Reduced Humoral Response of SARS-CoV-2 Antibodies following Vaccination in Patients with Inflammatory Rheumatic Diseases—An Interim Report from a Danish Prospective Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Patient Eligibility and Consent
2.2. User Involvement
2.3. Source Data
2.4. Antibody Testing
2.5. Statistics
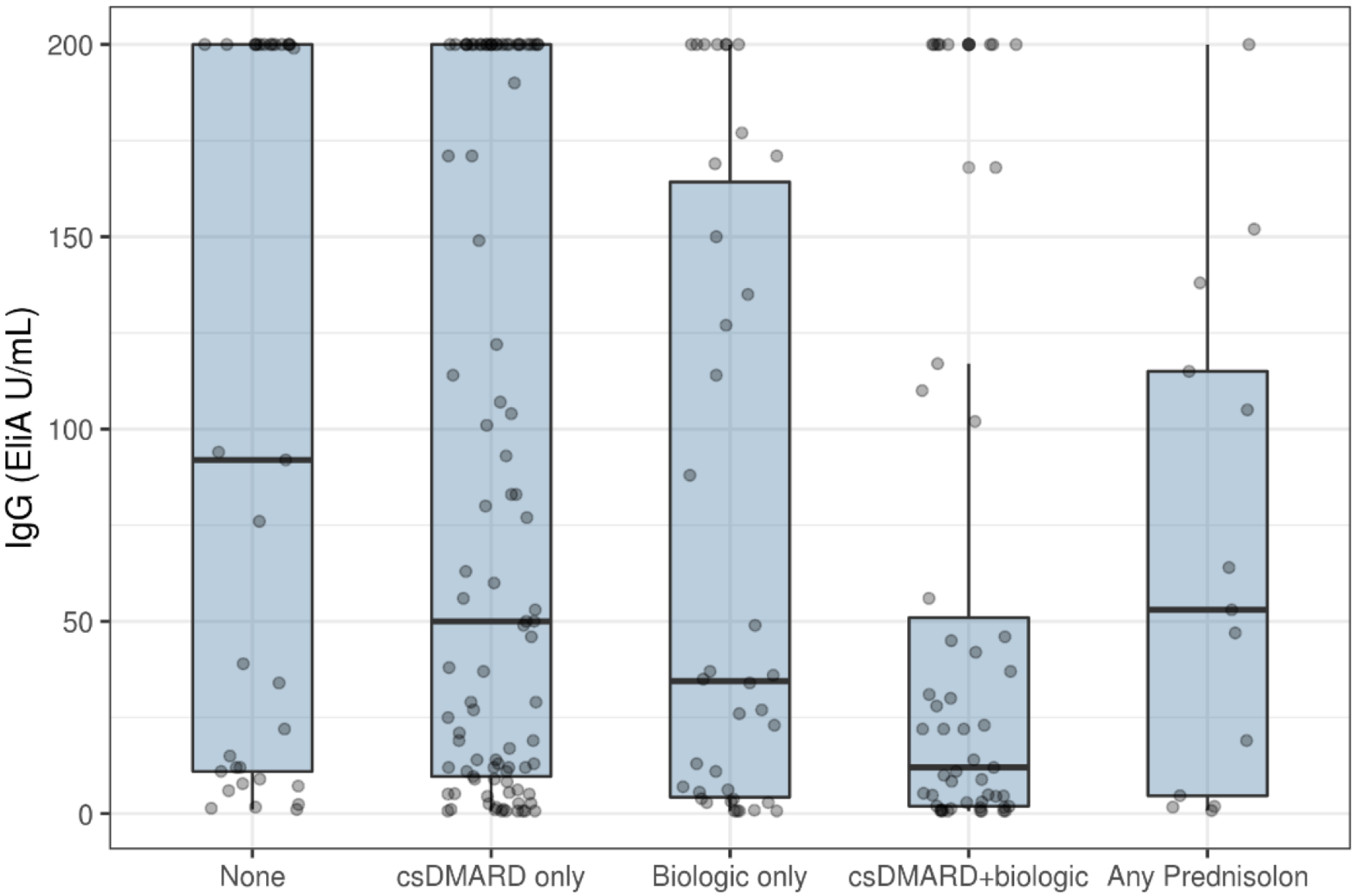
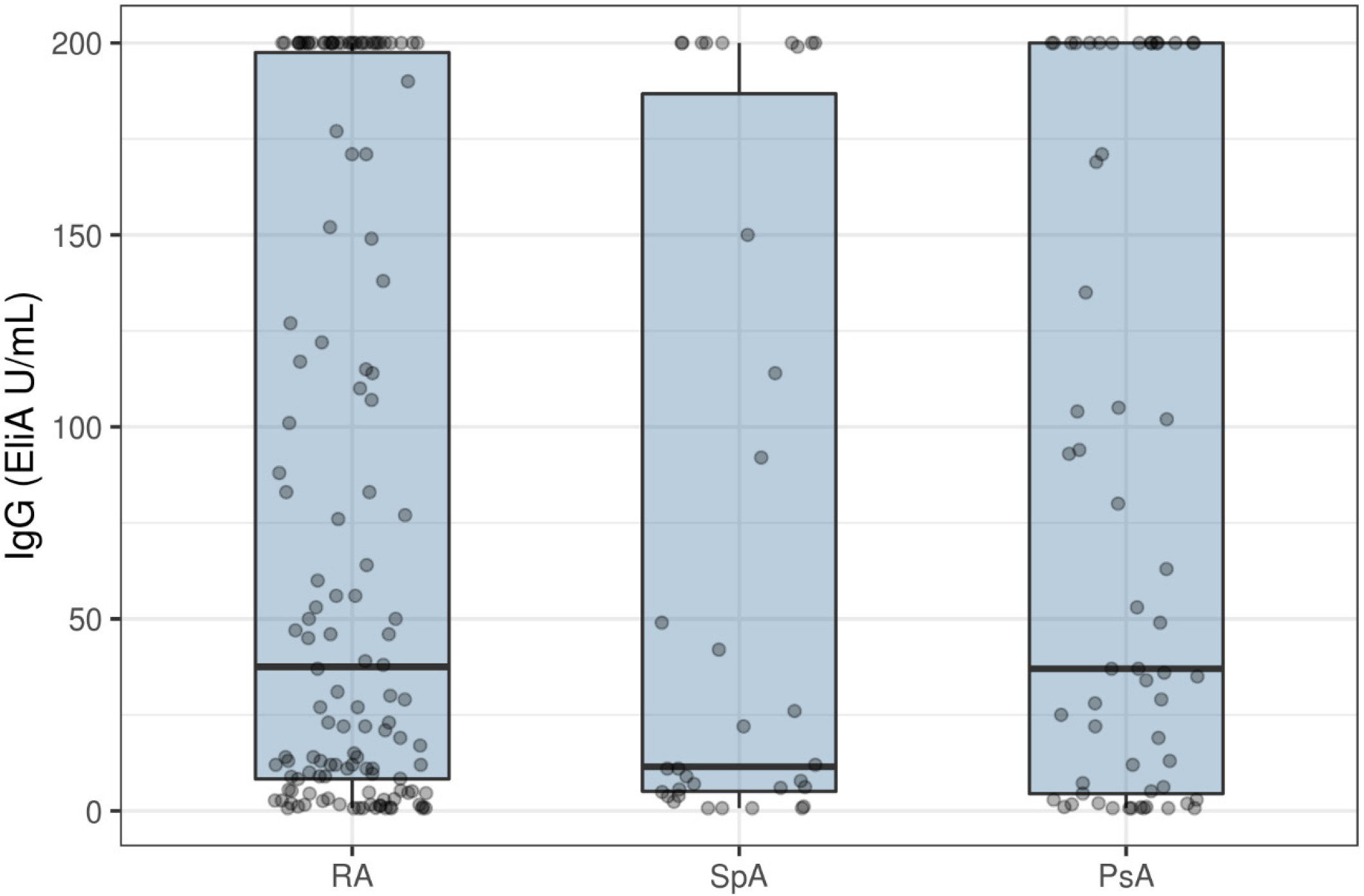
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Favalli, E.G.; Bugatti, S.; Klersy, C.; Biggioggero, M.; Rossi, S.; De Lucia, O.; Bobbio-Pallavicini, F.; Murgo, A.; Balduzzi, S.; Roberto Caporali & Carlomaurizio Montecucco. Impact of corticosteroids and immunosuppressive therapies on symptomatic SARS-CoV-2 infection in a large cohort of patients with chronic inflammatory arthritis. Arthritis Res. Ther. 2020, 22, 290. [Google Scholar] [CrossRef] [PubMed]
- Costantino, F.; Bahier, L.; Tarancón, L.C.; Leboime, A.; Vidal, F.; Bessalah, L.; Breban, M.; D’Agostino, M.-A. COVID-19 in French patients with chronic inflammatory rheumatic diseases: Clinical features, risk factors and treatment adherence. Joint Bone Spine 2021, 88, 105095. [Google Scholar] [CrossRef] [PubMed]
- England, B.R.; Roul, P.; Yang, Y.; Kalil, A.C.; Michaud, K.; Thiele, G.M.; Sauer, B.C.; Baker, J.F.; Mikuls, T.R. Risk of COVID-19 in Rheumatoid Arthritis: A National Veterans Affairs Matched Cohort Study in At-Risk Individuals. Arthritis Rheumatol. 2021, 73, 2179–2188. [Google Scholar] [CrossRef] [PubMed]
- Cordtz, R.; Kristensen, S.; Dalgaard, L.P.H.; Westermann, R.; Duch, K.; Lindhardsen, J.; Torp-Pedersen, C.; Dreyer, L. Incidence of COVID-19 Hospitalisation in Patients with Systemic Lupus Erythematosus: A Nationwide Cohort Study from Denmark. J. Clin. Med. 2021, 10, 3842. [Google Scholar] [CrossRef] [PubMed]
- Topless, R.K.; Phipps-Green, A.; Leask, M.; Dalbeth, N.; Stamp, L.K.; Robinson, P.C.; Merriman, T.R. Gout, Rheumatoid Arthritis, and the Risk of Death Related to Coronavirus Disease 2019: An Analysis of the UK Biobank. ACR Open Rheumatol. 2021, 3, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Marc, G.P.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; Sahly, H.M.E.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; DIemert, D.; Spector, A.S.; Rouphael, N.; Creech, B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Arkhipova-Jenkins, I.; Helfand, M.; Armstrong, C.; Gean, E.; Anderson, J.; Paynter, R.A.; Mackey, K. Antibody Response after SARS-CoV-2 Infection and Implications for Immunity. A Rapid Living Review. Ann. Int. Med. 2021, 20, 7547. [Google Scholar]
- Al-Janabi, A.; Littlewood, Z.; Griffiths, C.E.M.; Hunter, H.J.A.; Chinoy, H.; Moriarty, C.; Yiu, Z.Z.N.; Warren, R.B. Antibody responses to single-dose SARS-CoV-2 vaccination in patients receiving immunomodulators for immune-mediated inflammatory disease. Br. J. Dermatol. 2021, 185, 646–648. [Google Scholar] [CrossRef] [PubMed]
- Ibfelt, E.H.; Jensen, D.V.; Hetland, M.L. The Danish nationwide clinical register for patients with rheumatoid arthritis: DANBIO. Clin. Epidemiol. 2016, 8, 737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020, 383, 2427–2438. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Muik, A.; Derhovanessian, E.; Vogler, I.; Kranz, L.M.; Vormehr, M.; Baum, A.; Pascal, K.; Quandt, J.; Maurus, D.; et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020, 586, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Ruddy, J.A.; Connolly, C.M.; Boyarsky, B.J.; Werbel, W.A.; Christopher-Stine, L.; Garonzik-Wang, J.; Segev, D.L.; Paik, J.J. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann. Rheum. Dis. 2021, 80, 1351–1352. [Google Scholar] [CrossRef] [PubMed]
- Simon, D.; Tascilar, K.; Fagni, F.; Krönke, G.; Kleyer, A.; Meder, C.; Atreya, R.; Leppkes, M.; Kremer, A.E.; Ramming, A.; et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann. Rheum. Dis. 2021, 80, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Witte, T. Methotrexate as combination partner of TNF inhibitors and tocilizumab. What is reasonable from an immunological viewpoint. Clin. Rheumatol. 2015, 34, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- E.M.A. Comirnaty and Spikevax: EMA Recommendations on Extra Doses and Boosters. 2021. Available online: https://www.ema.europa.eu/en/news/comirnaty-spikevax-ema-recommendations-extra-doses-boosters (accessed on 18 October 2021).
- Prevention, C.D.C. CDC Statement on ACIP Booster Recommendations. 2021. Available online: https://www.cdc.gov/media/releases/2021/p0924-booster-recommendations-html (accessed on 18 October 2021).
| Number of Patients, N | 243 |
| Men/women | 135 (56%)/108 (44%) |
| Age, years, N (%) | |
| 30–49 | 24 (10%) |
| 50–59 | 54 (22%) |
| 60–69 | 75 (31%) |
| 70+ | 90 (37%) |
| Antirheumatic treatment, N (%) | |
| None | 33 (14%) |
| Conventional synthetic DMARD only | 105 (43%) |
| Biologic DMARD only | 41 (17%) |
| Conventional synthetic DMARD + biologic DMARD | 51 (21%) |
| Prednisolone monotherapy (varying doses) | 13 (5.3%) |
| Diagnosis: RA, n (%) | 142 (58%) |
| SpA, n (%) | 39 (16%) |
| PsA, n (%) | 60 (25%) |
| Vaccine given, n (%) | |
| Moderna * | 25 (10%) |
| Pfizer | 218 (90%) |
| DAS28crp +, n (%) | |
| <3 | 171 |
| 3+ | 27 |
| N/A | 45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schreiber, K.; Graversgaard, C.; Petersen, R.; Jakobsen, H.; Bojesen, A.B.; Krogh, N.S.; Glintborg, B.; Hetland, M.L.; Hendricks, O. Reduced Humoral Response of SARS-CoV-2 Antibodies following Vaccination in Patients with Inflammatory Rheumatic Diseases—An Interim Report from a Danish Prospective Cohort Study. Vaccines 2022, 10, 35. https://doi.org/10.3390/vaccines10010035
Schreiber K, Graversgaard C, Petersen R, Jakobsen H, Bojesen AB, Krogh NS, Glintborg B, Hetland ML, Hendricks O. Reduced Humoral Response of SARS-CoV-2 Antibodies following Vaccination in Patients with Inflammatory Rheumatic Diseases—An Interim Report from a Danish Prospective Cohort Study. Vaccines. 2022; 10(1):35. https://doi.org/10.3390/vaccines10010035
Chicago/Turabian StyleSchreiber, Karen, Christine Graversgaard, Randi Petersen, Henning Jakobsen, Anders Bo Bojesen, Niels Steen Krogh, Bente Glintborg, Merete Lund Hetland, and Oliver Hendricks. 2022. "Reduced Humoral Response of SARS-CoV-2 Antibodies following Vaccination in Patients with Inflammatory Rheumatic Diseases—An Interim Report from a Danish Prospective Cohort Study" Vaccines 10, no. 1: 35. https://doi.org/10.3390/vaccines10010035
APA StyleSchreiber, K., Graversgaard, C., Petersen, R., Jakobsen, H., Bojesen, A. B., Krogh, N. S., Glintborg, B., Hetland, M. L., & Hendricks, O. (2022). Reduced Humoral Response of SARS-CoV-2 Antibodies following Vaccination in Patients with Inflammatory Rheumatic Diseases—An Interim Report from a Danish Prospective Cohort Study. Vaccines, 10(1), 35. https://doi.org/10.3390/vaccines10010035






