Allergic Reactions to COVID-19 Vaccination in High-Risk Allergic Patients: The Experience of Trieste University Hospital (North-Eastern Italy)
Abstract
:1. Introduction
2. Materials and Methods
- History of allergic or anaphylactic reaction to multiple oral or injectable drugs or vaccines;
- History of idiopathic anaphylaxis;
- History of mast cell disorders;
- History of chronic urticaria;
- History of uncontrolled asthma.
- Prior anaphylactic reaction to any drug or vaccine;
- Multiple drug allergy without tolerance to PEG- or PS80-containing drugs;
- Mast cell disorders;
- Patients with uncontrolled asthma who had to be treated for their condition to achieve satisfactory control of their symptoms before being vaccinated for COVID-19.
3. Statistical Analysis
4. Results
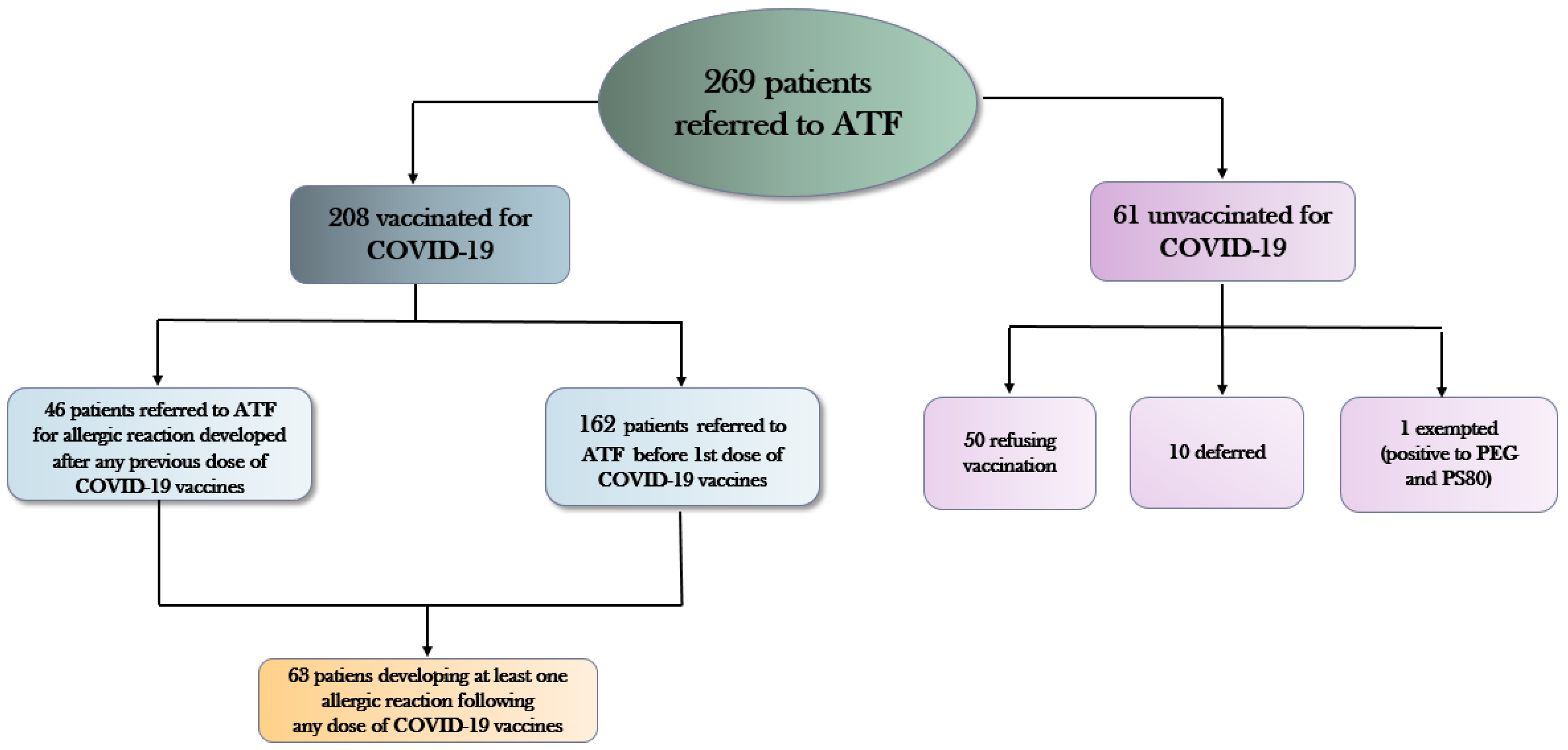
- 208 (77.3%) patients received COVID-19 vaccination;
- 50 (18.6%) patients refused to be immunized
- 10 (3.7%) patients were deferred for medical reasons (uncontrolled asthma, ongoing urticarial reactions, other acute allergic diseases);
- 1 patient tested positive for PEG and PS80 and was declared exempt from mandatory vaccination against COVID-19.
- 29 patients developed an allergic reaction only to the first vaccine dose (orange coloured), of whom 8 were referred to ATF medical examination before dose 1 and 21 between dose 1 and dose 2.
- 11 patients developed an allergic reaction only after the second vaccine dose (green coloured), of whom 3 were referred to ATF medical examination before dose 1 and 8 between dose 1 and dose 2.
- 3 patients developed an allergic reaction only to the third dose (yellow coloured), all referred to ATF medical examination before dose 1.
- 17 patients reacting to the first vaccine dose reacted also to the second (grey colored), of whom 5 were referred to ATF medical examination before the first dose and 12 between dose 1 and dose 2;
- 2 patients referring to AFT medical examination before the first dose did not develop any allergic reaction to the first dose, but reacted after both dose 2 as well as dose 3 (pink colored);
- 1 patient referring to ATF between first and second dose reacted against both dose 1 as well as dose 2;
- No patient was referred to ATF medical examination after the second dose of COVID-9 vaccine.
5. Discussion
Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Hurtman, A.; Lockhart, S. C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coleret, R.N.; mRNA-1273 Study Group. An mRNA vaccine against SARS-CoV-2—preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14–23, 2020. MMWR Morb Mortal Wkly Rep. 2021, 70, 46–51. [Google Scholar] [CrossRef]
- Shimabukuro, T.; Nair, N. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine. JAMA 2021, 325, 780–781. [Google Scholar] [CrossRef]
- Castells, M.C.; Phillips, E.J. Maintaining safety with SARS-CoV-2 vaccines. N. Engl. J. Med. 2021, 384, 643–649. [Google Scholar] [CrossRef]
- Banerji, A.; Wickner, P.G.; Saff, R.; Stone, C.A., Jr.; Robinson, L.B.; Long, A.A.; Wolfson, A.R.; Williams, P.; Khan, D.A.; Phillips, E.; et al. mRNA Vaccines to Prevent COVID-19 Disease and Reported Allergic Reactions: Current Evidence and Suggested Approach. J. Allergy Clin. Immunol. Pract. 2021, 9, 1423–1437. [Google Scholar] [CrossRef]
- Sokolowska, M.; Eiwegger, T.; Ollert, M.; Torres, M.J.; Barber, D.; Del Giacco, S.; Jutel, M.; Nadeau, K.C.; Palomares, O.; Rabin, R.L.; et al. EAACI statement on the diagnosis, management and prevention of severe allergic reactions to COVID-19 vaccines. Allergy 2021, 76, 1629–1639. [Google Scholar] [CrossRef]
- Klimek, L.; Jutel, M.; Akdis, C.A.; Bousquet, J.; Akdis, M.; Torres, M.J.; Agache, I.; Canonica, G.W.; Del Giacco, S.; O’Mahony, L.; et al. ARIA-EAACI statement on severe allergic reactions to COVID-19 vaccines—An EAACI-ARIA Position Paper. Allergy 2021, 76, 1624–1628. [Google Scholar] [CrossRef]
- Klimek, L.; Bergmann, K.C.; Brehler, R.; Pfützner, W.; Zuberbier, T.; Hartmann, K.; Jakob, T.; Novak, N.; Ring, J.; Merk, H.; et al. Practical handling of allergic reactions to COVID-19 vaccines: A position paper from German and Austrian Allergy Societies AeDA, DGAKI, GPA and ÖGAI. Allergo J. Int. 2021, 30, 79–95. [Google Scholar] [CrossRef]
- AAITO—Associazione degli Allergologi Italiani Territoriali e Ospedalieri. Linee di Indirizzo Per la Gestione da Parte Degli Allergologi dei Pazienti a Rischio di Reazioni Allergiche ai Vaccini Per COVID-19. Available online: https://www.aaiito.it/go/chisiamo/351/Emergenza%20COVID-19 (accessed on 12 July 2022).
- McMahon, D.E.; Amerson, E.; Rosenbach, M.; Lipoff, J.B.; Moustafa, D.; Tyagi, A.; Desai, S.R.; French, L.E.; Lim, H.W.; Thiers, B.H.; et al. Cutaneous reactions reported after Moderna and Pfizer COVID-19 vaccination: A registry-based study of 414 cases. J. Am. Acad Dermatol. 2021, 85, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.; Casale, T.B. The Relationship between IgE and Allergic Disease. Uptodate. Available online: https://www.uptodate.com/contents/the-relationship-between-ige-and-allergic-disease?search=atopy&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 6 September 2022).
- Pichler, W.J. Drug Hypersensitivity: Classification and Clinical Features. Uptodate. Available online: https://www.uptodate.com/contents/drug-hypersensitivity-classification-and-clinical-features?search=hypersensitivity&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 6 September 2022).
- Kim, H.; Warrington, R.; Watson, W. Practical guide for allergy and immunology in Canada. Allergy Asthma Clin. Immunol. 2011, 7, S1. [Google Scholar] [CrossRef] [PubMed]
- Filon, F.L.; Radman, G. Latex allergy: A follow up study of 1040 healthcare workers. Occup. Environ. Med. 2006, 63, 121–125. [Google Scholar] [CrossRef]
- Kemp, S.F. Pathophysiology of Anaphylaxis. Uptodate. Available online: https://www.uptodate.com/contents/pathophysiology-of-anaphylaxis?search=Anaphylactoid%20reaction&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 6 September 2022).
- Bian, S.; Lisha, L.; Wang, Z.; Cui, L.; Xu, Y.; Guan, K.; Zhao, B. Allergic Reactions After the Administration of COVID-19 Vaccine. Front Public Health 2022, 10, 878081. [Google Scholar] [CrossRef]
- Chen, W.; Mempel, M.; Schober, W.; Behrendt, H.; Ring, J. Gender difference, sex hormones, and immediate type hypersensitivity reactions. Allergy 2008, 63, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Shavit, R.; Maoz-Segal, R.; Iancovici-Kidon, M.; Offengenden, I.; Yahia, S.H.; Maayan, D.M.; Lifshitz-Tunitsky, Y.; Niznik, S.; Frizinsky, S.; Deutch, M.; et al. Prevalence of Allergic Reactions After Pfizer-BioNTech COVID-19 Vaccination Among Adults With High Allergy Risk. JAMA Netw. Open 2021, 4, e2122255. [Google Scholar] [CrossRef] [PubMed]
- De Blok, B.M.; Vlieg-Boerstra, B.J.; Oude Elberink, J.N.; Duiverman, E.J.; DunnGalvin, A.; Hourihane, J.O.; Cornelisse-Vermaat, J.R.; Frewer, L.J.; Mills, C.; Dubois, A.E. A framework for measuring the social impact of food allergy across Europe: A EuroPrevall state of the art paper. Allergy 2007, 62, 733–737. [Google Scholar] [CrossRef]
- Leynaert, B.; Sunyer, J.; Garcia-Esteban, R.; Svanes, C.; Jarvis, D.; Cerveri, I.; Dratva, J.; Gislason, T.; Heinrich, J.; Janson, C.; et al. Gender differences in prevalence, diagnosis and incidence of allergic and non-allergic asthma: A population-based cohort. Thorax 2012, 67, 625–631. [Google Scholar] [CrossRef]
- Ieven, T.; Vandebotermet, M.; Nuyttens, L.; Devolder, D.; Vandenberghe, P.; Bullens, D.; Schrijvers, R. COVID-19 Vaccination Safety and Tolerability in Patients Allegedly at High Risk for Immediate Hypersensitivity Reactions. Vaccines 2022, 10, 28. [Google Scholar]
- Gupta, R.; Sheikh, A.; Strachan, D.P.; Anderson, H.R. Time trends in allergic disorders in the UK. Thorax 2007, 62, 91–96. [Google Scholar] [CrossRef]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.; Blumenstock, J.A.; Davis, M.M.; Schleimeret, R.P.; Nadeau, K.C. Prevalence and severity of food allergies among US adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhopeshwarkar, N.; Sheikh, A.; Doan, R.; Topaz, M.; Bates, D.W.; Blumenthal, K.G.; Zhou, L. Drug-induced anaphylaxis documented in electronic health records. J. Allergy Clin. Immunol. Pract. 2019, 7, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.P.; Desai, A.P.; Loomis, G.J. Relationship between pre-existing allergies and anaphylactic reactions post mRNA COVID-19 vaccine administration. Vaccine 2021, 39, 4407–4409. [Google Scholar] [CrossRef]
- Nittner-Marszalska, M.; Rosiek-Biegus, M.; Kopeć, A.; Pawłowicz, R.; Kosińska, M.; Łata, A.; Szenborn, L. Pfizer-BioNTech COVID-19 Vaccine Tolerance in Allergic versus Non-Allergic Individuals. Vaccines 2021, 9, 553. [Google Scholar] [CrossRef]
- Klimek, L.; Novak, N.; Cabanillas, B.; Jutel, M.; Bousquet, J.; Akdis, C.A. Allergenic components of the mRNA-1273 vaccine for COVID-19: Possible involvement of polyethylene glycol and IgG-mediated complement activation. Allergy 2021, 76, 3307–3313. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, L.; Csuth, Á.; Storsaeter, J.; Garvey, L.H.; Jenmalm, M.C. Vaccine allergy: Evidence to consider for COVID-19 vaccines. Curr. Opin. Allergy Clin. Immunol. 2021, 21, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, A.R.; Robinson, L.B.; Li, L.; McMahon, A.E.; Cogan, A.S.; Fu, X.; Wickner, P.; Samarakoon, U.; Saff, R.R.; Blumenthal, K.G.; et al. First-Dose mRNA COVID-19 Vaccine Allergic Reactions: Limited Role for Excipient Skin Testing. J. Allergy Clin. Immunol. Pract. 2021, 9, 3308–3320.e3. [Google Scholar] [CrossRef]
- Barbaud, A.; Garvey, L.H.; Arcolaci, A.; Brockow, K.; Mori, F.; Mayorga, C.; Bonadonna, P.; Atanaskovic-Markovic, M.; Moral, L.; Zanoni, G.; et al. Allergies and COVID-19 vaccines: An ENDA/EAACI Position paper. Allergy 2022, 77, 2292–2312. [Google Scholar] [CrossRef]
- Kounis, N.G.; Koniari, I.; Mplani, V.; Plotas, P.; Velissaris, D. Hypersensitivity myocarditis and the pathogenetic conundrum of COVID 19 Vaccine Related Myocarditis. Cardiology, 2022; online ahead of print. [Google Scholar] [CrossRef]
- Turner, P.J.; Ansotegui, I.J.; Campbell, D.E.; Cardona, V.; Ebisawa, M.; El-Gamal, Y.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; et al. COVID-19 vaccine-associated anaphylaxis: A statement of the World Allergy Organization Anaphylaxis Committee WAO Anaphylaxis Committee. World Allergy Organ. J. 2021, 14, 100517. [Google Scholar] [CrossRef]
- Kelso, J.M. COVID-19: Allergic reactions to SARS-CoV-2 vaccines. Uptodate. Available online: https://www.uptodate.com/contents/covid-19-allergic-reactions-to-sars-cov-2-vaccines#:~:text=Delayed%20reactions%20(%3E2%20hours%20after,after%20vaccination%20(picture%201) (accessed on 5 September 2022).
- Seirafianpour, F.; Pourriyahi, H.; Gholizadeh Mesgarha, M.; Pour Mohammad, A.; Shaka, Z.; Goodarzi, A. A systematic review on mucocutaneous presentations after COVID-19 vaccination and expert recommendations about vaccination of important immune-mediated dermatologic disorders. Dermatol. Ther. 2022, 35, e15461. [Google Scholar] [CrossRef] [PubMed]
- Gambichler, T.; Boms, S.; Susok, L.; Dickel, H.; Finis, C.; Abu Rached, N.; Barras, M.; Stücker, M.; Kasakovski, D. Cutaneous findings following COVID-19 vaccination: Review of world literature and own experience. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.P.; Lewis, N.; Goddard, K.; Fireman, B.; Zerbo, O.; Hanson, K.E.; Donahue, J.G.; Kharbanda, E.O.; Naleway, A.; Nelson, J.C.; et al. Surveillance for Adverse Events After COVID-19 mRNA Vaccination. JAMA 2021, 326, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Shavit, R.; Maoz-Segal, R.; Offengenden, I.; Yahia, S.H.; Maayan, D.M.; Lifshitz, Y.; Niznik, S.; Deutch, M.; Elbaz, E.; Genaim, H.; et al. Assessment of immediate allergic reactions after immunization with the Pfizer BNT162b2 vaccine using intradermal skin testing with the COVID-19 vaccines. J. Allergy Clin. Immunol. Pract. 2022, 13, S2213-2198(22)00814-5. [Google Scholar] [CrossRef] [PubMed]

| FACTORS | Males N (Col %) | Females N (Col %) | Total N (Col %) | p-Value * | |||
|---|---|---|---|---|---|---|---|
| Total patients | 55 (20.4) | 214 (79.6) | 269 (100) | ||||
| Age (years) | Mean ± SD | 53.7 ± 17.7 | 54.1 ± 14.4 | 54.0 ± 15.1 | 0.439 | ||
| Median (IQR) | 53 (41–66) | 55 (45–64) | 55 (45–64) | 0.893 | |||
| Atopic dermatitis | No | 53 (86.4) | 213 (93.9) | 40 (94.4) | 0.482 | ||
| Yes | 2 (3.6) | 13 (6.1) | 15 (5.6) | ||||
| Allergy to inhalants | No | 37 (66.3) | 128 (59.8) | 165 (61.33) | 0.072 | ||
| yes | Others | 17 (30.9) | 86 (40.2) | 103 (38.3) | |||
| Anaphylaxis | 1 (1.8) | 0 (0) | 1 (0.37) | ||||
| Food allergy | No | 40 (72.7) | 153 (71.5) | 193 (71.8) | 0.159 | ||
| Yes | Others | 13 (23.6) | 56 (26.2) | 69 (25.6) | |||
| Anaphylaxis | 2 (3.6) | 5 (2.3) | 7 (2.6) | ||||
| Drug allergy | No | 21 (38.2) | 55 (25.7) | 76 (28.3) | 0.159 | ||
| Yes | Others | 24 (43.6) | 120 (56.1) | 144 (53.5) | |||
| Anaphylaxis | 10 (18.2) | 39 (18.2) | 49 (18.2) | ||||
| Hymenoptera allergy | No | 53 (96.4) | 200 (93.5) | 253 (94.0) | 0.001 | ||
| Yes | Others | 1 (1.8) | 11 (5.1) | 12 (4.5) | |||
| Anaphylaxis | 1 (1.8) | 3 (1.4) | 4 (1.5) | ||||
| Latex allergy | No | 54 (98.2) | 178 (83.2) | 232 (86.2) | 0.001 | ||
| Yes | Others | 0 (0) | 36 (16.8) | 36 (13.4) | |||
| Anaphylaxis | 1 (1.8) | 0 (0) | 1 (0.4) | ||||
| Contrast medium allergy | No | 50 (90.9) | 199 (94.0) | 249 (92.5) | 0.459 | ||
| Yes | Others | 3 (5.4) | 5 (2.3) | 8 (3.0) | |||
| Anaphylaxis | 2 (3.6) | 10 (4.7) | 12 (4.5) | ||||
| Asthma | No | 48 (85.4) | 166 (77.6) | ? | 0.046 | ||
| Yes | Controlled | 8 (14.6) | 44 (20.6) | 52 (19.3) | |||
| Uncontrolled | 0 (0) | 4 (1.9) | 4 (1.5) | ||||
| Chronic urticaria | No | 53 (96.4) | 96 (91.6) | 249 (92.6) | 0.229 | ||
| Yes | 2 (3.6) | 18 (8.4) | 20 (7.4) | ||||
| Angioedema | No | 50 (89.9) | 201 (93.9) | 251 (93.3) | 0.425 | ||
| Yes | 5 (9.1) | 13 (6.1) | 18 (6.7) | ||||
| Idiopathic anaphylaxis | No | 53 (89.9) | 201 (98.6) | 264 (98.1) | 0.274 | ||
| Yes | 2 (3.6) | 3 (1.4) | 5 (1.9) | ||||
| Suspect Macrogol allergy | No | 51 (92.7) | 206 (86.3) | 257 (95.5) | 0.257 | ||
| Yes | 4 (7.3) | 8 (3.7) | 12 (4.5) | ||||
| Suspect PS80 allergy | No | 54 (98.2) | 211 (98.6) | 265 (98.5) | 0.820 | ||
| Yes | 1 (1.8) | 3 (1.4) | 4 (1.5) | ||||
| Allergy test | No | 50 (89.9) | 198 (92.5) | 248 (92.2) | 0.195 | ||
| Yes | Macrogol | 4 (7.3) | 14 (6.5) | 18 (6.7) | |||
| Macrogol + PS80 | 1 (1.8) | 2 (0.9) | 3 (1.1) | ||||
| Positive reactions to Macrogol | 0 | 0 | 0 | NA | |||
| Positive reactions to PS80 | No | 0 | 2 (0.9) | 2 (0.75) | NA | ||
| Yes | 1 (1.8) | 0 (0) | 1 (0.4) | ||||
| COVID-19 vaccination under medical supervision | No | 30 (54.6) | 119 (54.6) | 149 (55.4) | 0.805 | ||
| Yes | 25 (45.4) | 95 (44.4) | 120 (44.6) | ||||
| COVID-19 vaccination status | Vaccinated (1+ doses) | 44 (80.0) | 164 (76.6) | 208 (77.3) | 0.030 | ||
| Refusing vaccination | 5 (9.1) | 44 (20.6) | 49 (18.2) | ||||
| Vaccination deferred before dose 1 | 4 (7.3) | 6 (2.8) | 10 (3.7) | ||||
| Exempted (allergy to PEG and PS80) | 1 (1.8) | 0 (0) | 1 (0.4) | ||||
| Allergy consultancy before COVID-19 vaccination | No | 4 (3.3) | 43 (19.3) | 47 (17.9) | 0.033 | ||
| Yes | 51 (92.7) | 171 (80.7) | 222 (83.1) | ||||
| Reactions to COVID-19 vaccine | No | 32 (72.7) | 92 (56.1) | 124 (59.6) | 0.046 | ||
| Yes | Any type of reaction | 12 (27.3) | 72 (43.9) | 84 (40.4) | |||
| At least one allergic reaction ** | Total | 7 (15.9) | 56 (34.2) | 63 (30.3) | 0.019 | ||
| <4 h since vaccination | 0 | 18 (11.0) | 18 (8.7) | 0.080 | |||
| >4 h since vaccination | 7 (15.9) | 38 (23.1) | 45 (21.6) | ||||
| FACTORS | UNIVARIABLE | MULTIVARIABLE | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | aOR (95% CI) | p-Value | |||
| Age (years, linear term) | 0.99 (0.97; 1.01) | 0.286 | ||||
| Sex | Males | reference | reference | |||
| Females | 2.74 (1.14; 6.54) | 0.023 | 3.05 (1.22; 7.65) | 0.017 | ||
| Atopic dermatitis | No | reference | ||||
| Yes | 1.34 (0.38; 4.73) | 0.653 | ||||
| Inhalant allergy | No | reference | ||||
| Yes | 1.31 (0.72; 2.39) | 0.375 | ||||
| Food allergy | No | reference | ||||
| Yes | 0.91 (0.47;1.76) | 0.771 | ||||
| Drug allergy | No | reference | reference | |||
| Yes | 0.33 (0.18; 0.62) | 0.001 | 0.30 (0.15; 0.58) | <0.001 | ||
| Venom allergy | No | reference | ||||
| Yes | 1.34 (0.38; 4.74) | 0.653 | ||||
| Latex allergy | No | reference | reference | |||
| Yes | 2.25 (1.00; 5.07) | 0.050 | 1.81 (0.77; 4.24) | 0.172 | ||
| Contrast medium allergy | No | reference | ||||
| Yes | 0.75 (0.23; 2.42) | 0.633 | ||||
| Asthma | No | reference | ||||
| Yes | Controlled | 1.15 (0.10; 12.9) | 0.910 | |||
| Uncontrolled | 0.98 (0.46; 2.01) | 0.910 | ||||
| Chronic urticaria | No | reference | ||||
| Yes | 0.19 (0.02; 1.56) | 0.123 | ||||
| Angioedema | No | reference | ||||
| Yes | 0.76 (0.20; 2.89) | 0.682 | ||||
| Number of allergic reactions (linear term) | 0.91 (0.68; 1.22) | 0.534 | ||||
| Vaccine type | Comirnaty | reference | ||||
| Spikevax | 0.96 (0.39; 2.31) | 0.957 | ||||
| Vaxzevria | 1.83 (0.29; 11.4) | 0.855 | ||||
| Patient Number | Sex | Age(years) | History of anaphylaxis | Skin Test | I dose | II dose | III dose | Vaccine type by dose administered | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allergic symptoms | Therapy | Allergic symptoms | Therapy | Allergic symptoms | Therapy | |||||||||||
| <4 h | >4 h | <4 h | >4 h | <4 h | >4 h | I Dose | II Dose | III Dose | ||||||||
| 1 | F | 53 | Urticaria | Urticaria | Comirnaty | Spikevax | ||||||||||
| 2 | F | 34 | Itching, erythema | Erythema | Comirnaty | Comirnaty | ||||||||||
| 3 | F | 52 | Urticaria | Urticaria | Cortisone and Antihistamine | Comirnaty | Comirnaty | |||||||||
| 4 | F | 50 | Angioedema | Urticaria | Antihistamine | Spikevax | Comirnaty | |||||||||
| 5 | F | 72 | Itching | Cortisone and Antihistamine | Itching | Cortisone and Antihistamine | Comirnaty | Comirnaty | ||||||||
| 6 | F | 62 | Urticaria | Antihistamine | Urticaria | Antihistamine | Comirnaty | Comirnaty | ||||||||
| 7 | F | 56 | Erythema | Antihistamine | Erythema | Spikevax | Spikevax | Spikevax | ||||||||
| 8 | F | 65 | Oculorinitis | Spikevax | Spikevax | Spikevax | ||||||||||
| 9 | F | 64 | Urticaria | Cortisone and Antihistamine | Comirnaty | Comirnaty | Comirnaty | |||||||||
| 10 | F | 49 | Angioedema | Comirnaty | Comirnaty | Comirnaty | ||||||||||
| 11 | M | 70 | Urticaria | Urticaria | Comirnaty | Comirnaty | Comirnaty | |||||||||
| 12 | F | 63 | Urticaria | Cortisone and Antihistamine | Urticaria | Cortisone and antihistamine | Spikevax | Spikevax | Spikevax | |||||||
| 13 | F | 78 | Urticaria | Antihistamine | Vaxzevria | Vaxzevria | Spikevax | |||||||||
| 14 | F | 45 | Urticaria | Antihistamine | Urticaria | Antihistamine | Spikevax | Spikevax | Spikevax | |||||||
| 15 | F | 28 | Yes (drugs) | Erythema | Cortisone ed Antihistamine | Comirnaty | ||||||||||
| 16 | F | 30 | Itching | Cortisone | Comirnaty | Comirnaty | Spikevax | |||||||||
| 17 | F | 56 | Angioedema | Cortisone | Comirnaty | |||||||||||
| 18 | F | 50 | Urticaria | Antihistamine | Comirnaty | Comirnaty | Spikevax | |||||||||
| 19 | F | 25 | Oculorinitis | Comirnaty | Comirnaty | Comirnaty | ||||||||||
| 20 | F | 70 | Angioedema | Cortisone ed Antihistamine | Vaxzevria | Comirnaty | Comirnaty | |||||||||
| 21 | F | 74 | Erythema | Vaxzevria | Comirnaty | Spikevax | ||||||||||
| 22 | F | 68 | Urticaria | Cortisone ed Antihistamine | Urticaria | Cortisone | Comirnaty | Comirnaty | Comirnaty | |||||||
| 23 | M | 62 | Urticaria | Vaxzevria | Comirnaty | |||||||||||
| 24 | F | 42 | Angioedema | Cortisone and Antihistamine | Spikevax | |||||||||||
| 25 | F | 51 | Yes (drugs) | Urticaria | Antihistamine | Comirnaty | ||||||||||
| 26 | F | 45 | Urticaria | Comirnaty | Comirnaty | |||||||||||
| 27 | F | 57 | Urticaria | Antihistamine | Comirnaty | Comirnaty | ||||||||||
| 28 | F | 44 | Urticaria | Cortisone | Urticaria | Cortisone | Vaxzevria | Comirnaty | ||||||||
| 29 | F | 58 | Urticaria | Antihistamine | Urticaria | Cortisone and Antihistamine | Comirnaty | Comirnaty | ||||||||
| 30 | F | 68 | Yes (drugs) | Erythema | Janssen | Comirnaty | ||||||||||
| 31 | F | 25 | PEG | Itching | Antihistamine | Comirnaty | Comirnaty | |||||||||
| 32 | F | 39 | PEG | Urticaria | Antihistamine | Comirnaty | Comirnaty | Comirnaty | ||||||||
| 33 | F | 71 | Yes (contrast medium) | Urticaria | Comirnaty | Comirnaty | ||||||||||
| 34 | F | 56 | Urticaria | Comirnaty | ||||||||||||
| 35 | F | 58 | Yes (drugs) | Urticaria | Cortisone | Comirnaty | ||||||||||
| 36 | F | 22 | Asthma | Comirnaty | ||||||||||||
| 37 | F | 32 | Urticaria | Comirnaty | ||||||||||||
| 38 | F | 66 | Asthma | Comirnaty | Comirnaty | |||||||||||
| 39 | M | 53 | Erythema | Spikevax | Spikevax | |||||||||||
| 40 | M | 52 | Erythema | Antihistamine | Spikevax | Spikevax | ||||||||||
| 41 | F | 49 | Yes (drugs, food, contrast medium) | Angioedema | Angioedema | Comirnaty | Comirnaty | |||||||||
| 42 | F | 78 | Yes (drugs) | Itching | Antihistamine | Comirnaty | Comirnaty | |||||||||
| 43 | M | 61 | Urticaria | Comirnaty | Comirnaty | |||||||||||
| 44 | F | 59 | Urticaria | Urticaria | Comirnaty | Comirnaty | Comirnaty | |||||||||
| 45 | F | 38 | Erythema | Comirnaty | Comirnaty | Comirnaty | ||||||||||
| 46 | F | 69 | Urticaria | Cortisone | Urticaria | Cortisone | Comirnaty | Comirnaty | Comirnaty | |||||||
| 47 | F | 27 | Erythema | Antihistamine | Comirnaty | Comirnaty | ||||||||||
| 48 | F | 73 | Erythema | Antihistamine | Vaxzevria | Vaxzevria | ||||||||||
| 49 | F | 55 | Angioedema | Cortisone | Angioedema | Cortisone | Comirnaty | Comirnaty | ||||||||
| 50 | F | 60 | Urticaria | Antihistamine | Urticaria, Asthma | Cortisone and Antihistamine | Spikevax | Spikevax | ||||||||
| 51 | M | 58 | Urticaria | Antihistamine | Comirnaty | Comirnaty | ||||||||||
| 52 | F | 60 | Urticaria | Spikevax | ||||||||||||
| 53 | F | 54 | PEG and PS80 | Erythema | Spikevax | |||||||||||
| 54 | F | 22 | Urticaria | Antihistamine | Comirnaty | Comirnaty | ||||||||||
| 55 | F | 37 | Asthma | Cortisone | Comirnaty | |||||||||||
| 56 | F | 51 | Yes (drugs) | Asthma | Cortisone | Comirnaty | Comirnaty | Comirnaty | ||||||||
| 57 | F | 42 | PEG | Urticaria | Cortisone and antihistamine | Oculorinitis | Spikevax | Comirnaty | ||||||||
| 58 | F | 56 | PEG | Erythema | Cortisone | Comirnaty | Novavax | |||||||||
| 59 | F | 64 | PEG | Angioedema | Janssen | Comirnaty | ||||||||||
| 60 | F | 60 | Yes (drugs) | PEG | Asthma | Comirnaty | Comirnaty | |||||||||
| 61 | M | 28 | Yes (drugs) | PEG | Urticaria | Comirnaty | ||||||||||
| 62 | F | 57 | Urticaria | Comirnaty | Comirnaty | |||||||||||
| 63 | F | 49 | Urticaria | Urticaria | Comirnaty | Comirnaty | ||||||||||
 ): N = 29. Reactions to doses I and II (
): N = 29. Reactions to doses I and II ( ): N = 17. Reactions only to dose II (
): N = 17. Reactions only to dose II ( ): N = 11. Reactions to doses II and III (
): N = 11. Reactions to doses II and III ( ): N = 2. Reactions only to dose III (
): N = 2. Reactions only to dose III ( ): N = 3. Reactions to doses I and III (
): N = 3. Reactions to doses I and III ( ): N = 1.
): N = 1.| I Dose (N) | II Dose (N) | III Dose (N) | Total Doses (N) | Total Adverse Reactions N (%) | Allergic Reactions * N (%) | |
|---|---|---|---|---|---|---|
| Vaxzevria (AstraZeneca) | 45,873 | 42,735 | - | 88,608 | 370 (0.41) | 64 (0.07) |
| Comirnaty (Pfizer BioNTech) | 215,426 | 201,266 | 98,510 | 515,202 | 1571 (0.30) | 223 (0.043) |
| Spikevax (Moderna) | 42,670 | 33,216 | 26,013 | 101,899 | 183 (0.18) | 44 (0.043) |
| Janssen (Johnson & Johnson) | 6936 | 15 (0.22) | 2 (0.029) | |||
| Unreported | 593 | 255 | 16 | 864 | - | |
| TOTAL | 311,498 | 277,472 | 124,539 | 706,573 | 2139 (0.30) | 333 (0.047) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filon, F.L.; Lazzarato, I.; Patriarca, E.; Iavernig, T.; Peratoner, A.; Perri, G.; Ponis, G.; Rocco, G.; Cegolon, L. Allergic Reactions to COVID-19 Vaccination in High-Risk Allergic Patients: The Experience of Trieste University Hospital (North-Eastern Italy). Vaccines 2022, 10, 1616. https://doi.org/10.3390/vaccines10101616
Filon FL, Lazzarato I, Patriarca E, Iavernig T, Peratoner A, Perri G, Ponis G, Rocco G, Cegolon L. Allergic Reactions to COVID-19 Vaccination in High-Risk Allergic Patients: The Experience of Trieste University Hospital (North-Eastern Italy). Vaccines. 2022; 10(10):1616. https://doi.org/10.3390/vaccines10101616
Chicago/Turabian StyleFilon, Francesca Larese, Ilaria Lazzarato, Emilia Patriarca, Thomas Iavernig, Alberto Peratoner, Giuseppe Perri, Giuliano Ponis, Giulio Rocco, and Luca Cegolon. 2022. "Allergic Reactions to COVID-19 Vaccination in High-Risk Allergic Patients: The Experience of Trieste University Hospital (North-Eastern Italy)" Vaccines 10, no. 10: 1616. https://doi.org/10.3390/vaccines10101616
APA StyleFilon, F. L., Lazzarato, I., Patriarca, E., Iavernig, T., Peratoner, A., Perri, G., Ponis, G., Rocco, G., & Cegolon, L. (2022). Allergic Reactions to COVID-19 Vaccination in High-Risk Allergic Patients: The Experience of Trieste University Hospital (North-Eastern Italy). Vaccines, 10(10), 1616. https://doi.org/10.3390/vaccines10101616







