Timing of the First Dose of the Hepatitis B Vaccine in Preterm Infants
Abstract
:1. Introduction
2. Methods
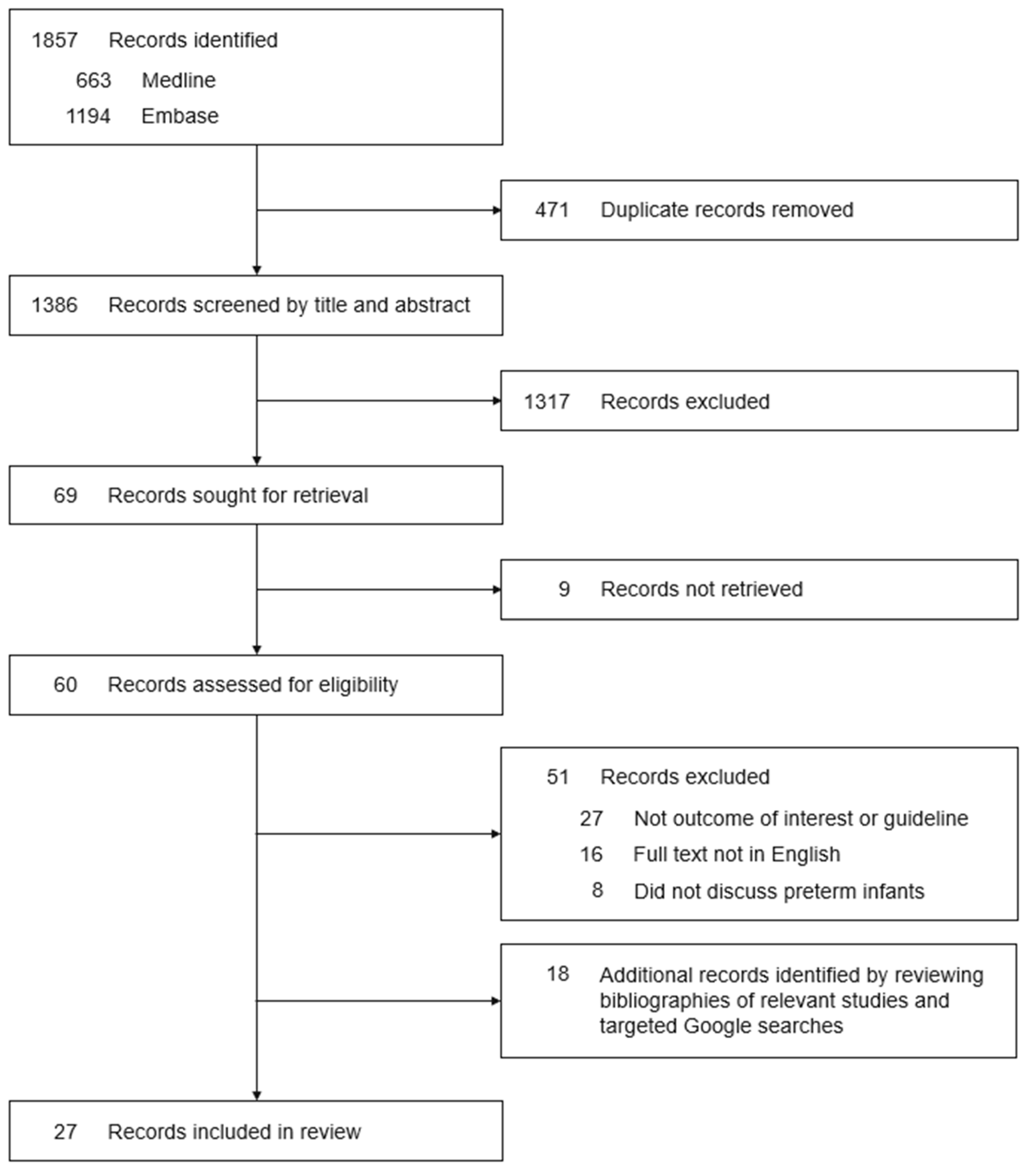
2.1. Search Strategy and Study Selection
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Systematic Review
3. Results
3.1. Nationwide Birth Dose
3.2. Nationwide Birth Dose Subject to Birth Weight
3.3. Nationwide No Universal Birth Dose
3.4. Countries/Regions with Varying Guidelines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Hepatitis B; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 12 July 2022).
- Department of Health and Aged Care. Pregnancy Care Guidelines. Hepatitis B; Australian Government: Canberra, Australia, 2022. Available online: https://www.health.gov.au/resources/pregnancy-care-guidelines/part-f-routine-maternal-health-tests/hepatitis-b (accessed on 1 October 2022).
- Umar, M.; Hamama-Tul-Bushra; Umar, S.; Khan, H. HBV perinatal transmission. Int. J. Hepatol. 2013, 2013, 875791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. About Global Hepatitis B; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2022. Available online: https://www.cdc.gov/globalhealth/immunization/diseases/hepatitis-b/about/index.html (accessed on 10 August 2022).
- World Health Organization. Global HIV Hepatitis and Sexually Transmitted Infections Programmes. Global Hepatitis Report; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Sioriki, A.A.; Gkentzi, D.; Papadimitriou, E.; Dimitriou, G.; Karatza, A. Vaccinations in infants born preterm: An update. Curr. Pediatr. Rev. 2020, 16, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, M.; Zhu, Y.M.; Zheng, Y.J. Immunogenicity of hepatitis B vaccine in preterm or low birth weight infants: A meta-analysis. Am. J. Prev. Med. 2020, 59, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Lao, J.C.; Bui, C.B.; Pang, M.A.; Cho, S.X.; Rudloff, I.; Elgass, K.; Schröder, J.; Maksimenko, A.; Mangan, N.E.; Starkey, M.R.; et al. Type 2 immune polarization is associated with cardiopulmonary disease in preterm infants. Sci. Transl. Med. 2022, 14, eaaz8454. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Vaccination Schedule for Hepatitis B; World Health Organization: Geneva, Switzerland, 2022; Available online: https://immunizationdata.who.int/pages/schedule-by-disease/hepatitisb.html (accessed on 1 October 2022).
- Department of Health and Aged Care. Hepatitis B; Australian Government: Canberra, Australia, 2021. Available online: https://immunisationhandbook.health.gov.au/contents/vaccine-preventable-diseases/hepatitis-b (accessed on 12 July 2022).
- Chaudhari, T. Vaccinations in the newborn. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 76, 66–82. [Google Scholar] [CrossRef] [PubMed]
- Department of Health and Aged Care. Vaccination for Preterm Infants; Australian Government: Canberra, Australia, 2020. Available online: https://immunisationhandbook.health.gov.au/contents/vaccination-for-special-risk-groups/vaccination-for-preterm-infants (accessed on 12 July 2022).
- Government of Canada. Recommended Immunization Schedules: Canadian Immunization Guide; Government of Canada: Ottawa, ON, Canada, 2021; Available online: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-1-key-immunization-information/page-13-recommended-immunization-schedules.html#p1c12a2. (accessed on 12 July 2022).
- Government of Canada. Immunization of Infants Born Prematurely: Canadian Immunization Guide; Government of Canada: Ottawa, ON, Canada, 2020; Available online: https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-3-vaccination-specific-populations/page-5-immunization-infants-born-prematurely.html (accessed on 12 July 2022).
- Zhou, Y.H.; Hu, Y.; Liu, X.; Yang, H. CSOG MFM committee guideline: Management of hepatitis B during pregnancy and prevention of mother-to-child transmission of hepatitis B virus (2020). Matern. Fetal Med. 2021, 3, 7–17. [Google Scholar] [CrossRef]
- Robert Koch Institute. Recommendations by the Standing Vaccination Committee (STIKO); Robert Koch Institute: Berlin, Germany, 2022. [Google Scholar]
- Aggarwal, M.K.; Machado, L. Immunization handbook for health workers. In WHO Country Office for India; Ministry of Health & Family Welfare: New Delhi, India, 2018. [Google Scholar]
- Ministry of Health & Family Welfare. Frequently Asked Questions on Immunization. In JSI; Ministry of Health & Family Welfare: New Delhi, India, 2017. [Google Scholar]
- Dutta, A.K. Immunization in practice—Clearing the cobwebs: Author’s reply. Indian J. Pediatr. 2014, 1, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Immunisation Advisory Committee. Hepatitis B; Department of Health, Health Service Executive: Dublin, Ireland, 2022. [Google Scholar]
- National Immunisation Advisory Committee. General Immunisation Procedures; Department of Health, Health Service Executive: Dublin, Ireland, 2022. [Google Scholar]
- Ministry of Health. Vaccines for Premature Babies; State of Israel: Jerusalem, Israel, 2022. Available online: https://www.health.gov.il/English/Topics/Pregnancy/Vaccination_of_infants/Pages/vaccine_premature_baby.aspx (accessed on 12 July 2022).
- Pan American Health Organization. Maternal and Neonatal Immunization Field Guide for Latin America and the Caribbean; Pan American Health Organization: Washington, DC, USA, 2017. [Google Scholar]
- Choi, K.; Wong, C.; Leong, K. Effectiveness of Macau hepatitis B vaccination programme for newborns from hepatitis B carrier mother. HKJ Paediatr. 2019, 24, 76–79. [Google Scholar]
- Rouers, E.D.; Berbers, G.A.; van Dongen, J.A.; Sanders, E.A.; Bruijning-Verhagen, P.; Priema Study Group. Timeliness of immunisations in preterm infants in the Netherlands. Vaccine 2019, 37, 5862–5867. [Google Scholar] [CrossRef]
- Scheepers, E.D.; van Lier, A.; Drijfhout, I.H.; Berbers, G.; van der Maas, N.A.; de Melker, H.E.; Knol, M.J. Dutch national immunization schedule: Compliance and associated characteristics for the primary series. Eur. J. Pediatr. 2017, 176, 769–778. [Google Scholar] [CrossRef]
- Ministry of Health. Immunisation Handbook 2020; New Zealand Government: Wellington, New Zealand, 2020. [Google Scholar]
- SNS24. National Vaccination Program. Porto, PT: SNS24. 2022. Available online: https://www.sns24.gov.pt/tema/vacinas/programa-nacional-de-vacinacao/ (accessed on 12 July 2022).
- Chen, C.Y.; Chen, H.L.; Chou, H.C.; Tsao, P.N.; Hsieh, W.S.; Chang, M.H. Weight-based policy of hepatitis B vaccination in very low birth weight infants in Taiwan: A retrospective cross-sectional study. PLoS ONE 2014, 9, e92271. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. Hepatitis B: The Green Book; UK Health Security Agency: London, UK, 2013; Chapter 18. [Google Scholar]
- Haber, P.; Schillie SHepatitis, B. The Pink Book; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2021. Available online: https://www.cdc.gov/vaccines/pubs/pinkbook/hepb.html#Schedule (accessed on 12 July 2022).
- Kimberlin, D.W.; Brady, M.T.; Jackson, M.A. Red Book (2018): Report of the Committee on Infectious Diseases; AAP Committee on Infectious Diseases: Itasca, IL, USA, 2018. [Google Scholar]
- Phillips, R.M.; Goldstein, M.; Hougland, K.; Nandyal, R.; Pizzica, A.; Santa-Donato, A.; Staebler, S.; Stark, A.R.; Treiger, T.M.; Yost, E. Multidisciplinary guidelines for the care of late preterm infants. J. Perinatol. 2013, 1, S5–S22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroger, A.T.; Atkinson, W.L.; Marcuse, E.K.; Pickering, L.K. General recommendations on immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recomm. Rep. 2016, 55, 1–48. [Google Scholar]
- Saari, T.N. American Academy of Pediatrics Committee on Infectious Diseases. Immunization of preterm and low birth weight infants. American Academy of Pediatrics Committee on Infectious Diseases. Pediatrics 2003, 112, 193–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, W.L.; Pickering, L.K.; Schwartz, B.; Weniger, B.; Iskander, J.K.; Watson, J.C. General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP). MMWR. Recomm. Rep. 2002, 51, 1–35. [Google Scholar]
- Wilck, M.B.; Jin Xu, Z.; Stek, J.E.; Goveia, M.G.; Lee, A.W. Protective immune responses against Haemophilus influenza type b elicited by a fully-liquid DTaP-IPV-Hib-HepB vaccine (VAXELISTM). Vaccine 2021, 39, 1428–1434. [Google Scholar] [CrossRef]
- The World Bank. Population, Total; The World Bank Group: Washington, DC, USA, 2022; Available online: https://data.worldbank.org/indicator/SP.POP.TOTL (accessed on 23 September 2022).
- Ramsay, M.; Gay, N.; Balogun, K.; Collins, M. Control of hepatitis B in the United Kingdom. Vaccine 1998, 16, S52–S55. [Google Scholar] [CrossRef]
- Siddiqui, M.R.; Gay, N.; Edmunds, W.J.; Ramsay, M. Economic evaluation of infant and adolescent hepatitis B vaccination in the UK. Vaccine 2011, 29, 466–475. [Google Scholar] [CrossRef]
- Ferreri, R.; Adinolfi, B.; Limardi, C.; Franco, E.; Matano, A. Hepatitis B vaccination: Evaluation of a short-interval dosing schedule in low-weight newborns. Curr. Ther.Res. Clin. Exp. 1992, 52, 493–497. [Google Scholar] [CrossRef]
- Ballesteros-Trujillo, A.; Vargas-Origel, A.; Alvarez-Munoz, T.; Aldana-Valenzuela, C. Response to hepatitis B vaccine in preterm infants: Four-dose schedule. Am. J. Perinatol. 2001, 18, 379–385. [Google Scholar] [CrossRef]
- Pourcyrous, M.; Korones, S.B.; Crouse, D.; Bada, H.S. Interleukin-6, C-reactive protein, and abnormal cardiorespiratory responses to immunization in premature infants. Pediatrics 1998, 101, E3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faldella, G.; Galletti, S.; Corvaglia, L.; Ancora, G.; Alessandroni, R. Safety of DTaP-IPV-HIb-HBV hexavalent vaccine in very premature infants. Vaccine 2007, 25, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Gidengil, C.; Lieu, T.A.; Payne, K.; Rusinak, D.; Messonnier, M.; Prosser, L.A. Parental and societal values for the risks and benefits of childhood combination vaccines. Vaccine 2012, 30, 3445–3452. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.S.; Happe, L.E.; Lunacsek, O.E.; Szymanski, M.D.; Woods, C.R.; Zahn, M.; Russell, A. Use of combination vaccines is associated with improved coverage rates. Pediatr. Infect. Dis. J. 2007, 26, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Marshall, H.; McIntyre, P.; Roberton, D.; Dinan, L.; Hardt, K. Primary and booster immunization with a diphtheria, tetanus, acellular pertussis, hepatitis B (DTPa-HBV) and Haemophilus influenzae type b (Hib) vaccine administered separately or together is safe and immunogenic. Int. J. Infect. Dis. 2010, 14, e41–e49. [Google Scholar] [CrossRef] [Green Version]
- Linder, N.; Vishne, T.H.; Levin, E.; Handsher, R.; Fink-Kremer, I.; Waldman, D.; Levine, A.; Ashkenazi, S.; Sirota, L. Hepatitis B vaccination: Long-term follow-up of the immune response of preterm infants and comparison of two vaccination protocols. Infection 2002, 30, 136–139. [Google Scholar] [CrossRef]
- Lau, Y.L.; Tam, A.Y.; Ng, K.; Tsoi, N.; Lam, B.; Lam, P.; Yeung, C. Response of preterm infants to hepatitis B vaccine. J. Pediatr. 1992, 121, 962–965. [Google Scholar] [CrossRef]
- Huang, F.-Y.; Lee, P.-I.; Lee, C.-Y.; Huang, L.-M.; Chang, L.-Y.; Liu, S.-C. Hepatitis B vaccination in preterm infants. Arch. Dis. Child. Fetal Neonatal Ed. 1997, 77, F135–F138. [Google Scholar] [CrossRef] [Green Version]
- Huynh, G.; Nguyen, T.B.; Cao, N.N.; Phan, M.H.; Dang, T.B.H.; Nguyen, T.N.H. Hepatitis B birth dose among children in District 2 Hospital, Ho Chi Minh City, Vietnam: Prevalence and associated factors. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 5680154. [Google Scholar] [CrossRef]
- Downing, S.G.; Lagani, W.; Guy, R.; Hellard, M. Barriers to the delivery of the hepatitis B birth dose: A study of five Papua New Guinean hospitals in 2007. PNG Med. J. 2008, 51, 47–55. [Google Scholar]

| Concept | Search Items | MeSH Terms |
|---|---|---|
| Vaccination | vaccin * OR immunis * OR immuniz * OR inoculat * | “vaccination”, “vaccines”, “viral vaccines” “immunization”, “immunization programs”, “vaccines, DNA” |
| Preterm | preterm OR prematur * | “infant, premature”, “premature birth” |
| Infant | infant OR neonat * OR baby OR babies | “infant, extremely premature”, “infant, premature, diseases” |
| Timing | timing OR time OR timetable OR schedule OR program * OR plan OR guideline * OR strateg * OR procedur * | “practice guideline” |
| Country/Region | References | Guideline |
|---|---|---|
| Australia | Australian Immunisation Handbook (2021) [10] Chaudhari (2021) [11] Australian Immunisation Handbook (2020) [12] | Preterm infants receive the first dose of Engerix-B 1 or H-B-Vax II 2 within 24 h of birth, if medically stable. Infants with a birth weight < 2000 g or gestation < 32 weeks receive an additional booster dose at 12 months of age. |
| Canada | Government of Canada (2021) [13] Government of Canada (2020) [14] | In jurisdictions where guidelines recommend infants to receive the first dose of Engerix-B 1 or Recombivax 3 at birth, this recommendation includes a delay of this first dose in infants with a birth weight < 2000 g until the infant reaches 2000 g or at hospital discharge, whichever occurs first. |
| China | Zhou et al. (2020) [15] | Preterm infants with a birth weight ≥ 2000 g receive the first dose at birth 4, if medically stable. Preterm infants who are not medically stable should receive appropriate management and be given the first dose 1 week after becoming medically stable. Infants with a birth weight < 2000 g receive the first dose 4 when the infant reaches 2000 g or at hospital discharge. |
| Germany | Robert Koch Institute (2022) [16] | Preterm infants receive the first dose 5 at 2 months of age as part of a combination vaccine (DTaP-IPA-Hib-HepB). |
| India | Ministry of Health & Family Welfare, Government of India (2018) [17] Ministry of Health & Family Welfare, Government of India (2017) [18] Dutta (2014) [19] | Preterm infants with a birth weight ≥ 2000 g receive the first dose 6 within 24 h of birth. Infants with a birth weight < 2000 g receive the first dose 6 at 30 days of age and when medically stable. |
| Ireland | Health Service Executive (2022) [20] Health Service Executive (2022) [21] | Preterm infants receive the first dose 7 at 2 months of age as part of a hexavalent vaccine. |
| Israel | Ministry of Health, State of Israel (2022) [22] | Preterm infants with a birth weight ≥ 2000 g receive the first dose shortly after birth 8. Preterm infants with a birth weight < 2000 g receive the first dose 8 when the infant reaches 2000 g or at hospital discharge or at 1 month of age, whichever occurs first. Infants discharged before reaching 1 month of age or 2000 g can receive the first dose 8 if medically stable and consistently gaining weight. |
| Latin America and the Caribbean | Pan American Health Organization (2017) [23] | Preterm infants receive the first dose 9 as soon as possible after birth, preferably within 24 h. Preterm infants with a birth weight < 2000 g require an additional booster dose. |
| Macau | Centro Hospitalar Conde de Sao Januario hospital guidelines as per Choi et al. (2019) [24] | Preterm infants receive the first dose at birth 10. Preterm infants with a birth weight < 2000 g receive a booster dose during their 2nd month of life, in addition to the routine schedule. |
| Netherlands | Rouers et al. (2019) [25] Scheepers et al. (2017) [26] | Preterm infants receive the first dose 11 between 6 and 9 weeks of age as part of a combination vaccine (DTaP-IPV-Hib-HepB). |
| New Zealand | Ministry of Health, New Zealand Government (2020) [27] | Preterm infants receive the first dose 7 at 6 weeks as part of a hexavalent vaccine, if medically stable. |
| Portugal | SNS24 (2022) [28] | Preterm infants with a birth weight ≥ 2000 g receive the first dose at birth 12. Preterm infants with a birth weight < 2000 g receive the first dose 12 when the infant reaches 2000 g or at 1 month of age, whichever occurs first. |
| Taiwan | Chen et al. (2014) [29] | Preterm infants with a birth weight ≥ 2000–2200 g receive the first dose at birth 13. Preterm infants with a birth weight < 2000–2200 g receive the first dose 13 when the infant reaches 2000–2200 g. |
| United Kingdom | UK Health Security Agency (2013) [30] | Preterm infants receive the first dose 7 at 8 weeks of age as part of a hexavalent vaccine. |
| United States of America | Centers for Disease Control and Prevention (2021) [31] AAP Committee on Infectious Diseases (2018) [32] Phillips et al. (2013) [33] Advisory Committee on Immunization Practices (2011) [34] AAP Committee on Infectious Diseases (2003) [35] Advisory Committee on Immunization Practices and American Academy of Family Physicians (2002) [36] | Preterm infants with a birth weight ≥ 2000 g receive the first dose within 24 h of birth 1,3. Preterm infants with a birth weight < 2000 g receive the first dose 1,3 when the infant reaches 1 month of age or at hospital discharge, whichever occurs first. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, D.; Miller, T.; Carr, J.; Buttery, J.; Nold-Petry, C.A.; Nold, M.F.; Malhotra, A. Timing of the First Dose of the Hepatitis B Vaccine in Preterm Infants. Vaccines 2022, 10, 1656. https://doi.org/10.3390/vaccines10101656
Lei D, Miller T, Carr J, Buttery J, Nold-Petry CA, Nold MF, Malhotra A. Timing of the First Dose of the Hepatitis B Vaccine in Preterm Infants. Vaccines. 2022; 10(10):1656. https://doi.org/10.3390/vaccines10101656
Chicago/Turabian StyleLei, Donna, Taryn Miller, Jeremy Carr, Jim Buttery, Claudia A. Nold-Petry, Marcel F. Nold, and Atul Malhotra. 2022. "Timing of the First Dose of the Hepatitis B Vaccine in Preterm Infants" Vaccines 10, no. 10: 1656. https://doi.org/10.3390/vaccines10101656
APA StyleLei, D., Miller, T., Carr, J., Buttery, J., Nold-Petry, C. A., Nold, M. F., & Malhotra, A. (2022). Timing of the First Dose of the Hepatitis B Vaccine in Preterm Infants. Vaccines, 10(10), 1656. https://doi.org/10.3390/vaccines10101656





