Comprehensive Highlights of the Universal Efforts towards the Development of COVID-19 Vaccine
Abstract
:1. Introduction
2. The COVID-19 Vaccine Scenario
2.1. The Race for Development
2.2. Clinical Trial Reports
2.3. Computational Roles in Vaccine Development
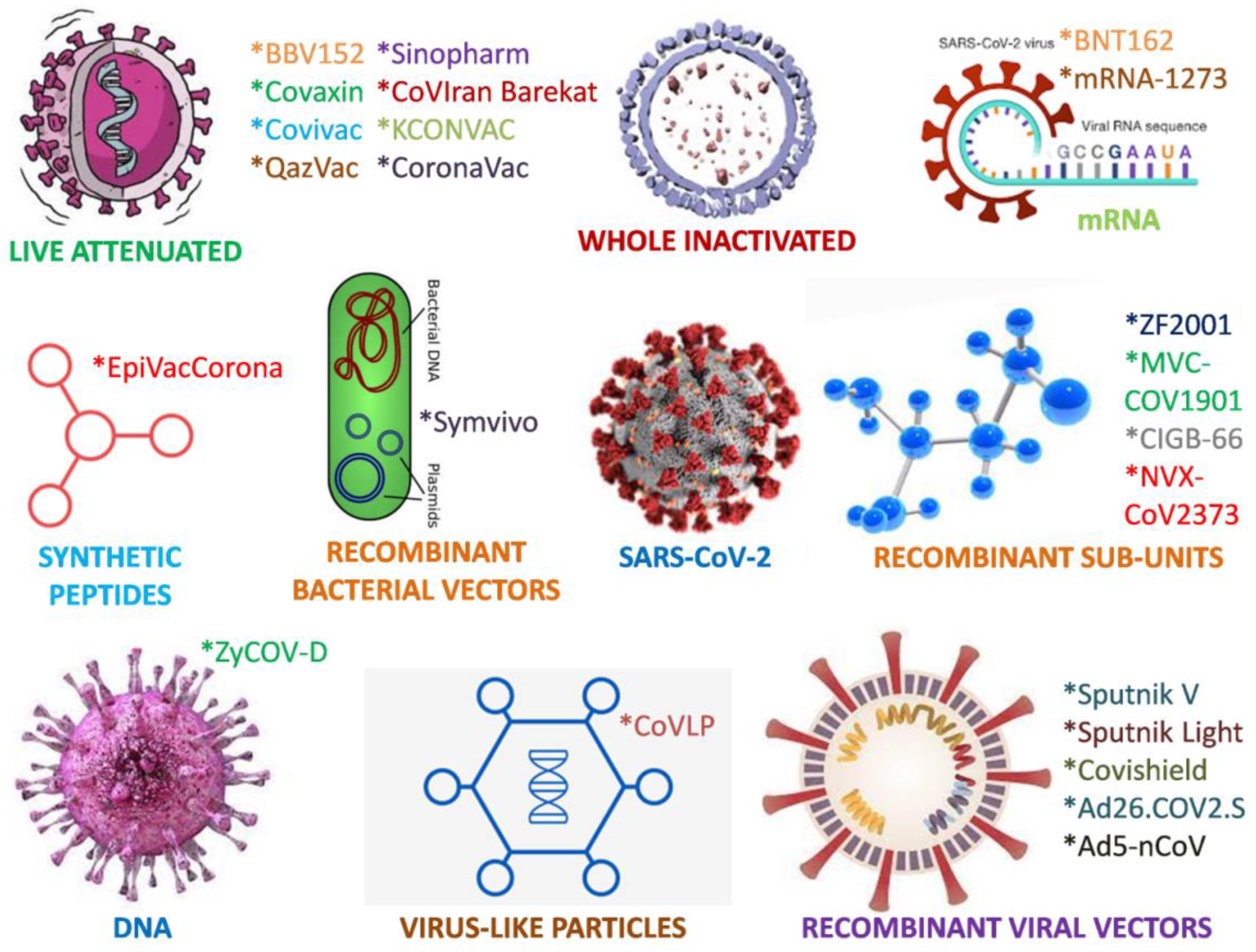
3. Vaccines
3.1. Conventional Whole-Virus Vaccines
3.1.1. Live-Attenuated Vaccines
New Vaccine
3.1.2. Inactivated Vaccines
Clinical Trials
CoronaVac
BBIBP-CorV
BBV152
3.2. Recombinant Viral Protein-Based Vaccines
3.2.1. Protein Subunit Vaccines
Clinical Trials Report
NVX-CoV2373
UB-612
Cuban Abdala
3.2.2. Virus-Like Particle (VLP) Vaccines
3.3. Viral Vector Vaccines
3.3.1. Types of Viral Vector Vaccines
Nonreplicating Viral Vector Vaccines
Ad5 nCoV
JNJ-78436735
AZD1222
VXA-CoV-2-1
Sputnik V
Replicating Viral Vector Vaccines
Clinical Trials
3.4. Nucleic Acid Vaccines
3.4.1. DNA Vaccines
Clinical Trials
INO-4800
AG0301 and AG0302
3.4.2. mRNA Vaccines
Clinical Trials
3.5. Promising Vaccine Candidates
3.6. Vaccines Used under Emergency Authorization
3.6.1. Sinopharm
3.6.2. Sinovac
3.6.3. Covaxin
3.6.4. CoviVac
3.6.5. QazVac
3.6.6. COVIranBarekat
3.6.7. KCONVAC
3.6.8. Sputnik V
Clinical Trials
3.6.9. Oxford/AstraZeneca
Clinical Trials
3.6.10. CanSino
3.6.11. Pfizer/BioNTech
Clinical Trials
3.6.12. Moderna
Clinical Trials
3.6.13. Ad26.COV2. S
3.6.14. ZyCoV-D
3.6.15. EpiVacCorona
3.6.16. ZF2001
3.6.17. CIGB-66
3.6.18. MVC-COV1901
4. Adjuvants
4.1. Role of Adjuvants
4.2. Natural Products
4.3. Global Market
5. mRNA Sequence Modification
6. Delivery Vehicles
6.1. Nanotechnology
6.2. Lipid Nanoparticles
6.3. Self-Assembling Nanoparticles
7. Imperative Aspects and Their Applications in COVID-19 Vaccines
7.1. Regulatory Scenario
7.2. Mutations
7.3. Storage
8. COVID-19 Variants as Challenges to Vaccines
9. Vaccination: Protection
10. Vaccination Challenges
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anand, U.; Jakhmola, S.; Indari, O.; Chandra Jha, H.; Chen, Z.S.; Tripathi, V.; Pérez de la Lastra, J.M. Potential therapeutic targets and vaccine development for COVID-19 management: A review on the recent update. Front. Immunol. 2021, 12, 2454. [Google Scholar] [CrossRef]
- Izda, V.; Jeffries, M.A.; Sawalha, A.H. COVID-19: A review of therapeutic strategies and vaccine candidates. Clin. Immunol. 2021, 222, 108634. [Google Scholar] [CrossRef]
- Shahzamani, K.; Mahmoudian, F.; Ahangarzadeh, S.; Ranjbar, M.M.; Beikmohammadi, L.; Bahrami, S.; Mohammadi, E.; Esfandyari, S.; Alibakhshi, A.; Javanmard, S.H. Vaccine design and delivery approaches for COVID-19. Int. Immunopharmacol. 2021, 100, 108086. [Google Scholar] [CrossRef]
- Mellet, J.; Pepper, M.S. A COVID-19 vaccine: Big strides come with big challenges. Vaccines 2021, 9, 39. [Google Scholar] [CrossRef]
- Kaur, R.J.; Dutta, S.; Bhardwaj, P.; Charan, J.; Dhingra, S.; Mitra, P.; Singh, K.; Yadav, D.; Sharma, P.; Misra, S. Adverse Events Reported from COVID-19 Vaccine Trials: A Systematic Review. Indian J. Clin. Biochem. 2021, 36, 427–439. [Google Scholar] [CrossRef]
- Wang, J.; Peng, Y.; Xu, H.; Cui, Z.; Williams, R.O. The COVID-19 vaccine race: Challenges and opportunities in vaccine formulation. AAPS Pharm. Sci. Tech. 2020, 21, 225. [Google Scholar] [CrossRef]
- Krause, P.R.; Fleming, T.R.; Peto, R.; Longini, I.M.; Figueroa, J.P.; Sterne, J.A.; Cravioto, A.; Rees, H.; Higgins, J.P.; Boutron, I.; et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet 2021, 398, 1377–1380. [Google Scholar] [CrossRef]
- Chevallier, C.; Hacquin, A.S.; Mercier, H. COVID-19 vaccine hesitancy: Shortening the last mile. Trend Cogn. Sci. 2021, 25, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K. Vaccine against Covid-19 disease–present status of development. Indian J. Pediatr. 2020, 87, 810–816. [Google Scholar] [CrossRef]
- Attia, Y.A.; El-Saadony, M.T.; Swelum, A.A.; Qattan, S.Y.; Al-Qurashi, A.D.; Asiry, K.A.; Shafi, M.E.; Elbestawy, A.R.; Gado, A.R.; Khafaga, A.F.; et al. COVID-19: Pathogenesis, advances in treatment and vaccine development and environmental impact—An updated review. Environ. Sci. Pollut. Res. 2021, 28, 22241–22264. [Google Scholar] [CrossRef] [PubMed]
- Vasireddy, D.; Vanaparthy, R.; Mohan, G.; Malayala, S.V.; Atluri, P. Review of COVID-19 Variants and COVID-19 Vaccine Efficacy: What the Clinician Should Know? J. Clin. Med. Res. 2021, 13, 317. [Google Scholar] [CrossRef]
- Moradi, M.; Golmohammadi, R.; Najafi, A.; Moosazadeh-Moghadam, M.; Fasihi-Ramandi, M.; Mirnejad, R. A contemporary review on the important role of in silico approaches for managing different aspects of COVID-19 crisis. Informat. Med. Unlock. 2022, 28, 100862. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Pennisi, M.; Fichera, E.; Motta, S.; Raciti, G.; Viceconti, M.; Pappalardo, F. In silico trial to test COVID-19 candidate vaccines: A case study with UISS platform. BMC Bioinformat. 2020, 21, 527. [Google Scholar] [CrossRef]
- Belete, T.M. A review on Promising vaccine development progress for COVID-19 disease. Vacunas 2020, 21, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Haidere, M.F.; Ratan, Z.A.; Nowroz, S.; Zaman, S.B.; Jung, Y.J.; Hosseinzadeh, H.; Cho, J.Y. COVID-19 vaccine: Critical questions with complicated answers. Biomol. Therapeut. 2021, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. COVID-19: Where are we on vaccines and variants? BMJ 2021, 372, n597. [Google Scholar] [CrossRef]
- Prüβ, B.M. Current state of the first COVID-19 vaccines. Vaccines 2021, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.K.; Kayarohanam, S.; Fuloria, S.; Fuloria, N.K.; Janakiraman, A.K.; Djearamane, S.; Wu, Y.S.; Chakravarthi, S.; Subramaniyan, V. COVID-19 vaccine candidates under clinical evaluation-a review. Int. J. Pharm. Res. 2021, 13, 4588–4598. [Google Scholar]
- Yang, L.; Tian, D.; Liu, W. Strategies for vaccine development of COVID-19. Chin. J. Biotechnol. 2020, 36, 593–604. [Google Scholar]
- Wibawa, T. COVID-19 vaccine research and development: Ethical issues. Trop. Med. Int. Health 2021, 26, 14–19. [Google Scholar] [CrossRef]
- Teo, S.P. Review of COVID-19 Vaccines and Their Evidence in Older Adults. Annal. Geriatr. Med. Res. 2021, 25, 4. [Google Scholar] [CrossRef]
- Teo, S.P. Review of COVID-19 mRNA Vaccines: BNT162b2 and mRNA-1273. J. Pharm. Pract. 2021, 8971900211009650. [Google Scholar] [CrossRef]
- Luchsinger, L.L.; Hillyer, C.D. Vaccine efficacy probable against COVID-19 variants. Science 2021, 371, 1116. [Google Scholar] [CrossRef]
- Vitiello, A.; Ferrara, F. Brief review of the mRNA vaccines COVID-19. Inflammopharmacology 2021, 29, 645–649. [Google Scholar] [CrossRef]
- Shahcheraghi, S.H.; Ayatollahi, J.; Aljabali, A.A.; Shastri, M.D.; Shukla, S.D.; Chellappan, D.K.; Jha, N.K.; Anand, K.; Katari, N.K.; Mehta, M.; et al. An overview of vaccine development for COVID-19. Ther. Deliv. 2021, 12, 235–244. [Google Scholar] [CrossRef]
- Khan, S.F. A review on how exactly COVID-19 vaccination works. GSC Biol. Pharm. Sci. 2021, 14, 75–81. [Google Scholar] [CrossRef]
- Meena, L.S. A comprehensive review of COVID-19 in India: A frequent catch of the information. Biotechnol. Appl. Biochem. 2021, 68, 700–711. [Google Scholar]
- Sharma, O.; Sultan, A.A.; Ding, H.; Triggle, C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020, 11, 585354. [Google Scholar] [CrossRef]
- Sallam, M. COVID-19 vaccine hesitancy worldwide: A concise systematic review of vaccine acceptance rates. Vaccines 2021, 9, 160. [Google Scholar] [CrossRef]
- Forchette, L.; Sebastian, W.; Liu, T. A comprehensive review of COVID-19 virology, vaccines, variants, and therapeutics. Curr. Med. Sci. 2021, 41, 1037–1051. [Google Scholar] [CrossRef]
- Kamidani, S.; Rostad, C.A.; Anderson, E.J. COVID-19 vaccine development: A pediatric perspective. Curr. Opin. Pediatr. 2021, 33, 144–151. [Google Scholar] [CrossRef]
- Nagy, A.; Alhatlani, B. An overview of current COVID-19 vaccine platforms. Comp. Struct. Biotechnol. J. 2021, 19, 2508–2517. [Google Scholar] [CrossRef]
- Shah, S.M.; Alsaab, H.O.; Rawas-Qalaji, M.M.; Uddin, M.N. A review on current COVID-19 vaccines and evaluation of particulate vaccine delivery systems. Vaccines 2021, 9, 1086. [Google Scholar] [CrossRef]
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020, 288, 198114. [Google Scholar] [CrossRef]
- Saha, R.P.; Sharma, A.R.; Singh, M.K.; Samanta, S.; Bhakta, S.; Mandal, S.; Bhattacharya, M.; Lee, S.S.; Chakraborty, C. Repurposing drugs, ongoing vaccine, and new therapeutic development initiatives against COVID-19. Front. Pharmacol. 2020, 11, 1258. [Google Scholar] [CrossRef]
- Borah, P.; Deb, P.K.; Al-Shar’i, N.A.; Dahabiyeh, L.A.; Venugopala, K.N.; Singh, V.; Shinu, P.; Hussain, S.; Deka, S.; Chandrasekaran, B.; et al. Perspectives on RNA vaccine candidates for COVID-19. Front. Mol. Biosci. 2021, 8, 30. [Google Scholar] [CrossRef]
- Más-Bermejo, P.I.; Dickinson-Meneses, F.O.; Almenares-Rodríguez, K.; Sanchez, L.; Guinovart-Díaz, R.; Vidal-Ledo, M.; Galbán-García, E.; Olivera-Nodarse, Y.; Morgado-Vega, I.; Dueñas-Carrera, S.; et al. Cuban Abdala Vaccine: Effectiveness in Preventing Severe Disease and Death from COVID-19 in Havana, Cuba; a Cohort Study. Lancet Reg. Health 2022, 16, 100366. [Google Scholar]
- ElBagoury, M.; Tolba, M.M.; Nasser, H.A.; Jabbar, A.; Elagouz, A.M.; Aktham, Y.; Hutchinson, A. The find of COVID-19 vaccine: Challenges and opportunities. J. Infect. Public Health 2021, 14, 389–416. [Google Scholar] [CrossRef]
- Li, Y.; Tenchov, R.; Smoot, J.; Liu, C.; Watkins, S.; Zhou, Q. A comprehensive review of the global efforts on COVID-19 vaccine development. ACS Cent. Sci. 2021, 7, 512–533. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Al-Ahmed, S.H.; Sah, R.; Al-Tawfiq, J.A.; Al-Qaaneh, A.M.; Al-Jamea, L.H.; Woodman, A.; Al-Qahtani, M.; Haque, S.; Harapan, H.; et al. Recent advances in vaccine and immunotherapy for COVID-19. Hum. Vaccine Immunother. 2020, 16, 3011–3022. [Google Scholar] [CrossRef]
- Forman, R.; Shah, S.; Jeurissen, P.; Jit, M.; Mossialos, E. COVID-19 vaccine challenges: What have we learned thus far and what remains to be done? Health Policy 2021, 125, 553–567. [Google Scholar] [CrossRef]
- Won, J.H.; Lee, H. The current status of drug repositioning and vaccine developments for the COVID-19 pandemic. Int. J. Mol. Sci. 2020, 21, 9775. [Google Scholar] [CrossRef]
- Li, Y.D.; Chi, W.Y.; Su, J.H.; Ferrall, L.; Hung, C.F.; Wu, T.C. Coronavirus vaccine development: From SARS and MERS to COVID-19. J. Biomed. Sci. 2020, 27, 104. [Google Scholar] [CrossRef]
- Jeyanathan, M.; Afkhami, S.; Smaill, F.; Miller, M.S.; Lichty, B.D.; Xing, Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Rev. Immunol. 2020, 20, 615–632. [Google Scholar] [CrossRef]
- Soleimanpour, S.; Yaghoubi, A. COVID-19 vaccine: Where are we now and where should we go? Exp. Rev. Vaccine 2021, 20, 23–44. [Google Scholar] [CrossRef]
- Francis, A.I.; Ghany, S.; Gilkes, T.; Umakanthan, S. Review of COVID-19 vaccine subtypes, efficacy and geographical distributions. Postgrad. Med. J. 2022, 98, 389–394. [Google Scholar] [CrossRef]
- Vashi, A.P.; Coiado, O.C. The future of COVID-19: A vaccine review. J. Infect. Public Health 2021, 14, 1461–1465. [Google Scholar] [CrossRef]
- Marian, A.J. Current state of vaccine development and targeted therapies for COVID-19: Impact of basic science discoveries. Cardiovasc. Pathol. 2021, 50, 107278. [Google Scholar] [CrossRef]
- Burgos, R.M.; Badowski, M.E.; Drwiega, E.; Ghassemi, S.; Griffith, N.; Herald, F.; Johnson, M.; Smith, R.O.; Michienzi, S.M. The race to a COVID-19 vaccine: Opportunities and challenges in development and distribution. Drugs Context 2021, 10, 2020-12-2. [Google Scholar] [CrossRef]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 vaccine effort: Viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef]
- Shekhar, R.; Garg, I.; Pal, S.; Kottewar, S.; Sheikh, A.B. COVID-19 vaccine booster: To boost or not to boost. Infect. Dis. Rep. 2021, 13, 924–929. [Google Scholar] [CrossRef]
- Haque, A.; Pant, A.B. Efforts at COVID-19 vaccine development: Challenges and successes. Vaccines 2020, 8, 739. [Google Scholar] [CrossRef]
- Burckhardt, R.M.; Dennehy, J.J.; Poon, L.L.; Saif, L.J.; Enquist, L.W. Are COVID-19 vaccine boosters needed? The science behind boosters. J. Virol. 2022, 96, e0197321. [Google Scholar] [CrossRef]
- Ullah, I.; Khan, K.S.; Tahir, M.J.; Ahmed, A.; Harapan, H. Myths and conspiracy theories on vaccines and COVID-19: Potential effect on global vaccine refusals. Vacunas 2021, 22, 93–97. [Google Scholar] [CrossRef]
- Al-Kassmy, J.; Pedersen, J.; Kobinger, G. Vaccine candidates against coronavirus infections. Where does COVID-19 stand? Viruses 2020, 12, 861. [Google Scholar] [CrossRef]
- Zhu, J.; Ananthaswamy, N.; Jain, S.; Batra, H.; Tang, W.C.; Lewry, D.A.; Richards, M.L.; David, S.A.; Kilgore, P.B.; Sha, J.; et al. A universal bacteriophage T4 nanoparticle platform to design multiplex SARS-CoV-2 vaccine candidates by CRISPR engineering. Sci. Adv. 2021, 7, eabh1547. [Google Scholar] [CrossRef]
- Batra, H.; Zhu, J.; Jain, S.; Ananthaswamy, N.; Mahalingam, M.; Tao, P.; Lange, C.; Zhong, C.; Kearney, M.F.; Hu, H.; et al. Engineered Bacteriophage T4 Nanoparticle as a Potential Targeted Activator of HIV-1 Latency in CD4 + Human T-cells. bioRxiv 2021. [Google Scholar] [CrossRef]

| Vaccine Platform | Organization | Candidate | Immune Response | Country Involved | Key Trials |
|---|---|---|---|---|---|
| RNA-based vaccine | BioNTech/Pfizer | BNT162(3LNP-mRNAs) | Spike protein; S-2P (full-length with proline substitutions, K986P and V987P) | Germany/United States | Phase 3 NCT04368728 |
| Moderna/National Institute of Allergy and Infectious Diseases | mRNA-1273 | Spike protein; S-2P (full-length with proline substitutions, K986P and V987P) | United States | Phase 3 NCT04470427 | |
| CureVac | CVnCoV Vaccine | Prefusion stabilized full-length spike protein | Germany | Phase 3 NCT04674189 | |
| DNA-based vaccine | Inovio Pharmaceuticals + International Vaccine Institute + Advaccine Biopharmaceuticals | INO-4800 + Electroporation | Full-length spike protein | United States | Phase 2/3 NCT04642638 |
| AnGes + Takara Bio + Osaka University | AG0301-COVID19 | Spike protein | Japan | Phase2/3 NCT04655625 | |
| ZydusCadila | nCov vaccine (ZyCoV-D) | Spike protein | India | Phase 1/2 CTRI/2020/07/026352 | |
| Non-Replicating Viral Vector | CanSino | Novel coronavirus vaccine(adenovirus type 5 vector) | Full-length spike protein | China | Phase 3 NCT04526990 |
| Gamaleya Research Institute | Sputnik V Gam-COVID-Vacadeno-based (rAd26-S + rAd5-S) | Full-length spike protein | Russia | Phase 3 NCT04530396 | |
| Janssen | Ad26.COV2.S | Full-length S with two proline substitutions (K986P and V987P) and two mutations at the furin cleavage site (R682S and R685G) | United States | Phase 3 NCT04505722 | |
| University of Oxford/AstraZeneca | ChAdOx1-S-(AZD1222) | Full-length spike protein | UK | Phase 3 NCT04516746 | |
| Replicating Viral Vectors | Jiangsu Provincial Center for Disease Prevention and Control | DelNS1-2019-nCoV-RBD-OPT1 | Spike protein | China | Phase 2 ChiCTR2000039715 |
| Protein Subunit | Anhui Zhifei | Recombinant SARS-CoV-2 vaccine (CHO cell) | RBD dimer (as tandem repeat residues 319–537) | China | Phase 3 NCT04445194 |
| CIGB | CIGB 66 | RBD + aluminum hydroxide | Cuba | Phase 1/2 RPCEC00000345 | |
| Medigen | MVC-COV1901 | Spike protein with aluminum hydroxide and CpG1018 | Taiwan | Phase 2 NCT04695652 | |
| Novavax | SARS-CoV-2 rS (CHO)/Matrix M1 adjuvant (NVX-CoV2373) | Full-length spike protein with two proline substitutions(K986P and V987P) and threemutations at cleavage site (R682Q, R683Q, R685Q) | United States | Phase 3 NCT04611802 | |
| Clover Biopharm/GSK | SCB 2019 + AS03 or CpG 1018 adjuvant plus alum adjuvant | Ectodomain of wild-type S with fusion to trimer-tag | Australia | Phase 2/3 NCT04672395 | |
| Covaxx + United Biomedical Inc | UB 162 | Multitope S1-RBD peptide based | China | Phase 2/3 NCT04683224 | |
| Live Attenuated Virus | Codagenix/Serum Institute India | COVI-VAC | Whole virus | India | Phase 1 NCT04619628 |
| Inactivated Virus | Sinopharm/Beijing Institute of Biological Products/Wuhan Institute of Biological Products | SARS-CoV-2 vaccine | Whole virus | China | Phase 3 ChiCTR2000034780 |
| Bharat Biotech | Whole virion inactivated SARS-CoV-2 vaccine (BBV152) | Whole virus | India | Phase 3 NCT04641481 | |
| Institute of Medical Biology/Chinese Academy of Medical Sciences | SARS-CoV-2 vaccine | Whole virus | China | Phase 3 NCT04659239 | |
| Sinovac | CoronaVac | Whole virus | China | Phase 3 NCT04456595 | |
| Chumakov Center | CoviVac | Whole virus | Russia | - | |
| Shifa Pharmed | COVIranBarekat | Whole virus | Iran | Phase 2/3 IRCT20201202049567N3 | |
| Minhai Co | KCONVAC | Whole virus | China | Phase 3 NCT04852705 | |
| Research Institute for Biological Safety Problems | QazCovid-in | Whole virus | Kazakhstan | Phase 3 NCT04530357 | |
| Virus-like Particles | Medicago/GSK | Coronavirus-like particle COVID-19 (CoVLP) with AS03 adjuvant | Living plant-based platform to produce noninfectious VLP | Canada | Phase 2/3 NCT04636697 |
| Bacterial vector | Symvivo | bacTRL-Spike Vaccine | Spike protein | Canada | Phase 1 NCT04334980 |
| Synthetic peptide | Vektor State Research Center of Virology and Biotechnology | EpiVacCorona | Spike protein | Russia | Phase1/2 NCT04527575 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaik, R.A.; Ahmad, M.S.; Alzahrani, M.; Alzerwi, N.A.N.; Alnemare, A.K.; Reyzah, M.; Albar, H.M.; Alshagrawi, S.; Elkhalifa, A.M.E.; Alzahrani, R.; et al. Comprehensive Highlights of the Universal Efforts towards the Development of COVID-19 Vaccine. Vaccines 2022, 10, 1689. https://doi.org/10.3390/vaccines10101689
Shaik RA, Ahmad MS, Alzahrani M, Alzerwi NAN, Alnemare AK, Reyzah M, Albar HM, Alshagrawi S, Elkhalifa AME, Alzahrani R, et al. Comprehensive Highlights of the Universal Efforts towards the Development of COVID-19 Vaccine. Vaccines. 2022; 10(10):1689. https://doi.org/10.3390/vaccines10101689
Chicago/Turabian StyleShaik, Riyaz Ahamed, Mohammed Shakil Ahmad, Mansour Alzahrani, Nasser A. N. Alzerwi, Ahmad K. Alnemare, Musaed Reyzah, Haitham M. Albar, Salah Alshagrawi, Ahmed M. E. Elkhalifa, Raed Alzahrani, and et al. 2022. "Comprehensive Highlights of the Universal Efforts towards the Development of COVID-19 Vaccine" Vaccines 10, no. 10: 1689. https://doi.org/10.3390/vaccines10101689
APA StyleShaik, R. A., Ahmad, M. S., Alzahrani, M., Alzerwi, N. A. N., Alnemare, A. K., Reyzah, M., Albar, H. M., Alshagrawi, S., Elkhalifa, A. M. E., Alzahrani, R., Alrohaimi, Y., Mahfoz, T. M. B., Ahmad, R. K., Alahmdi, R. A., & Al-baradie, N. R. S. (2022). Comprehensive Highlights of the Universal Efforts towards the Development of COVID-19 Vaccine. Vaccines, 10(10), 1689. https://doi.org/10.3390/vaccines10101689









