang, H.; Wang, D.; Tang, D. Small extracellular vesicles: From mediating cancer cell metastasis to therapeutic value in pancreatic cancer. Cell Commun. Signal. 2022, 20, 1. [Google Scholar] [CrossRef]
- Xing, Y.; Sun, X.; Dou, Y.; Wang, M.; Zhao, Y.; Yang, Q.; Zhao, Y. The Immuno-Modulation Effect of Macrophage-Derived Extracellular Vesicles in Chronic Inflammatory Diseases. Front. Immunol. 2021, 12, 785728. [Google Scholar] [CrossRef]
- Zhu, M.; Li, S.; Li, S.; Wang, H.; Xu, J.; Wang, Y.; Liang, G. Strategies for Engineering Exosomes and Their Applications in Drug Delivery. J. Biomed. Nanotechnol. 2021, 17, 2271–2297. [Google Scholar] [CrossRef]
- Zelli, V.; Compagnoni, C.; Capelli, R.; Corrente, A.; Di Vito Nolfi, M.; Zazzeroni, F.; Alesse, E.; Tessitore, A. Role of exosomal microRNAs in cancer therapy and drug resistance mechanisms: Focus on hepatocellular carcinoma. Front. Oncol. 2022, 12, 940056. [Google Scholar] [CrossRef]
- Canning, P.; Alwan, A.; Khalil, F.; Zhang, Y.; Opara, E.C. Perspectives and Challenges on the Potential Use of Exosomes in Bioartificial Pancreas Engineering. Ann. Biomed. Eng. 2022; in press. [Google Scholar] [CrossRef]
- Zheng, C.; Xie, L.; Qin, H.; Liu, X.; Chen, X.; Lv, F.; Wang, L.; Zhu, X.; Xu, J. The Role of Extracellular Vesicles in Systemic Lupus Erythematosus. Front. Cell Dev. Biol. 2022, 10, 835566. [Google Scholar] [CrossRef]
- Wang, J.; Yue, B.L.; Huang, Y.Z.; Lan, X.Y.; Liu, W.J.; Chen, H. Exosomal RNAs: Novel Potential Biomarkers for Diseases-A Review. Int. J. Mol. Sci. 2022, 23, 2461. [Google Scholar] [CrossRef]
- Abreu, C.M.; Costa-Silva, B.; Reis, R.L.; Kundu, S.C.; Caballero, D. Microfluidic platforms for extracellular vesicle isolation, analysis and therapy in cancer. Lab. Chip 2022, 22, 1093–1125. [Google Scholar] [CrossRef]
- Lampropoulou, D.I.; Pliakou, E.; Aravantinos, G.; Filippou, D.; Gazouli, M. The Role of Exosomal Non-Coding RNAs in Colorectal Cancer Drug Resistance. Int. J. Mol. Sci. 2022, 23, 1473. [Google Scholar] [CrossRef]
- Moon, B.; Chang, S. Exosome as a Delivery Vehicle for Cancer Therapy. Cells 2022, 11, 316. [Google Scholar] [CrossRef]
- Heo, J.; Kang, H. Exosome-Based Treatment for Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 1002. [Google Scholar] [CrossRef]
- Liu, C.; He, D.; Li, L.; Zhang, S.; Wang, L.; Fan, Z.; Wang, Y. Extracellular vesicles in pancreatic cancer immune escape: Emerging roles and mechanisms. Pharmacol. Res. 2022, 183, 106364. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Y.; Li, L.; He, D.; Chi, J.; Li, Q.; Wu, Y.; Zhao, Y.; Zhang, S.; Wang, L.; et al. Engineered extracellular vesicles and their mimetics for cancer immunotherapy. J. Control. Release 2022, 349, 679–698. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef]
- Matsuzaka, Y.; Yashiro, R. Immune Modulation Using Extracellular Vesicles Encapsulated with MicroRNAs as Novel Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 5658. [Google Scholar] [CrossRef]
- Wu, R.; Fan, X.; Wang, Y.; Shen, M.; Zheng, Y.; Zhao, S.; Yang, L. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Liver Immunity and Therapy. Front. Immunol. 2022, 13, 833878. [Google Scholar] [CrossRef]
- Parveen, S.; Subramanian, K. Emerging Roles of Extracellular Vesicles in Pneumococcal Infections: Immunomodulators to Potential Novel Vaccine Candidates. Front. Cell Infect. Microbiol. 2022, 12, 836070. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Wang, D.; Zhang, H.; Shi, Q.; Zhang, Y.; Wang, M.; Ding, Z.; Xu, S.; Gao, B.; et al. Exosomes Immunity Strategy: A Novel Approach for Ameliorating Intervertebral Disc Degeneration. Front. Cell Dev. Biol. 2022, 9, 822149. [Google Scholar] [CrossRef]
- Chen, X.; Chi, H.; Zhao, X.; Pan, R.; Wei, Y.; Han, Y. Role of Exosomes in Immune Microenvironment of Hepatocellular Carcinoma. J. Oncol. 2022, 2022, 2521025. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Liang, Q.; Xu, Z.; Cai, Y.; Peng, B.; Li, J.; Zhang, W.; Kang, F.; Hong, Q.; Yan, Y.; et al. Current Understanding of Exosomal MicroRNAs in Glioma Immune Regulation and Therapeutic Responses. Front. Immunol. 2022, 12, 813747. [Google Scholar] [CrossRef]
- Liu, J.; Peng, X.; Yang, S.; Li, X.; Huang, M.; Wei, S.; Zhang, S.; He, G.; Zheng, H.; Fan, Q.; et al. Extracellular vesicle PD-L1 in reshaping tumor immune microenvironment: Biological function and potential therapy strategies. Cell Commun. Signal 2022, 20, 14. [Google Scholar] [CrossRef]
- Jiang, C.; Fu, Y.; Liu, G.; Shu, B.; Davis, J.; Tofaris, G.K. Multiplexed Profiling of Extracellular Vesicles for Biomarker Development. Nano-Micro Lett. 2021, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, S.; Baccarelli, A.; Prada, D. Role of brain extracellular vesicles in air pollution-related cognitive impairment and neurodegeneration. Environ. Res. 2022, 204, 112316. [Google Scholar] [CrossRef]
- Belkozhayev, A.M.; Al-Yozbaki, M.; George, A.; Ye Niyazova, R.; Sharipov, K.O.; Byrne, L.J.; Wilson, C.M. Extracellular Vesicles, Stem Cells and the Role of miRNAs in Neurodegeneration. Curr. Neuropharmacol. 2022, 20, 1450–1478. [Google Scholar] [CrossRef]
- Picca, A.; Guerra, F.; Calvani, R.; Coelho-Junior, H.J.; Bucci, C.; Marzetti, E. Circulating extracellular vesicles: Friends and foes in neurodegeneration. Neural. Regen. Res. 2022, 17, 534–542. [Google Scholar] [CrossRef]
- Mishra, L.C.; Pandey, U.; Gupta, A.; Gupta, J.; Sharma, M.; Mishra, G. Alternating exosomes and their mimetics as an emergent strategy for targeted cancer therapy. Front. Mol. Biosci. 2022, 9, 939050. [Google Scholar] [CrossRef]
- Baldasici, O.; Pileczki, V.; Cruceriu, D.; Gavrilas, L.I.; Tudoran, O.; Balacescu, L.; Vlase, L.; Balacescu, O. Breast Cancer-Delivered Exosomal miRNA as Liquid Biopsy Biomarkers for Metastasis Prediction: A Focus on Translational Research with Clinical Applicability. Int. J. Mol. Sci. 2022, 23, 9371. [Google Scholar] [CrossRef]
- Lopatina, T.; Sarcinella, A.; Brizzi, M.F. Tumour Derived Extracellular Vesicles: Challenging Target to Blunt Tumour Immune Evasion. Cancers 2022, 14, 4020. [Google Scholar] [CrossRef]
- Meng, L.; Song, K.; Li, S.; Kang, Y. xosomes: Small Vesicles with Important Roles in the Development, Metastasis and Treatment of Breast Cancer. Membranes 2022, 12, 775. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Xia, Y.Q.; Zheng, S.Y. Extruded small extracellular vesicles: Splinters of circulating tumour cells may promote cancer metastasis? Br. J. Cancer, 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Onukwugha, N.E.; Kang, Y.T.; Nagrath, S. Emerging micro-nanotechnologies for extracellular vesicles in immuno-oncology: From target specific isolations to immunomodulation. Lab. Chip, 2022; in press. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, J.; Wu, B.; Wang, X.; Jiang, Y.; Zhu, D. Role of extracellular vesicles in osteosarcoma. Int. J. Med. Sci. 2022, 19, 1216–1226. [Google Scholar] [CrossRef]
- Wu, M.; Wang, M.; Jia, H.; Wu, P. Extracellular vesicles: Emerging anti-cancer drugs and advanced functionalization platforms for cancer therapy. Drug Deliv. 2022, 29, 2513–2538. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, T.; Zhou, M. Immune-Cell-Derived Exosomes for Cancer Therapy. Mol. Pharm. 2022; in press. [Google Scholar] [CrossRef]
- Bie, N.; Yong, T.; Wei, Z.; Gan, L.; Yang, X. Extracellular vesicles for improved tumor accumulation and penetration. Adv. Drug Deliv. Rev. 2022, 188, 114450. [Google Scholar] [CrossRef]
- Wang, H.; Yu, L.; Huang, P.; Zhou, Y.; Zheng, W.; Meng, N.; He, R.; Xu, Y.; Keong, T.S.; Cui, Y. Tumor-associated Exosomes Are Involved in Hepatocellular Carcinoma Tumorigenesis, Diagnosis, and Treatment. J. Clin. Transl. Hepatol. 2022, 10, 496–508. [Google Scholar] [CrossRef]
- Pompili, S.; Vetuschi, A.; Sferra, R.; Cappariello, A. Extracellular Vesicles and Resistance to Anticancer Drugs: A Tumor Skeleton Key for Unhinging Chemotherapies. Front. Oncol. 2022, 12, 933675. [Google Scholar] [CrossRef]
- Gulati, R.; Nandi, D.; Sarkar, K.; Venkataraman, P.; Ramkumar, K.M.; Ranjan, P.; Janardhanan, R. Exosomes as Theranostic Targets: Implications for the Clinical Prognosis of Aggressive Cancers. Front. Mol. Biosci. 2022, 9, 890768. [Google Scholar] [CrossRef]
- Lattmann, E.; Levesque, M.P. The Role of Extracellular Vesicles in Melanoma Progression. Cancers 2022, 14, 3086. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Chen, F.; Zhu, J.; Ding, Y.; Zhang, Q. Advances of Exosomal microRNAs and Proteins in Ovarian Cancer Diagnosis, Prognosis, and Treatment. Curr. Mol. Med. 2022; in press. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, X.; Jiang, L.; Li, Y.; Zheng, Q. Tumor endothelial cell-derived extracellular vesicles contribute to tumor microenvironment remodeling. Cell Commun. Signal 2022, 20, 97. [Google Scholar] [CrossRef]
- Babaker, M.A.; Aljoud, F.A.; Alkhilaiwi, F.; Algarni, A.; Ahmed, A.; Khan, M.I.; Saadeldin, I.M.; Alzahrani, F.A. The Therapeutic Potential of Milk Extracellular Vesicles on Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 6812. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Chaiswing, L.; St Clair, D.K. Extracellular Vesicles and Cancer Therapy: Insights into the Role of Oxidative Stress. Antioxidants 2022, 11, 1194. [Google Scholar] [CrossRef]
- Yu, Z.L.; Liu, J.Y.; Chen, G. Small extracellular vesicle PD-L1 in cancer: The knowns and unknowns. NPJ Precis. Oncol. 2022, 6, 42. [Google Scholar] [CrossRef]
- Yong, T.; Wei, Z.; Gan, L.; Yang, X. Extracellular Vesicle-Based Drug Delivery Systems for Enhanced Anti-Tumor Therapies through Modulating Cancer-Immunity Cycle. Adv. Mater. 2022, 20, e2201054. [Google Scholar] [CrossRef]
- Glass, S.E.; Coffey, R.J. Recent Advances in the Study of Extracellular Vesicles in Colorectal Cancer. Gastroenterology, 2022; in press. [Google Scholar] [CrossRef]
- Chen, H.; Sun, T.; Jiang, C. Extracellular vesicle-based macromolecule delivery systems in cancer immunotherapy. J. Control. Release 2022, 348, 572–589. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Q.; Zhang, X.; Huang, H.; Tang, S.; Chai, Y.; Xu, Z.; Li, M.; Chen, X.; Liu, J.; et al. Recent advances in exosome-mediated nucleic acid delivery for cancer therapy. J. Nanobiotechnol. 2022, 20, 279. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Zhang, X.; Shao, T.; Luo, Y.; Wang, W.; Han, Y. Extracellular Vesicles and Hepatocellular Carcinoma: Opportunities and Challenges. Front. Oncol. 2022, 12, 884369. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.E. Extracellular vesicles in cancer therapy. Semin. Cancer Biol. 2022; in press. [Google Scholar] [CrossRef]
- Khan, M.I.; Alsayed, R.K.M.E.; Choudhry, H.; Ahmad, A. Exosome-Mediated Response to Cancer Therapy: Modulation of Epigenetic Machinery. Int. J. Mol. Sci. 2022, 23, 6222. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, X.; Jiang, Z. Small Extracellular Vesicles: Key Forces Mediating the Development and Metastasis of Colorectal Cancer. Cells 2022, 11, 1780. [Google Scholar] [CrossRef]
- Tuo, B.; Chen, Z.; Dang, Q.; Chen, C.; Zhang, H.; Hu, S.; Sun, Z. Roles of exosomal circRNAs in tumour immunity and cancer progression. Cell Death Dis. 2022, 13, 539. [Google Scholar] [CrossRef]
- Yang, S.; Wang, J.; Wang, S.; Zhou, A.; Zhao, G.; Li, P. Roles of small extracellular vesicles in the development, diagnosis and possible treatment strategies for hepatocellular carcinoma (Review). Int. J. Oncol. 2022, 61, 91. [Google Scholar] [CrossRef]
- Nan, W.; Zhang, C.; Wang, H.; Chen, H.; Ji, S. Direct Modification of Extracellular Vesicles and Its Applications for Cancer Therapy: A Mini-Review. Front. Chem. 2022, 10, 910341. [Google Scholar] [CrossRef]
- Shan, Y.; Zhou, P.; Zhou, Q.; Yang, L. Extracellular Vesicles in the Progression and Therapeutic Resistance of Nasopharyngeal Carcinoma. Cancers 2022, 14, 2289. [Google Scholar] [CrossRef]
- Fridman, E.S.; Ginini, L.; Gil, Z. The Role of Extracellular Vesicles in Metabolic Reprogramming of the Tumor Microenvironment. Cells 2022, 11, 1433. [Google Scholar] [CrossRef]
- Tang, D.; Liu, S.; Shen, H.; Deng, G.; Zeng, S. Extracellular Vesicles Promote the Formation of Pre-Metastasis Niche in Gastric Cancer. Front. Immunol. 2022, 13, 813015. [Google Scholar] [CrossRef]
- Tămaș, F.; Bălașa, R.; Manu, D.; Gyorki, G.; Chinezu, R.; Tămaș, C.; Bălașa, A. The Importance of Small Extracellular Vesicles in the Cerebral Metastatic Process. Int. J. Mol. Sci. 2022, 23, 1449. [Google Scholar] [CrossRef]
- Chang, L.C.; Chiu, H.M.; Wu, M.S.; Shen, T.L. The Role of Small Extracellular Vesicles in the Progression of Colorectal Cancer and Its Clinical Applications. Int. J. Mol. Sci. 2022, 23, 1379. [Google Scholar] [CrossRef]
- Bai, S.; Wei, Y.; Liu, R.; Xu, R.; Xiang, L.; Du, J. Role of tumour-derived exosomes in metastasis. Biomed. Pharmacother. 2022, 147, 112657. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Huang, Q.; Chen, Y.; Wang, Q.; Sang, R.; Wang, L.; Xie, Y.; Chen, W. Tumor-Derived Extracellular Vesicles Regulate Cancer Progression in the Tumor Microenvironment. Front. Mol. Biosci. 2022, 8, 796385. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wang, D.; Han, Y.; Huang, T.; He, X.; Wang, J.; Ou, C. Emerging Role of Cancer-Associated Fibroblasts-Derived Exosomes in Tumorigenesis. Front. Immunol. 2022, 12, 795372. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Lei, N.; Zhou, J.; Chen, M.; Guo, R.; Qin, B.; Li, Y.; Chang, L. Extracellular vesicles in ovarian cancer chemoresistance, metastasis, and immune evasion. Cell Death Dis. 2022, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Sheta, M.; Fujii, M.; Calderwood, S.K. Cancer extracellular vesicles, tumoroid models, and tumor microenvironment. Semin. Cancer Biol. 2022; in press. [Google Scholar] [CrossRef]
- Tan, K.L.; Chia, W.C.; How, C.W.; Tor, Y.S.; Show, P.L.; Looi, Q.H.D.; Foo, J.B. Benchtop Isolation and Characterisation of Small Extracellular Vesicles from Human Mesenchymal Stem Cells. Mol. Biotechnol. 2021, 63, 780–791. [Google Scholar] [CrossRef]
- Mojtahedin, S.; Nasimi, F.S.; Tajalli, H.; Ebrahimi, S.; Alimohammadzadeh, B.; Rahbarghazi, R.; Mahdipour, M. Light-emitting diode photomodulation of uterine adenocarcinoma cells inhibited angiogenesis capacity via the regulation of exosome biogenesis. Lasers Med. Sci. 2022; in press. [Google Scholar] [CrossRef]
- Ferreira, J.V.; da Rosa Soares, A.; Ramalho, J.; Máximo Carvalho, C.; Cardoso, M.H.; Pintado, P.; Carvalho, A.S.; Beck, H.C.; Matthiesen, R.; Zuzarte, M.; et al. LAMP2A regulates the loading of proteins into exosomes. Sci. Adv. 2022, 8, eabm1140. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, J.; Tong, L.; Liu, S.; Zhang, Y. The role of exosomes from BALF in lung disease. J. Cell Physiol. 2022, 237, 161–168. [Google Scholar] [CrossRef]
- Rincón-Riveros, A.; Lopez, L.; Villegas, E.V.; Antonia Rodriguez, J. Regulation of Antitumor Immune Responses by Exosomes Derived from Tumor and Immune Cells. Cancers 2021, 13, 847. [Google Scholar] [CrossRef]
- Pavlic, A.; Bahram Sangani, N.; Kerins, J.; Nicolaes, G.; Schurgers, L.; Reutelingsperger, C. Vascular Smooth Muscle Cell Neutral Sphingomyelinase 2 in the Release of Exosomes and Vascular Calcification. Int. J. Mol. Sci. 2022, 23, 9178. [Google Scholar] [CrossRef]
- Landa, S.; Verlov, N.; Fedorova, N.; Filatov, M.; Pantina, R.; Burdakov, V.; Varfolomeeva, E.; Emanuel, V. Extracellular Particles as Carriers of Cholesterol Not Associated with Lipoproteins. Membranes 2022, 12, 618. [Google Scholar] [CrossRef]
- Liangsupree, T.; Multia, E.; Forssén, P.; Fornstedt, T.; Riekkola, M.L. Kinetics and interaction studies of anti-tetraspanin antibodies and ICAM-1 with extracellular vesicle subpopulations using continuous flow quartz crystal microbalance biosensor. Biosens. Bioelectron. 2022, 206, 114151. [Google Scholar] [CrossRef]
- Chand, S.; Gowen, A.; Savine, M.; Moore, D.; Clark, A.; Huynh, W.; Wu, N.; Odegaard, K.; Weyrich, L.; Bevins, R.A.; et al. A comprehensive study to delineate the role of an extracellular vesicle-associated microRNA-29a in chronic methamphetamine use disorder. J. Extracell. Vesicles 2021, 10, e12177. [Google Scholar] [CrossRef]
- Zhang, Q.; Jeppesen, D.K.; Higginbotham, J.N.; Graves-Deal, R.; Trinh, V.Q.; Ramirez, M.A.; Sohn, Y.; Neininger, A.C.; Taneja, N.; McKinley, E.T.; et al. Supermeres are functional extracellular nanoparticles replete with disease biomarkers and therapeutic targets. Nat. Cell Biol. 2021, 23, 1240–1254. [Google Scholar] [CrossRef]
- Hsiao, Y.P.; Chen, C.; Lee, C.M.; Chen, P.Y.; Chung, W.H.; Wang, Y.P.; Hung, Y.C.; Cheng, C.M.; Chen, C.; Ko, B.H.; et al. Differences in the Quantity and Composition of Extracellular Vesicles in the Aqueous Humor of Patients with Retinal Neovascular Diseases. Diagnostics 2021, 11, 1276. [Google Scholar] [CrossRef]
- Anand, S.; Samuel, M.; Mathivanan, S. Exomeres: A New Member of Extracellular Vesicles Family. Subcell. Biochem. 2021, 97, 89–97. [Google Scholar] [CrossRef]
- Zhang, Q.; Higginbotham, J.N.; Jeppesen, D.K.; Yang, Y.P.; Li, W.; McKinley, E.T.; Graves-Deal, R.; Ping, J.; Britain, C.M.; Dorsett, K.A.; et al. Transfer of Functional Cargo in Exomeres. Cell Rep. 2019, 27, 940–954.e6. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef]
- Zaldivia, M.T.K.; McFadyen, J.D.; Lim, B.; Wang, X.; Peter, K. Platelet-Derived Microvesicles in Cardiovascular Diseases. Front. Cardiovasc. Med. 2017, 4, 74. [Google Scholar] [CrossRef]
- Ståhl, A.L.; Johansson, K.; Mossberg, M.; Kahn, R.; Karpman, D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr. Nephrol. 2019, 34, 11–30. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, P.; Lin, J.; Zeng, H. The Role of Extracellular Vesicles in Osteoporosis: A Scoping Review. Membranes 2022, 12, 324. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; She, D.; Sun, C.; Gong, M.; Rong, Y. Dumbbell structure probe-triggered rolling circle amplification (RCA)-based detection scaffold for sensitive and specific neonatal infection-related small extracellular vesicle (sEV) detection. Anal. Methods 2022, 14, 1534–1539. [Google Scholar] [CrossRef]
- Gamperl, H.; Plattfaut, C.; Freund, A.; Quecke, T.; Theophil, F.; Gieseler, F. Extracellular vesicles from malignant effusions induce tumor cell migration: Inhibitory effect of LMWH tinzaparin. Cell Biol. Int. 2016, 40, 1050–1061. [Google Scholar] [CrossRef]
- Asea, A.; Jean-Pierre, C.; Kaur, P.; Rao, P.; Linhares, I.M.; Skupski, D.; Witkin, S.S. Heat shock protein-containing exosomes in mid-trimester amniotic fluids. J. Reprod. Immunol. 2008, 79, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Calvo, V.; Izquierdo, M. Inducible Polarized Secretion of Exosomes in T and B Lymphocytes. Int. J. Mol. Sci. 2020, 21, 2631. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liu, X. Macrophage-Derived Small Extracellular Vesicles in Multiple Diseases: Biogenesis, Function, and Therapeutic Applications. Front. Cell Dev. Biol. 2022, 10, 913110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, D.; Song, Y.; He, R.; Wang, T. Research Progress in the Application of Exosomes in Immunotherapy. Front. Immunol. 2022, 13, 731516. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Xie, M.; Hun, M.; She, Z.; Li, C.; Luo, S.; Chen, X.; Wan, W.; Wen, C.; Tian, J. Natural Killer Cell-Derived Extracellular Vesicles: Novel Players in Cancer Immunotherapy. Front. Immunol. 2021, 12, 658698. [Google Scholar] [CrossRef]
- Skogberg, G.; Telemo, E.; Ekwall, O. Exosomes in the Thymus: Antigen Transfer and Vesicles. Front. Immunol. 2015, 6, 366. [Google Scholar] [CrossRef]
- Martinez-Usatorre, A.; De Palma, M. Dendritic cell cross-dressing and tumor immunity. EMBO Mol. Med. 2022, 12, e16523. [Google Scholar] [CrossRef]
- Mo, L.H.; Han, H.Y.; Jin, Q.R.; Song, Y.N.; Wu, G.H.; Zhang, Y.; Yang, L.T.; Liu, T.; Liu, Z.G.; Feng, Y.; et al. T cell activator-carrying extracellular vesicles induce antigen-specific regulatory T cells. Clin. Exp. Immunol. 2021, 206, 129–140. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, W.; Wang, B.; Yang, J.; Yang, J.; Yu, Z.; Qin, Z.; Shi, A.; Xu, W.; Zheng, C.; et al. ICAM-1-mediated adhesion is a prerequisite for exosome-induced T cell suppression. Dev. Cell 2022, 57, 329–343.e7. [Google Scholar] [CrossRef]
- Han, J.M.; Song, H.Y.; Lim, S.T.; Kim, K.I.; Seo, H.S.; Byun, E.B. Immunostimulatory Potential of Extracellular Vesicles Isolated from an Edible Plant, Petasites japonicus, via the Induction of Murine Dendritic Cell Maturation. Int. J. Mol. Sci. 2021, 22, 10634. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, H.; Li, Y. Roles of exosomes and exosome-derived miRNAs in pulmonary fibrosis. Front. Pharmacol. 2022, 13, 928933. [Google Scholar] [CrossRef]
- Zou, J.; Peng, H.; Liu, Y. The Roles of Exosomes in Immunoregulation and Autoimmune Thyroid Diseases. Front. Immunol. 2021, 12, 757674. [Google Scholar] [CrossRef]
- Fu, C.; Peng, P.; Loschko, J.; Feng, L.; Pham, P.; Cui, W.; Lee, K.P.; Krug, A.B.; Jiang, A. Plasmacytoid dendritic cells cross-prime naive CD8 T cells by transferring antigen to conventional dendritic cells through exosomes. Proc. Natl. Acad. Sci. USA 2020, 117, 23730–23741. [Google Scholar] [CrossRef]
- Pfister, H. Neutrophil Extracellular Traps and Neutrophil-Derived Extracellular Vesicles: Common Players in Neutrophil Effector Functions. Diagnostics 2022, 12, 1715. [Google Scholar] [CrossRef] [PubMed]
- Ou, Q.; Dou, X.; Tang, J.; Wu, P.; Pan, D. Small extracellular vesicles derived from PD-L1-modified mesenchymal stem cell promote Tregs differentiation and prolong allograft survival. Cell Tissue Res. 2022; in press. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.; Almeida, F. Exosome-Based Vaccines: History, Current State, and Clinical Trials. Front. Immunol. 2021, 12, 711565. [Google Scholar] [CrossRef]
- Fernández-Delgado, I.; Calzada-Fraile, D.; Sánchez-Madrid, F. Immune Regulation by Dendritic Cell Extracellular Vesicles in Cancer Immunotherapy and Vaccines. Cancers 2020, 12, 3558. [Google Scholar] [CrossRef]
- Nikfarjam, S.; Rezaie, J.; Kashanchi, F.; Jafari, R. Dexosomes as a cell-free vaccine for cancer immunotherapy. J. Exp. Clin. Cancer Res. 2020, 39, 258. [Google Scholar] [CrossRef] [PubMed]
- Sabanovic, B.; Piva, F.; Cecati, M.; Giulietti, M. Promising Extracellular Vesicle-Based Vaccines against Viruses, Including SARS-CoV-2. Biology 2021, 10, 94. [Google Scholar] [CrossRef]
- Teshima, T.; Yuchi, Y.; Suzuki, R.; Matsumoto, H.; Koyama, H. Immunomodulatory Effects of Canine Adipose Tissue Mesenchymal Stem Cell-Derived Extracellular Vesicles on Stimulated CD4+ T Cells Isolated from Peripheral Blood Mononuclear Cells. J. Immunol. Res. 2021, 2021, 2993043. [Google Scholar] [CrossRef]
- Zhang, J.; Li, P.; Zhao, G.; He, S.; Xu, D.; Jiang, W.; Peng, Q.; Li, Z.; Xie, Z.; Zhang, H.; et al. Mesenchymal stem cell-derived extracellular vesicles protect retina in a mouse model of retinitis pigmentosa by anti-inflammation through miR-146a-Nr4a3 axis. Stem Cell Res. Ther. 2022, 13, 394. [Google Scholar] [CrossRef]
- Wang, C.; Börger, V.; Mohamud Yusuf, A.; Tertel, T.; Stambouli, O.; Murke, F.; Freund, N.; Kleinschnitz, C.; Herz, J.; Gunzer, M.; et al. Postischemic Neuroprotection Associated With Anti-Inflammatory Effects by Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles in Aged Mice. Stroke 2022, 53, e14–e18. [Google Scholar] [CrossRef]
- Ha, D.H.; Kim, H.K.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef]
- Lu, T.; Zhang, Z.; Zhang, J.; Pan, X.; Zhu, X.; Wang, X.; Li, Z.; Ruan, M.; Li, H.; Chen, W.; et al. CD73 in small extracellular vesicles derived from HNSCC defines tumour-associated immunosuppression mediated by macrophages in the microenvironment. J. Extracell. Vesicles 2022, 11, e12218. [Google Scholar] [CrossRef] [PubMed]
- Buzas, E.I. The roles of extracellular vesicles in the immune system. Nat. Rev. Immunol. 2022; in press. [Google Scholar] [CrossRef]
- Fu, C.; Ma, T.; Zhou, L.; Mi, Q.S.; Jiang, A. Dendritic Cell-Based Vaccines Against Cancer: Challenges, Advances and Future Opportunities. Immunol. Investig. 2022, 10, 1–26. [Google Scholar] [CrossRef]
- Chi, H.; Hao, Y.; Wang, X.; Tang, L.; Deng, Y.; Chen, X.; Gao, F.; Sha, O.; Jin, G. A Therapeutic Whole-Tumor-Cell Vaccine Covalently Conjugated with a TLR7 Agonist. Cells 2022, 11, 1986. [Google Scholar] [CrossRef]
- Dhandapani, H.; Jayakumar, H.; Seetharaman, A.; Singh, S.S.; Ganeshrajah, S.; Jagadish, N.; Suri, A.; Thangarajan, R.; Ramanathan, P. Dendritic cells matured with recombinant human sperm associated antigen 9 (rhSPAG9) induce CD4+, CD8+ T cells and activate NK cells: A potential candidate molecule for immunotherapy in cervical cancer. Cancer Cell Int. 2021, 21, 473. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Jia, H.; Zheng, M.; Wang, H.; Yang, W.; Gao, L.; Zhang, Z.; Xue, J.; Xu, B.; Yang, W.; et al. Trp2 Peptide-Assembled Nanoparticles with Intrinsically Self-Chelating 64Cu Properties for PET Imaging Tracking and Dendritic Cell-Based Immunotherapy against Melanoma. ACS Appl. Bio. Mater. 2021, 4, 5707–5716. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y.; Okazaki, T.; Katakai, T. Motility Dynamics of T Cells in Tumor-Draining Lymph Nodes: A Rational Indicator of Antitumor Response and Immune Checkpoint Blockade. Cancers 2021, 13, 4616. [Google Scholar] [CrossRef]
- Kotsias, F.; Cebrian, I.; Alloatti, A. Antigen processing and presentation. Int. Rev. Cell Mol. Biol. 2019, 348, 69–121. [Google Scholar] [CrossRef]
- Han, J.H.; Shin, H.E.; Lee, J.; Kang, J.M.; Park, J.H.; Park, C.G.; Han, D.K.; Kim, I.H.; Park, W. Combination of Metal-Phenolic Network-Based Immunoactive Nanoparticles and Bipolar Irreversible Electroporation for Effective Cancer Immunotherapy. Small 2022, 18, e2200316. [Google Scholar] [CrossRef]
- Rolig, A.S.; Rose, D.C.; McGee, G.H.; Rubas, W.; Kivimäe, S.; Redmond, W.L. Combining bempegaldesleukin (CD122-preferential IL-2 pathway agonist) and NKTR-262 (TLR7/8 agonist) improves systemic antitumor CD8+ T cell cytotoxicity over BEMPEG+RT. J. Immunother. Cancer 2022, 10, e004218. [Google Scholar] [CrossRef]
- Marashi, H.; Beihaghi, M.; Chaboksavar, M.; Khaksar, S.; Tehrani, H.; Abiri, A. In silico analysis and in planta production of recombinant ccl21/IL1β protein and characterization of its in vitro anti-tumor and immunogenic activity. PLoS ONE 2022, 17, e0261101. [Google Scholar] [CrossRef]
- Millán-Salanova, M.; Vicente-Manzanares, M. The interface between biochemical signaling and cell mechanics shapes T lymphocyte migration and activation. Eur. J. Cell Biol. 2022, 101, 151236. [Google Scholar] [CrossRef] [PubMed]
- Baljon, J.J.; Wilson, J.T. Bioinspired vaccines to enhance MHC class-I antigen cross-presentation. Curr. Opin. Immunol. 2022, 77, 102215. [Google Scholar] [CrossRef] [PubMed]
- Jongsma, M.L.M.; Neefjes, J.; Spaapen, R.M. Playing hide and seek: Tumor cells in control of MHC class I antigen presentation. Mol. Immunol. 2021, 136, 36–44. [Google Scholar] [CrossRef]
- Weishaupt, C.; Steinert, M.; Brunner, G.; Schulze, H.J.; Fuhlbrigge, R.C.; Goerge, T.; Loser, K. Activation of human vascular endothelium in melanoma metastases induces ICAM-1 and E-selectin expression and results in increased infiltration with effector lymphocytes. Exp. Dermatol. 2019, 28, 1258–1269. [Google Scholar] [CrossRef]
- Li, S.J.; Chen, J.X.; Sun, Z.J. Improving antitumor immunity using antiangiogenic agents: Mechanistic insights, current progress, and clinical challenges. Cancer Commun. 2021, 41, 830–850. [Google Scholar] [CrossRef]
- Zhou, X.; Li, C.; Zhang, Z.; Li, D.Y.; Du, J.; Ding, P.; Meng, H.; Xu, H.; Li, R.; Ho, E.; et al. Kinetics of plasma cfDNA predicts clinical response in non-small cell lung cancer patients. Sci. Rep. 2021, 11, 7633. [Google Scholar] [CrossRef]
- Bauer, S.M.; Williams, M.A.; Howell, A.P.; Schwarz, E.; Smith, E.S.; Zauderer, M. Maximizing immune responses: The effects of covalent peptide linkage to beta-2-microglobulin. Oncol. Res. 2008, 17, 20–216. [Google Scholar] [CrossRef]
- Chang, C.C.; Ogino, T.; Mullins, D.W.; Oliver, J.L.; Yamshchikov, G.V.; Bandoh, N.; Slingluff, C.L., Jr.; Ferrone, S. Defective human leukocyte antigen class I-associated antigen presentation caused by a novel beta2-microglobulin loss-of-function in melanoma cells. J. Biol. Chem. 2006, 281, 18763–18773. [Google Scholar] [CrossRef]
- Guerreiro-Cacais, A.O.; Uzunel, M.; Levitskaya, J.; Levitsky, V. Inhibition of heavy chain and beta2-microglobulin synthesis as a mechanism of major histocompatibility complex class I downregulation during Epstein-Barr virus replication. J. Virol. 2007, 81, 1390–1400. [Google Scholar] [CrossRef]
- Li, X.; Xiang, Y.; Li, F.; Yin, C.; Li, B.; Ke, X. WNT/β-Catenin Signaling Pathway Regulating T Cell-Inflammation in the Tumor Microenvironment. Front. Immunol. 2019, 10, 2293. [Google Scholar] [CrossRef]
- Gerhard, G.M.; Bill, R.; Messemaker, M.; Klein, A.M.; Pittet, M.J. Tumor-infiltrating dendritic cell states are conserved across solid human cancers. J. Exp. Med. 2021, 218, e20200264. [Google Scholar] [CrossRef]
- Saito, Y.; Komori, S.; Kotani, T.; Murata, Y.; Matozaki, T. The Role of Type-2 Conventional Dendritic Cells in the Regulation of Tumor Immunity. Cancers 2022, 14, 1976. [Google Scholar] [CrossRef]
- Kobayashi, T.; Oishi, K.; Okamura, A.; Maeda, S.; Komuro, A.; Hamaguchi, Y.; Fujimoto, M.; Takehara, K.; Matsushita, T. Regulatory B1a Cells Suppress Melanoma Tumor Immunity via IL-10 Production and Inhibiting T Helper Type 1 Cytokine Production in Tumor-Infiltrating CD8+ T Cells. J. Investig. Dermatol. 2019, 139, 1535–1544.e1. [Google Scholar] [CrossRef]
- Bellmann, L.; Cappellano, G.; Schachtl-Riess, J.F.; Prokopi, A.; Seretis, A.; Ortner, D.; Tripp, C.H.; Brinckerhoff, C.E.; Mullins, D.W.; Stoitzner, P. A TLR7 agonist strengthens T and NK cell function during BRAF-targeted therapy in a preclinical melanoma model. Int. J. Cancer 2020, 146, 1409–1420. [Google Scholar] [CrossRef] [PubMed]
- Petretto, A.; Carbotti, G.; Inglese, E.; Lavarello, C.; Pistillo, M.P.; Rigo, V.; Croce, M.; Longo, L.; Martini, S.; Vacca, P.; et al. Proteomic analysis uncovers common effects of IFN-γ and IL-27 on the HLA class I antigen presentation machinery in human cancer cells. Oncotarget 2016, 7, 72518–72536. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, L.; Zhu, J.; Zhang, Y.; Yang, R.; Yan, J.; Huang, R.; Zheng, C.; Xiao, W.; Huang, C.; et al. Predicting the herbal medicine triggering innate anti-tumor immunity from a system pharmacology perspective. Biomed. Pharmacother. 2021, 143, 112105. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Z.; Li, T.F.; Wang, C.; Ma, Y.; Liu, Y.; Zheng, M.Y.; Liu, Z.J.; Chen, J.B.; Li, K.; Sun, S.K.; et al. Synergy of nanodiamond-doxorubicin conjugates and PD-L1 blockade effectively turns tumor-associated macrophages against tumor cells. J. Nanobiotechnol. 2021, 19, 268. [Google Scholar] [CrossRef]
- Li, H.; Somiya, M.; Kuroda, S. Enhancing antibody-dependent cellular phagocytosis by Re-education of tumor-associated macrophages with resiquimod-encapsulated liposomes. Biomaterials 2021, 268, 120601. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Khan, M.N.; Shi, L.; Wang, Z.; Zheng, F.; Gong, F.; Fang, M. HMGB1 exacerbates experimental mouse colitis by enhancing innate lymphoid cells 3 inflammatory responses via promoted IL-23 production. Innate Immun. 2016, 22, 696–705. [Google Scholar] [CrossRef]
- Gao, Q.; Li, F.; Wang, S.; Shen, Z.; Cheng, S.; Ping, Y.; Qin, G.; Chen, X.; Yang, L.; Cao, L.; et al. A cycle involving HMGB1, IFN-γ and dendritic cells plays a putative role in anti-tumor immunity. Cell Immunol. 2019, 343, 103850. [Google Scholar] [CrossRef]
- Liu, H.; Innamarato, P.P.; Kodumudi, K.; Weber, A.; Nemoto, S.; Robinson, J.L.; Crago, G.; McCardle, T.; Royster, E.; Sarnaik, A.A.; et al. Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1. Oncotarget 2016, 7, 37893–37905. [Google Scholar] [CrossRef]
- Lau, C.M.; Tiniakou, I.; Perez, O.A.; Kirkling, M.E.; Yap, G.S.; Hock, H.; Reizis, B. Transcription factor Etv6 regulates functional differentiation of cross-presenting classical dendritic cells. J. Exp. Med. 2018, 215, 2265–2278. [Google Scholar] [CrossRef]
- Nizza, S.T.; Campbell, J.J. CD11b+ migratory dendritic cells mediate CD8 T cell cross-priming and cutaneous imprinting after topical immunization. PLoS ONE 2014, 9, e91054. [Google Scholar] [CrossRef]
- Bergamaschi, C.; Pandit, H.; Nagy, B.A.; Stellas, D.; Jensen, S.M.; Bear, J.; Cam, M.; Valentin, A.; Fox, B.A.; Felber, B.K.; et al. Heterodimeric IL-15 delays tumor growth and promotes intratumoral CTL and dendritic cell accumulation by a cytokine network involving XCL1, IFN-γ, CXCL9 and CXCL10. J. Immunother. Cancer 2020, 8, e000599. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e14. [Google Scholar] [CrossRef]
- Abdi, K.; Laky, K.; Abshari, M.; Hill, E.M.; Lantz, L.; Singh, N.J.; Long, E.O. Dendritic cells Trigger IFN-γ secretion by NK cells independent of IL-12 and IL-18. Eur. J. Immunol. 2022; in press. [Google Scholar] [CrossRef]
- Anderko, R.R.; Rinaldo, C.R.; Mailliard, R.B. IL-18 Responsiveness Defines Limitations in Immune Help for Specialized FcRγ− NK Cells. J. Immunol. 2020, 205, 3429–3442. [Google Scholar] [CrossRef]
- Ziblat, A.; Nuñez, S.Y.; Raffo Iraolagoitia, X.L.; Spallanzani, R.G.; Torres, N.I.; Sierra, J.M.; Secchiari, F.; Domaica, C.I.; Fuertes, M.B.; Zwirner, N.W. Interleukin (IL)-23 Stimulates IFN-γ Secretion by CD56bright Natural Killer Cells and Enhances IL-18-Driven Dendritic Cells Activation. Front. Immunol. 2018, 8, 1959. [Google Scholar] [CrossRef] [PubMed]
- Artinger, M.; Gerken, O.J.; Purvanov, V.; Legler, D.F. Distinct Fates of Chemokine and Surrogate Molecule Gradients: Consequences for CCR7-Guided Dendritic Cell Migration. Front. Immunol. 2022, 13, 913366. [Google Scholar] [CrossRef] [PubMed]
- Brandum, E.P.; Jørgensen, A.S.; Rosenkilde, M.M.; Hjortø, G.M. Dendritic Cells and CCR7 Expression: An Important Factor for Autoimmune Diseases, Chronic Inflammation, and Cancer. Int. J. Mol. Sci. 2021, 22, 8340. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kadam, P.; Dubinett, S. CCL21 Programs Immune Activity in Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1231, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, A.S.; Rosenkilde, M.M.; Hjortø, G.M. Biased signaling of G protein-coupled receptors—From a chemokine receptor CCR7 perspective. Gen. Comp. Endocrinol. 2018, 258, 4–14. [Google Scholar] [CrossRef]
- Ulvmar, M.H.; Werth, K.; Braun, A.; Kelay, P.; Hub, E.; Eller, K.; Chan, L.; Lucas, B.; Novitzky-Basso, I.; Nakamura, K.; et al. The atypical chemokine receptor CCRL1 shapes functional CCL21 gradients in lymph nodes. Nat. Immunol. 2014, 15, 623–630. [Google Scholar] [CrossRef]
- Ploix, C.C.; Noor, S.; Crane, J.; Masek, K.; Carter, W.; Lo, D.D.; Wilson, E.H.; Carson, M.J. CNS-derived CCL21 is both sufficient to drive homeostatic CD4+ T cell proliferation and necessary for efficient CD4+ T cell migration into the CNS parenchyma following Toxoplasma gondii infection. Brain Behav. Immun. 2011, 25, 883–896. [Google Scholar] [CrossRef]
- Jiang, K.; Zhang, Q.; Fan, Y.; Li, J.; Zhang, J.; Wang, W.; Fan, J.; Guo, Y.; Liu, S.; Hao, D.; et al. MYC inhibition reprograms tumor immune microenvironment by recruiting T lymphocytes and activating the CD40/CD40L system in osteosarcoma. Cell Death Discov. 2022, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Müerköster, S.; Laman, J.D.; Rocha, M.; Umansky, V.; Schirrmacher, V. Functional and in situ evidence for nitric oxide production driven by CD40-CD40L interactions in graft-versus-leukemia reactivity. Clin. Cancer Res. 2000, 6, 1988–1996. [Google Scholar] [PubMed]
- Zhang, X.; Zheng, P.; Prestwood, T.R.; Zhang, H.; Carmi, Y.; Tolentino, L.L.; Wu, N.; Choi, O.; Winer, D.A.; Strober, S.; et al. Human Regulatory Dendritic Cells Develop From Monocytes in Response to Signals From Regulatory and Helper T Cells. Front. Immunol. 2020, 11, 1982. [Google Scholar] [CrossRef] [PubMed]
- Heidegger, S.; Kreppel, D.; Bscheider, M.; Stritzke, F.; Nedelko, T.; Wintges, A.; Bek, S.; Fischer, J.C.; Graalmann, T.; Kalinke, U.; et al. RIG-I activating immunostimulatory RNA boosts the efficacy of anticancer vaccines and synergizes with immune checkpoint blockade. EBioMedicine 2019, 41, 146–155. [Google Scholar] [CrossRef]
- Hu, C.; Lei, T.; Wang, Y.; Cao, J.; Yang, X.; Qin, L.; Liu, R.; Zhou, Y.; Tong, F.; Umeshappa, C.S.; et al. Phagocyte-membrane-coated and laser-responsive nanoparticles control primary and metastatic cancer by inducing anti-tumor immunity. Biomaterials 2020, 255, 120159. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Mallik, S.B.; Gupta, P.; Iyer, A. Targeting Tumour-Associated Fibroblasts in Cancers. Front. Oncol. 2022, 12, 908156. [Google Scholar] [CrossRef]
- Ghahremanifard, P.; Chanda, A.; Bonni, S.; Bose, P. TGF-β Mediated Immune Evasion in Cancer-Spotlight on Cancer-Associated Fibroblasts. Cancers 2020, 12, 3650. [Google Scholar] [CrossRef]
- Affo, S.; Yu, L.X.; Schwabe, R.F. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annu. Rev. Pathol. 2017, 12, 153–186. [Google Scholar] [CrossRef]
- Dou, X.; Hua, Y.; Chen, Z.; Chao, F.; Li, M. Extracellular vesicles containing PD-L1 contribute to CD8+ T-cell immune suppression and predict poor outcomes in small cell lung cancer. Clin. Exp. Immunol. 2022, 207, 307–317. [Google Scholar] [CrossRef]
- Xu, M.; Zhou, C.; Weng, J.; Chen, Z.; Zhou, Q.; Gao, J.; Shi, G.; Ke, A.; Ren, N.; Sun, H.; et al. Tumor associated macrophages-derived exosomes facilitate hepatocellular carcinoma malignance by transferring lncMMPA to tumor cells and activating glycolysis pathway. J. Exp. Clin. Cancer Res. 2022, 41, 253. [Google Scholar] [CrossRef]
- Wang, M.; Cai, W.; Yang, A.J.; Wang, C.Y.; Zhang, C.L.; Liu, W.; Xie, X.F.; Gong, Y.Y.; Zhao, Y.Y.; Wu, W.C.; et al. Gastric cancer cell-derived extracellular vesicles disrupt endothelial integrity and promote metastasis. Cancer Lett. 2022, 545, 215827. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, S.; Zhang, P. Tumor-derived exosomes: Immune properties and clinical application in lung cancer. Cancer Drug Resist. 2022, 5, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi Najafabadi, S.A.; Bolhassani, A.; Aghasadeghi, M.R. Tumor cell-based vaccine: An effective strategy for eradication of cancer cells. Immunotherapy 2022, 14, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Mukherjee, S.; Ali, S.; Bhardwaj, U.; Choudhary, R.K.; Balakrishnan, S.; Naseem, A.; Mir, S.A.; Banawas, S.; Alaidarous, M.; et al. Pioneer Role of Extracellular Vesicles as Modulators of Cancer Initiation in Progression, Drug Therapy, and Vaccine Prospects. Cells 2022, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Parra, D.C.; Motallebnejad, P.; Brocchi, M.; Chen, H.J. Exosomes: Small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact. Mater. 2021, 10, 281–294. [Google Scholar] [CrossRef]
- Guo, W.; Qiao, T.; Dong, B.; Li, T.; Liu, Q.; Xu, X. The Effect of Hypoxia-Induced Exosomes on Anti-Tumor Immunity and Its Implication for Immunotherapy. Front. Immunol. 2022, 13, 915985. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, G.N.; Bhatta, M.; Bankert, R.B. Tumor-Associated Exosomes: A Potential Therapeutic Target for Restoring Anti-Tumor T Cell Responses in Human Tumor Microenvironments. Cells 2021, 10, 3155. [Google Scholar] [CrossRef]
- Whiteside, T.L. The Role of Tumor-Derived Exosomes (TEX) in Shaping Anti-Tumor Immune Competence. Cells 2021, 10, 3054. [Google Scholar] [CrossRef]
- Kowal, J.; Tkach, M. Dendritic cell extracellular vesicles. Int. Rev. Cell Mol. Biol. 2019, 349, 213–249. [Google Scholar] [CrossRef]
- Zeng, F.; Morelli, A.E. Extracellular vesicle-mediated MHC cross-dressing in immune homeostasis, transplantation, infectious diseases, and cancer. Semin. Immunopathol. 2018, 40, 477–490. [Google Scholar] [CrossRef]
- Hodge, A.L.; Baxter, A.A.; Poon, I.K.H. Gift bags from the sentinel cells of the immune system: The diverse role of dendritic cell-derived extracellular vesicles. J. Leukoc. Biol. 2022, 111, 903–920. [Google Scholar] [CrossRef]
- Quaglia, M.; Dellepiane, S.; Guglielmetti, G.; Merlotti, G.; Castellano, G.; Cantaluppi, V. Extracellular Vesicles as Mediators of Cellular Crosstalk Between Immune System and Kidney Graft. Front. Immunol. 2020, 11, 74. [Google Scholar] [CrossRef]
- Lindenbergh, M.F.S.; Stoorvogel, W. Antigen Presentation by Extracellular Vesicles from Professional Antigen-Presenting Cells. Annu. Rev. Immunol. 2018, 36, 435–459. [Google Scholar] [CrossRef]
- Morishita, M.; Takahashi, Y.; Nishikawa, M.; Ariizumi, R.; Takakura, Y. Enhanced Class I Tumor Antigen Presentation via Cytosolic Delivery of Exosomal Cargos by Tumor-Cell-Derived Exosomes Displaying a pH-Sensitive Fusogenic Peptide. Mol. Pharm. 2017, 14, 4079–4086. [Google Scholar] [CrossRef]
- Arima, Y.; Liu, W.; Takahashi, Y.; Nishikawa, M.; Takakura, Y. Effects of Localization of Antigen Proteins in Antigen-Loaded Exosomes on Efficiency of Antigen Presentation. Mol. Pharm. 2019, 16, 2309–2314. [Google Scholar] [CrossRef] [PubMed]
- Morishita, M.; Takahashi, Y.; Matsumoto, A.; Nishikawa, M.; Takakura, Y. Exosome-based tumor antigens-adjuvant co-delivery utilizing genetically engineered tumor cell-derived exosomes with immunostimulatory CpG DNA. Biomaterials 2016, 111, 55–65. [Google Scholar] [CrossRef]
- Liu, W.; Takahashi, Y.; Morishita, M.; Nishikawa, M.; Takakura, Y. Development of CD40L-modified tumor small extracellular vesicles for effective induction of antitumor immune response. Nanomedicine 2020, 15, 1641–1652. [Google Scholar] [CrossRef]
- Amodio, G.; Cichy, J.; Conde, P.; Matteoli, G.; Moreau, A.; Ochando, J.; Oral, B.H.; Pekarova, M.; Ryan, E.J.; Roth, J.; et al. Role of myeloid regulatory cells (MRCs) in maintaining tissue homeostasis and promoting tolerance in autoimmunity, inflammatory disease and transplantation. Cancer Immunol. Immunother. 2019, 68, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Mahmoudi, M.; Farhadi, E. Dendritic Cells Currently under the Spotlight; Classification and Subset Based upon New Markers. Immunol. Investig. 2021, 50, 646–661. [Google Scholar] [CrossRef] [PubMed]
- Morishita, M.; Takahashi, Y.; Nishikawa, M.; Sano, K.; Kato, K.; Yamashita, T.; Imai, T.; Saji, H.; Takakura, Y. Quantitative analysis of tissue distribution of the B16BL6-derived exosomes using a streptavidin-lactadherin fusion protein and iodine-125-labeled biotin derivative after intravenous injection in mice. J. Pharm. Sci. 2015, 104, 705–713. [Google Scholar] [CrossRef]
- Peng, Y.; Bai, W.; Wang, Z.; Yu, H. TLR9/NF-κB Pathway Regulates Brucella CpG DNA-mediated Cytokine Response in Human Peripheral Blood Mononuclear Cells. Iran. J. Immunol. 2021, 18, 268–278. [Google Scholar] [CrossRef]
- Tang, Y.; Ma, D.; Ming, S.; Zhang, L.; Zhou, J.; Shan, G.; Chen, Z.; Lu, X.; Zuo, D. Mannan-binding lectin reduces CpG DNA-induced inflammatory cytokine production by human monocytes. Microbiol. Immunol. 2015, 59, 231–237. [Google Scholar] [CrossRef]
- He, W.; Yu, Q.; Zhou, Z.; Wang, P. CpG oligonucleotides induce an immune response of odontoblasts through the TLR9, MyD88 and NF-kappaB pathways. Biochem. Biophys. Res. Commun. 2010, 399, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, T.H.; Wu, G.; Park, B.K.; Ha, J.H.; Kim, Y.S.; Lee, K.; Lee, Y.; Kwon, H.J. Extracellular Release of CD11b by TLR9 Stimulation in Macrophages. PLoS ONE 2016, 11, e0150677. [Google Scholar] [CrossRef]
- Kitai, Y.; Kawasaki, T.; Sueyoshi, T.; Kobiyama, K.; Ishii, K.J.; Zou, J.; Akira, S.; Matsuda, T.; Kawai, T. DNA-Containing Exosomes Derived from Cancer Cells Treated with Topotecan Activate a STING-Dependent Pathway and Reinforce Antitumor Immunity. J. Immunol. 2017, 198, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Hazrati, A.; Soudi, S.; Malekpour, K.; Mahmoudi, M.; Rahimi, A.; Hashemi, S.M.; Varma, R.S. Immune cells-derived exosomes function as a double-edged sword: Role in disease progression and their therapeutic applications. Biomark. Res. 2022, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Lindenbergh, M.F.S.; Wubbolts, R.; Borg, E.G.F.; van’t Veld, E.M.; Boes, M.; Stoorvogel, W. Dendritic cells release exosomes together with phagocytosed pathogen; potential implications for the role of exosomes in antigen presentation. J. Extracell. Vesicles 2020, 9, 1798606. [Google Scholar] [CrossRef]
- Deng, C.J.; Liu, L.; Liu, L.Z.; Wang, Q.Q.; Guo, X.L.; Lee, W.H.; Li, S.A.; Zhang, Y. A secreted pore-forming protein modulates cellular endolysosomes to augment antigen presentation. FASEB J. 2020, 34, 13609–13625. [Google Scholar] [CrossRef]
- Hao, S.; Liu, Y.; Yuan, J.; Zhang, X.; He, T.; Wu, X.; Wei, Y.; Sun, D.; Xiang, J. Novel exosome-targeted CD4+ T cell vaccine counteracting CD4+25+ regulatory T cell-mediated immune suppression and stimulating efficient central memory CD8+ CTL responses. J. Immunol. 2007, 179, 2731–2740. [Google Scholar] [CrossRef]
- Utsugi-Kobukai, S.; Fujimaki, H.; Hotta, C.; Nakazawa, M.; Minami, M. MHC class I-mediated exogenous antigen presentation by exosomes secreted from immature and mature bone marrow derived dendritic cells. Immunol. Lett. 2003, 89, 125–131. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Long, Y.; Chen, Y. Extracellular Vesicle Derived From Mesenchymal Stem Cells Have Bidirectional Effects on the Development of Lung Cancer. Front. Oncol. 2022, 12, 914832. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Shi, Y.; Wang, X.; Li, X. Advances in the application of low-intensity pulsed ultrasound to mesenchymal stem cells. Stem Cell Res. Ther. 2022, 13, 214. [Google Scholar] [CrossRef]
- Chen, L.; Qu, J.; Kalyani, F.S.; Zhang, Q.; Fan, L.; Fang, Y.; Li, Y.; Xiang, C. Mesenchymal stem cell-based treatments for COVID-19: Status and future perspectives for clinical applications. Cell Mol. Life Sci. 2022, 79, 142. [Google Scholar] [CrossRef]
- Vatsa, P.; Negi, R.; Ansari, U.A.; Khanna, V.K.; Pant, A.B. Insights of Extracellular Vesicles of Mesenchymal Stem Cells: A Prospective Cell-Free Regenerative Medicine for Neurodegenerative Disorders. Mol. Neurobiol. 2022, 59, 459–474. [Google Scholar] [CrossRef]
- Wu, M.C.; Meng, Q.H. Current understanding of mesenchymal stem cells in liver diseases. World J. Stem Cells 2021, 13, 1349–1359. [Google Scholar] [CrossRef]
- Hassanshahi, G.; Roohi, M.A.; Esmaeili, S.A.; Pourghadamyari, H.; Nosratabadi, R. Involvement of various chemokine/chemokine receptor axes in trafficking and oriented locomotion of mesenchymal stem cells in multiple sclerosis patients. Cytokine 2021, 148, 155706. [Google Scholar] [CrossRef]
- Johnson, J.; Shojaee, M.; Mitchell Crow, J.; Khanabdali, R. From Mesenchymal Stromal Cells to Engineered Extracellular Vesicles: A New Therapeutic Paradigm. Front. Cell Dev. Biol. 2021, 9, 705676. [Google Scholar] [CrossRef]
- Asgari Taei, A.; Khodabakhsh, P.; Nasoohi, S.; Farahmandfar, M.; Dargahi, L. Paracrine Effects of Mesenchymal Stem Cells in Ischemic Stroke: Opportunities and Challenges. Mol. Neurobiol. 2022; in press. [Google Scholar] [CrossRef]
- Ahmed, L.; Al-Massri, K. New Approaches for Enhancement of the Efficacy of Mesenchymal Stem Cell-Derived Exosomes in Cardiovascular Diseases. Tissue Eng. Regen. Med. 2022; in press. [Google Scholar] [CrossRef]
- Fujii, S.; Miura, Y. Immunomodulatory and regenerative effects of MSC-derived extracellular vesicles to treat acute GVHD. Stem Cells 2022, 5, sxac057. [Google Scholar] [CrossRef]
- Sheikholeslami, A.; Fazaeli, H.; Khoshandam, M.; Kalhor, N.; Eshaghhosseini, S.J.; Sheykhhasan, M. Use of Mesenchymal Stem Cells in Crohn’s Disease and Perianal Fistulas: A Narrative Review. Curr. Stem Cell Res. Ther. 2021; in press. [Google Scholar] [CrossRef]
- Arabpour, M.; Saghazadeh, A.; Rezaei, N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int. Immunopharmacol. 2021, 97, 107823. [Google Scholar] [CrossRef]
- Botello-Flores, Y.A.; Yocupicio-Monroy, M.; Balderrábano-Saucedo, N.; Contreras-Ramos, A. A systematic review on the role of MSC-derived exosomal miRNAs in the treatment of heart failure. Mol. Biol. Rep. 2022, in press. [CrossRef] [PubMed]
- Xiong, J.; Hu, H.; Guo, R.; Wang, H.; Jiang, H. Mesenchymal Stem Cell Exosomes as a New Strategy for the Treatment of Diabetes Complications. Front. Endocrinol. 2021, 12, 646233. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Kita, S.; Tanaka, Y.; Fukuda, S.; Obata, Y.; Okita, T.; Nishida, H.; Takahashi, Y.; Kawachi, Y.; Tsugawa-Shimizu, Y.; et al. Adiponectin Stimulates Exosome Release to Enhance Mesenchymal Stem-Cell-Driven Therapy of Heart Failure in Mice. Mol. Ther. 2020, 28, 2203–2219. [Google Scholar] [CrossRef]
- Kawada-Horitani, E.; Kita, S.; Okita, T.; Nakamura, Y.; Nishida, H.; Honma, Y.; Fukuda, S.; Tsugawa-Shimizu, Y.; Kozawa, J.; Sakaue, T.; et al. Human adipose-derived mesenchymal stem cells prevent type 1 diabetes induced by immune checkpoint blockade. Diabetologia 2022, 65, 1185–1197. [Google Scholar] [CrossRef]
- Xu, H.Y.; Li, N.; Yao, N.; Xu, X.F.; Wang, H.X.; Liu, X.Y.; Zhang, Y. CD8+ T cells stimulated by exosomes derived from RenCa cells mediate specific immune responses through the FasL/Fas signaling pathway and, combined with GM-CSF and IL-12, enhance the anti-renal cortical adenocarcinoma effect. Oncol. Rep. 2019, 42, 866–879. [Google Scholar] [CrossRef]
- Qin, Q.; Song, R.; Du, P.; Gao, C.; Yao, Q.; Zhang, J.A. Systemic Proteomic Analysis Reveals Distinct Exosomal Protein Profiles in Rheumatoid Arthritis. J. Immunol. Res. 2021, 2021, 9421720. [Google Scholar] [CrossRef]
- Takamura, Y.; Aoki, W.; Satomura, A.; Shibasaki, S.; Ueda, M. Small RNAs detected in exosomes derived from the MH7A synovial fibroblast cell line with TNF-α stimulation. PLoS ONE 2018, 13, e0201851. [Google Scholar] [CrossRef]
- Hosseini, R.; Sarvnaz, H.; Arabpour, M.; Ramshe, S.M.; Asef-Kabiri, L.; Yousefi, H.; Akbari, M.E.; Eskandari, N. Cancer exosomes and natural killer cells dysfunction: Biological roles, clinical significance and implications for immunotherapy. Mol. Cancer 2022, 21, 15. [Google Scholar] [CrossRef]
- Ponath, V.; Hoffmann, N.; Bergmann, L.; Mäder, C.; Alashkar, A.B.; Preußer, C.; Pogge von Strandmann, E. Secreted Ligands of the NK Cell Receptor NKp30: B7-H6 Is in Contrast to BAG6 Only Marginally Released via Extracellular Vesicles. Int. J. Mol. Sci. 2021, 22, 2189. [Google Scholar] [CrossRef]
- Ferguson Bennit, H.R.; Gonda, A.; Kabagwira, J.; Oppegard, L.; Chi, D.; Licero Campbell, J.; De Leon, M.; Wall, N.R. Natural Killer Cell Phenotype and Functionality Affected by Exposure to Extracellular Survivin and Lymphoma-Derived Exosomes. Int. J. Mol. Sci. 2021, 22, 1255. [Google Scholar] [CrossRef]
- Zhu, X.; Qin, X.; Wang, X.; Wang, Y.; Cao, W.; Zhang, J.; Chen, W. Oral cancer cell-derived exosomes modulate natural killer cell activity by regulating the receptors on these cells. Int. J. Mol. Med. 2020, 46, 2115–2125. [Google Scholar] [CrossRef]
- Jafarzadeh, N.; Safari, Z.; Pornour, M.; Amirizadeh, N.; Forouzandeh, M.M.; Sadeghizadeh, M. Alteration of cellular and immune-related properties of bone marrow mesenchymal stem cells and macrophages by K562 chronic myeloid leukemia cell derived exosomes. J. Cell Physiol. 2019, 234, 3697–3710. [Google Scholar] [CrossRef]
- Koh, E.; Lee, E.J.; Nam, G.H.; Hong, Y.; Cho, E.; Yang, Y.; Kim, I.S. Exosome-SIRPα, a CD47 blockade increases cancer cell phagocytosis. Biomaterials 2017, 121, 121–129. [Google Scholar] [CrossRef]
- Du, J.; Wan, Z.; Wang, C.; Lu, F.; Wei, M.; Wang, D.; Hao, Q. Designer exosomes for targeted and efficient ferroptosis induction in cancer via chemo-photodynamic therapy. Theranostics 2021, 11, 8185–8196. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Sawada, K.; Kobayashi, M.; Yamamoto, M.; Yagi, T.; Kinose, Y.; Kodama, M.; Hashimoto, K.; Kimura, T. Exosomal CD47 Plays an Essential Role in Immune Evasion in Ovarian Cancer. Mol. Cancer Res. 2021, 19, 1583–1595. [Google Scholar] [CrossRef]
- Kamerkar, S.; LeBleu, V.S.; Sugimoto, H.; Yang, S.; Ruivo, C.F.; Melo, S.A.; Lee, J.J.; Kalluri, R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 2017, 546, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liu, X.; Wu, F.; Ge, C.; Ye, H.; Chen, X.; Wei, Y.; Zhou, R.; Duan, S.; Zhu, R.; et al. Platelet Pharmacytes for the Hierarchical Amplification of Antitumor Immunity in Response to Self-Generated Immune Signals. Adv. Mater. 2022, 34, e2109517. [Google Scholar] [CrossRef] [PubMed]
- Rolfes, V.; Idel, C.; Pries, R.; Plötze-Martin, K.; Habermann, J.; Gemoll, T.; Bohnet, S.; Latz, E.; Ribbat-Idel, J.; Franklin, B.S.; et al. PD-L1 is expressed on human platelets and is affected by immune checkpoint therapy. Oncotarget 2018, 9, 27460–27470. [Google Scholar] [CrossRef] [PubMed]
- Ning, H.; Chen, H.; Deng, J.; Xiao, C.; Xu, M.; Shan, L.; Yang, C.; Zhang, Z. Exosomes secreted by FNDC5-BMMSCs protect myocardial infarction by anti-inflammation and macrophage polarization via NF-κB signaling pathway and Nrf2/HO-1 axis. Stem Cell Res. Ther. 2021, 12, 519. [Google Scholar] [CrossRef]
- Li, C.; Li, X.; Shi, Z.; Wu, P.; Fu, J.; Tang, J.; Qing, L. Exosomes from LPS-preconditioned bone marrow MSCs accelerated peripheral nerve regeneration via M2 macrophage polarization: Involvement of TSG-6/NF-κB/NLRP3 signaling pathway. Exp. Neurol. 2022, 356, 114139. [Google Scholar] [CrossRef]
- Hu, Y.; Qu, H.; He, J.; Zhong, H.; He, S.; Zhao, P.; Zhang, L.; Chen, J.; Deng, C. Human placental mesenchymal stem cell derived exosomes exhibit anti-inflammatory effects via TLR4-mediated NF-κB/MAPK and PI3K signaling pathways. Pharmazie 2022, 77, 112–117. [Google Scholar] [CrossRef]
- Fan, L.; Dong, J.; He, X.; Zhang, C.; Zhang, T. Bone marrow mesenchymal stem cells-derived exosomes reduce apoptosis and inflammatory response during spinal cord injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway. Hum. Exp. Toxicol. 2021, 40, 1612–1623. [Google Scholar] [CrossRef]
- Mentkowski, K.I.; Mursleen, A.; Snitzer, J.D.; Euscher, L.M.; Lang, J.K. CDC-derived extracellular vesicles reprogram inflammatory macrophages to an arginase 1-dependent proangiogenic phenotype. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1447–H1460. [Google Scholar] [CrossRef]
- Hattori, H.; Takaoka, K.; Ueta, M.; Oshitani, M.; Tamaoka, J.; Noguchi, K.; Kishimoto, H. Senescent RAW264.7 cells exhibit increased production of nitric oxide and release inducible nitric oxide synthase in exo-somes. Mol. Med. Rep. 2021, 24, 681. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).