Effect of a Community-Based Hepatitis B Virus Infection Detection Combined with Vaccination Program in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
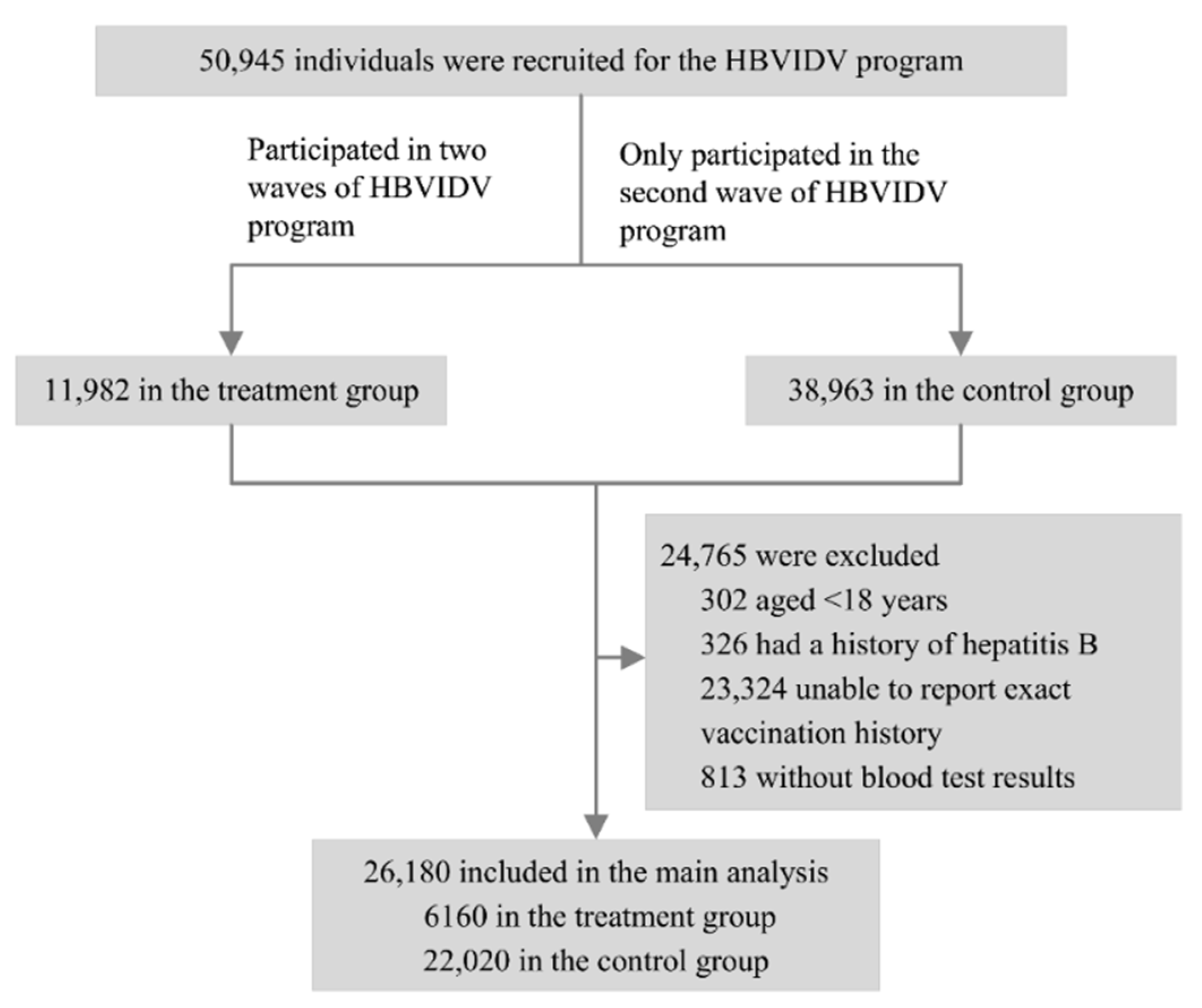
2.3. Study Population
2.4. Measurements
2.4.1. Covariates
2.4.2. Outcome Definition
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
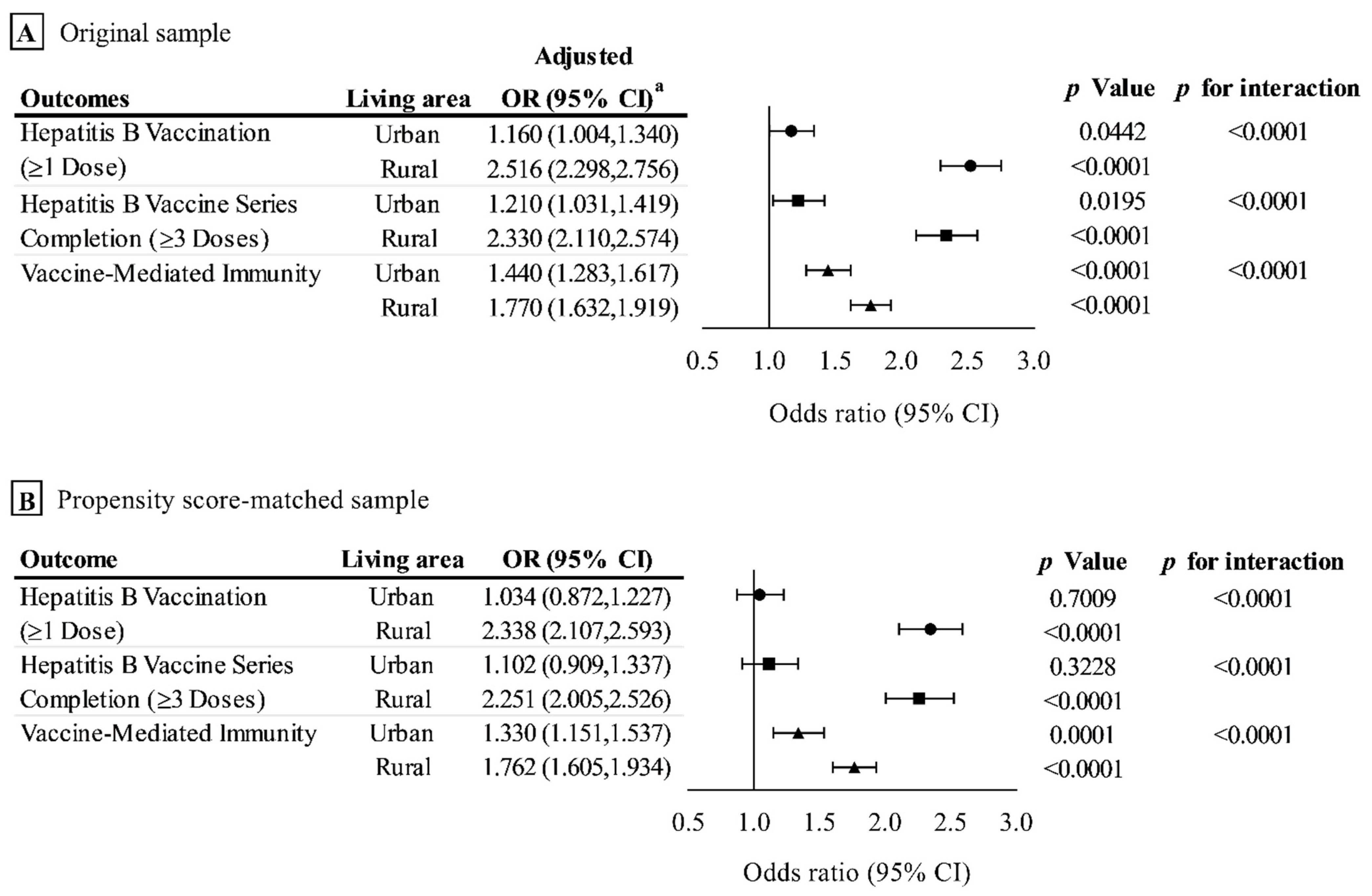
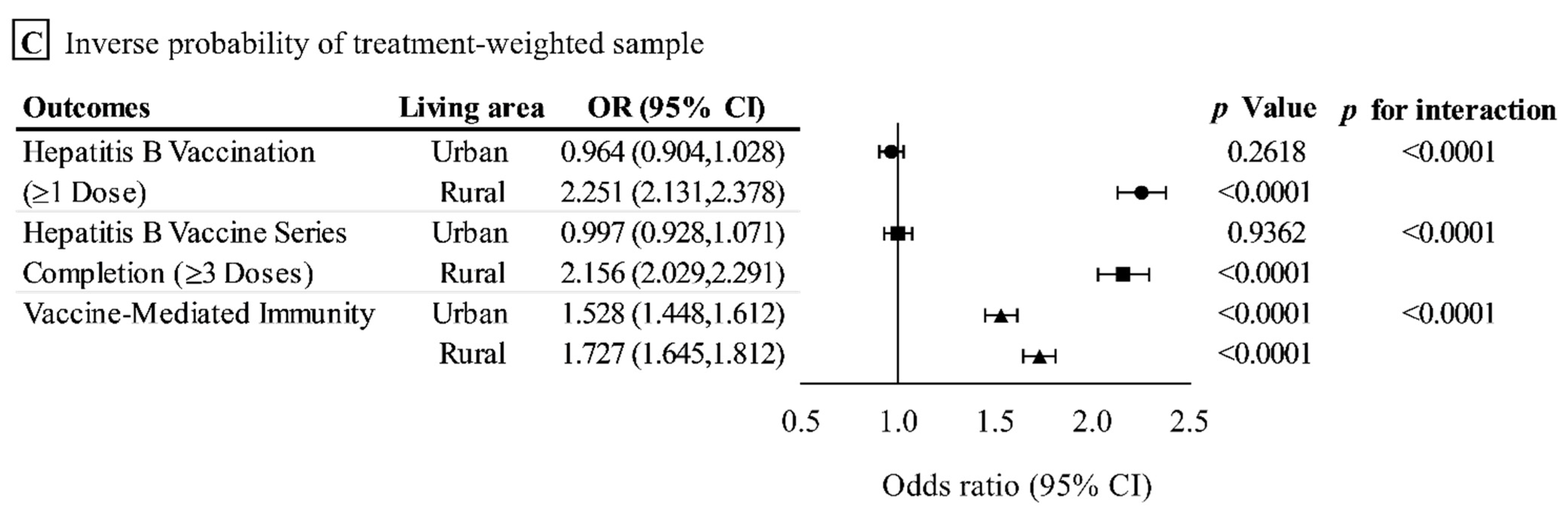
3.2. Outcome Analysis in the Original Sample
3.3. Outcome Analysis in the Propensity Score-Matched Sample
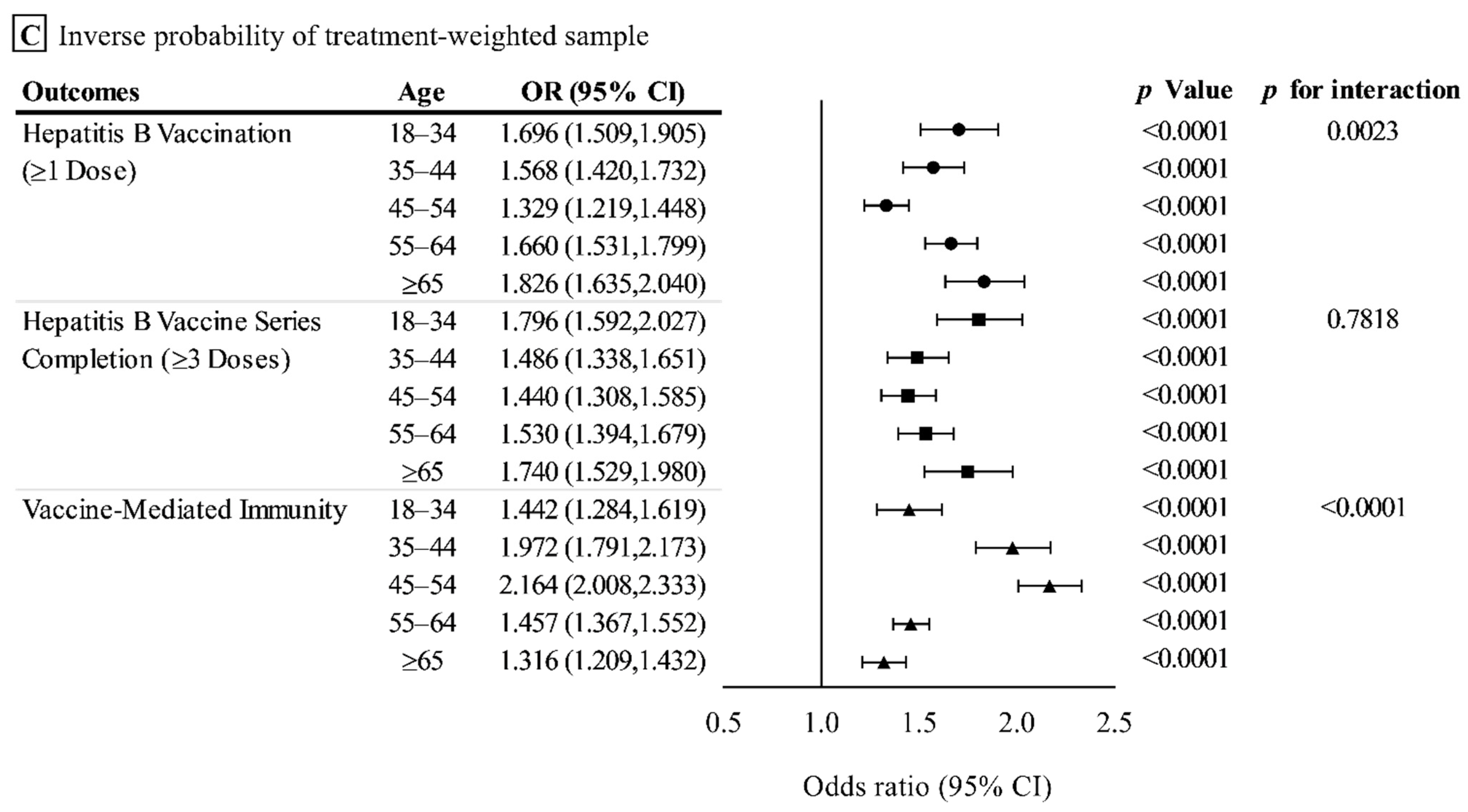
3.3.1. Effect on Hepatitis B Vaccination (≥1 Dose)
3.3.2. Effect on the Hepatitis B Vaccine Series Completion (≥3 Doses)
3.3.3. Effect on the Prevalence of Vaccine-Mediated Immunity
3.4. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016–2021. 2016. Available online: http://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/ (accessed on 3 November 2021).
- The Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol. Hepatol. 2018, 3, 383–403. [Google Scholar] [CrossRef]
- Cui, F.; Shen, L.; Li, L.; Wang, H.; Wang, F.; Bi, S.; Liu, J.; Zhang, G.; Wang, F.; Zheng, H.; et al. Prevention of Chronic Hepatitis B after 3 Decades of Escalating Vaccination Policy, China. Emerg. Infect. Dis. 2017, 23, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Mao, W.; Guo, L.; Zhang, J.; Tang, S. Combating hepatitis B and C by 2030: Achievements, gaps, and options for actions in China. BMJ Glob. Health 2020, 5, e002306. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, W.; Jing, W.; Liu, M. Countdown to 2030: Eliminating hepatitis B disease, China. Bull. World Health Organ. 2019, 97, 230–238. [Google Scholar] [CrossRef]
- Woodring, J.; Pastore, R.; Brink, A.; Ishikawa, N.; Takashima, Y.; Tohme, R.A. Progress Toward Hepatitis B Control and Elimination of Mother-to-Child Transmission of Hepatitis B Virus—Western Pacific Region, 2005–2017. MMWR. Morb. Mortal. Wkly. Rep. 2019, 68, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zheng, H.; Sun, X.; Miao, N.; Zhang, G.; Yin, Z. Achievements and prospects for hepatitis B prevention and control in China. Chin. J. Vaccines Immun. 2019, 25, 487–492. [Google Scholar]
- World Health Organization. Up to 10 Million People in China Could Die from Chronic Hepatitis by 2030—Urgent Action Needed to Bring an End to the ‘Silent Epidemic’. 2016. Available online: https://www.who.int/china/news/detail/26-07-2016-up-to-10-million-people-in-china-could-die-from-chronic-hepatitis-by-2030-urgent-action-needed-to-bring-an-end-to-the-silent-epidemic- (accessed on 3 November 2021).
- World Health Organization. Guidelines on Hepatitis B and C Testing. 2017. Available online: https://www.who.int/publications/i/item/9789241549981 (accessed on 3 November 2021).
- Zheng, H.; Wang, F.-Z.; Zhang, G.-M.; Cui, F.-Q.; Wu, Z.-H.; Miao, N.; Sun, X.-J.; Liang, X.-F.; Li, L. An economic analysis of adult hepatitis B vaccination in China. Vaccine 2015, 33, 6831–6839. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, C.; Jiang, L.; Peng, C.; Pan, L.; Zhang, X.; Shen, W.; Chen, L.; Lou, Z.; Xu, K.; et al. Cost-Effectiveness of Hepatitis B Mass Screening and Management in High-Prevalent Rural China: A Model Study From 2020 to 2049. Int. J. Health Policy Manag. 2021. [Google Scholar] [CrossRef]
- Yan, Y.-P.; Su, H.-X.; Ji, Z.-H.; Shao, Z.-J.; Pu, Z.-S. Epidemiology of Hepatitis B Virus Infection in China: Current Status and Challenges. J. Clin. Transl. Hepatol. 2014, 2, 15–22. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Initiative, F.T.S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and Elaboration. PLoS Med. 2007, 4, e297–e835. [Google Scholar] [CrossRef]
- Overview of Chaoyang District. Available online: https://english.bjchy.gov.cn/about-chaoyang (accessed on 3 November 2021).
- Zhang, X.-C.; Pang, X.-H.; Zhang, W.; Han, L.-L.; Lin, C.-Y.; Ma, J.-X.; Wu, K.; Li, S.-M.; Wang, Q.-Y.; Li, L.-Q.; et al. Prevalence of hepatitis B in Chaoyang district, Beijing in 2010. Chin. J. Prev. Med. 2012, 46, 623–626. [Google Scholar]
- Li, Q.; Liu, Y.; Ma, J.; Yu, H.; Shao, H.; Liao, W.; Zhang, B.; Pang, X.; Liao, S. Sero-epidemiological survey of hepatitis B virus in Chaoyang district of Beijing, 2015. Dis. Surveill. 2017, 32, 372–376. [Google Scholar] [CrossRef]
- Yao, S.; Cao, G.-Y.; Han, L.; Chen, Z.-S.; Huang, Z.-T.; Gong, P.; Hu, Y.; Xu, B. Prevalence and Patterns of Multimorbidity in a Nationally Representative Sample of Older Chinese: Results from the China Health and Retirement Longitudinal Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2020, 75, 1974–1980. [Google Scholar] [CrossRef]
- Le, M.; Yeo, Y.H.; Cheung, R.; Henry, L.; Lok, A.S.; Nguyen, M.H. Chronic Hepatitis B Prevalence Among Foreign-Born and U.S.-Born Adults in the United States, 1999–2016. Hepatology 2020, 71, 431–443. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef] [PubMed]
- Baliunas, D.; Selby, P.; de Oliveira, C.; Kurdyak, P.; Rosella, L.; Zawertailo, L.; Fu, L.; Sutradhar, R. Primary care-based smoking cessation treatment and subsequent healthcare service utilisation: A matched cohort study of smokers using linked administrative healthcare data. Tobacco Control 2021. [Google Scholar] [CrossRef] [PubMed]
- Marshall, I.B. Screening and Vaccination for Hepatitis B in Hong Kong University Students. J. Am. Coll. Health 1995, 44, 59–62. [Google Scholar] [CrossRef]
- Rein, D.B.; Lesesne, S.B.; Leese, P.J.; Weinbaum, C.M. Community-based hepatitis B screening programs in the United States in 2008. J. Viral Hepat. 2009, 17, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Pollack, H.; Wang, S.; Wyatt, L.; Peng, C.-H.; Wan, K.; Trinh-Shevrin, C.; Chun, K.; Tsang, T.; Kwon, S. A Comprehensive Screening and Treatment Model for Reducing Disparities in Hepatitis B. Health Aff. 2011, 30, 1974–1983. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Heil, J.; Hoebe, C.J.P.A.; Cals, J.; Ter Waarbeek, H.L.G.; Van Loo, I.H.M.; Dukers-Muijrers, N.H.T.M. Detecting Hepatitis B and C by Combined Public Health and Primary Care Birth Cohort Testing. Ann. Fam. Med. 2018, 16, 21–27. [Google Scholar] [CrossRef]
- Zhu, L.; Zhai, X.; Zhu, Y.; Xu, W.; Bao, C.; Peng, H.; Bian, Q.; Yang, H.; Wang, H.; Hu, Z.; et al. Evaluation of the Impact of Hepatitis B Vaccination in Adults in Jiangsu Province, China. PLoS ONE 2014, 9, e101501. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Bai, X.; Liu, X.; Zhang, Z.; Pang, X.; Nie, L.; Qiu, W.; Zhao, W.; Hu, G. Hepatitis B Vaccination Coverage Rates and Associated Factors: A Community-Based, Cross-Sectional Study Conducted in Beijing, 2019–2020. Vaccines 2021, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Shankar, H.; Blanas, D.; Bichoupan, K.; Ndiaye, D.; Carmody, E.; Martel-Laferriere, V.; Culpepper-Morgan, J.; Dieterich, D.T.; Branch, A.D.; Bekele, M.; et al. A Novel Collaborative Community-Based Hepatitis B Screening and Linkage to Care Program for African Immigrants. Clin. Infect. Dis. 2016, 62 (Suppl. 4), S289–S297. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, E.; Kim, K.; Kaur, R.; Song, S. A comparison of effectiveness of hepatitis B screening and linkage to care among foreign-born populations in clinical and nonclinical settings. J. Multidiscip. Health 2015, 8, 1–9. [Google Scholar] [CrossRef]
- Beckett, G.A.; Ramirez, G.; Vanderhoff, A.; Nichols, K.; Chute, S.M.; Wyles, D.L.; Schoenbachler, B.T.; Bedell, D.T.; Cabral, R.; Ward, J.W. Early identification and linkage to care of persons with chronic hepatitis B virus infection—Three U.S. sites, 2012–2014. MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 399–401. [Google Scholar] [PubMed]
- Perumalswami, P.V.; Factor, S.H.; Kapelusznik, L.; Friedman, S.L.; Pan, C.Q.; Chang, C.; Di Clemente, F.; Dieterich, D.T. Hepatitis Outreach Network: A practical strategy for hepatitis screening with linkage to care in foreign-born communities. J. Hepatol. 2013, 58, 890–897. [Google Scholar] [CrossRef]
- Lemoine, M.; Shimakawa, Y.; Njie, R.; Taal, M.; Ndow, G.; Chemin, I.; Ghosh, S.; Njai, H.F.; Jeng, A.; Sow, A.; et al. Acceptability and feasibility of a screen-and-treat programme for hepatitis B virus infection in The Gambia: The Prevention of Liver Fibrosis and Cancer in Africa (PROLIFICA) study. Lancet Glob. Health 2016, 4, e559–e567. [Google Scholar] [CrossRef]
- Zeng, F.; Guo, P.; Huang, Y.; Xin, W.; Du, Z.; Zhu, S.; Deng, Y.; Zhang, D.; Hao, Y. Epidemiology of hepatitis B virus infection: Results from a community-based study of 0.15 million residents in South China. Sci. Rep. 2016, 6, 36186. [Google Scholar] [CrossRef]
- Guo, Y.; Gao, P.; Wang, H.; Wu, J.; Bai, Q.; Huang, L.; Li, S.; Lv, M.; Shi, X. Risk factors of hepatitis B virus infection between vaccinated and unvaccinated groups among spouses in 2006 and 2014: A cross-sectional study in Beijing. Hum. Vaccines Immunother. 2020, 16, 148–157. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, T.; Shao, Z.; Huang, D.; Wang, A.; Guo, Z.; Long, Y.; Zhang, L.; Su, H.; Zhang, Q.; et al. A Population-Based Study Examining Hepatitis B Virus Infection and Immunization Rates in Northwest China. PLoS ONE 2014, 9, e97474. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, S.; Wang, Q.; Shen, H.; Zhang, M.; Zhang, Y.; Yan, D.; Liu, M. Seroepidemiology of hepatitis B virus infection in 2 million men aged 21–49 years in rural China: A population-based, cross-sectional study. Lancet Infect. Dis. 2016, 16, 80–86. [Google Scholar] [CrossRef]
- Xin, X.; Wang, Y.; Cheng, J.; Zhang, Y.; Peng, Z.; Xu, J.; Yang, Y.; He, Y.; Ma, X. Seroepidemiological survey of hepatitis B virus infection among 764,460 women of childbearing age in rural China: A cross-sectional study. J. Clin. Virol. 2016, 81, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Zhang, W.; Yue, H.; Zhu, G.; Wu, W.; Gong, W.; Fang, H.; He, G.; Hu, X.; Zhao, H.; et al. Prevalence of Hepatitis B Virus Infection in Shenzhen, China, 2015–2018. Sci. Rep. 2019, 9, 13948. [Google Scholar] [CrossRef]
- Liu, A.; Le, A.; Zhang, J.; Wong, C.; Wong, C.; Henry, L.; Nguyen, M.H. Increasing co-morbidities in chronic hepatitis B patients: Experience in primary care and referral practices during 2000–2015. Clin. Transl. Gastroenterol. 2018, 9, e141. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, Q.; Shi, O.; Ye, W.; Chen, X.; Zhang, T. The epidemiology of hepatitis B and hepatitis C infections in China from 2004 to 2014: An observational population-based study. J. Viral Hepat. 2018, 25, 1543–1554. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Wong, G.; Gane, E.; Kao, J.-H.; Dusheiko, G. Hepatitis B Virus: Advances in Prevention, Diagnosis, and Therapy. Clin. Microbiol. Rev. 2020, 33, e00046-19. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Lim, J.K.; Ozbay, A.B.; Fraysse, J.; Liou, I.; Meyer, N.; Dusheiko, G.; Gordon, S.C. Advancing Age and Comorbidity in a US Insured Population-Based Cohort of Patients with Chronic Hepatitis B. Hepatology 2019, 69, 959–973. [Google Scholar] [CrossRef]
- Wang, H.; Men, P.; Xiao, Y.; Gao, P.; Lv, M.; Yuan, Q.; Chen, W.; Bai, S.; Wu, J. Hepatitis B infection in the general population of China: A systematic review and meta-analysis. BMC Infect. Dis. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Chen, H.; Liu, N.; Ji, Z.H.; Pu, Z.S.; Guo, Z.W.; Gao, J.; Shao, Z.J.; Liu, Y.W.; Yan, Y.P. Assessment on the Effects of Hepatitis B Prevention and Control Measures in Western China: A Comparison of Three Population-based Serosurveys. Biomed. Environ. Sci. 2020, 33, 735–744. [Google Scholar]
- Zhu, D.; Guo, N.; Wang, J.; Nicholas, S.; Wang, Z.; Zhang, G.; Shi, L.; Wangen, K.R. Socioeconomic inequality in Hepatitis B vaccination of rural adults in China. Hum. Vaccines Immunother. 2018, 14, 464–470. [Google Scholar] [CrossRef]
- Wangen, K.R.; Zhu, D.; Wang, J. Hepatitis B vaccination among 1997–2011 birth cohorts in rural China: The potential for further catch-up vaccination and factors associated with infant coverage rates. Hum. Vaccines Immunother. 2019, 15, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.S.; Wang, D.; Tan, W.J.; Allameen, N.A.; Fong, N.P. Facilitators and barriers of Hepatitis B screening and vaccination. Vaccine 2020, 38, 5447–5453. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Total Sample (N = 26,180) | Original Sample | Propensity 1:1 Matching | IPTW Sample | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Group (N = 6160) | Control Group (N = 20,020) | SMD | Treatment Group (N = 5793) | Control Group (N = 5793) | SMD | Treatment Group (N = 6160) | Control Group (N = 20,020) | SMD | ||
| Age | 0.348 | 0.068 | 0.026 | |||||||
| 18–34 | 2625 (10.0) | 248 (4.0) | 2377 (11.9) | 248 (4.3) | 283 (4.9) | 2062.4 (7.5) | 2682.9 (10.4) | |||
| 35–44 | 2992 (11.4) | 604 (9.8) | 2388 (11.9) | 604 (10.4) | 587 (10.1) | 4354.8 (15.9) | 2848.1 (11.0) | |||
| 45–54 | 5826 (22.3) | 1241 (20.2) | 4585 (22.9) | 1241 (21.4) | 1323 (22.8) | 7043.7 (25.7) | 5811.3 (22.5) | |||
| 55–64 | 9616 (36.7) | 2400 (39.0) | 7216 (36.0) | 2222 (38.4) | 2397 (41.4) | 8485.0 (31.0) | 9799.8 (37.9) | |||
| ≥65 | 5121 (19.6) | 1667 (27.1) | 3454 (17.3) | 1478 (25.5) | 1203 (20.8) | 5431.0 (19.8) | 4745.4 (18.3) | |||
| Gender, male | 9593 (36.6) | 2075 (33.7) | 7518 (37.6) | 0.081 | 1965 (33.9) | 1868 (32.3) | 0.036 | 9812.8 (35.8) | 9471.5 (36.6) | 0.015 |
| Education level | 0.255 | 0.052 | 0.085 | |||||||
| Illiterate | 514 (2.0) | 135 (2.2) | 379 (1.9) | 135 (2.3) | 117 (2.0) | 476.1 (1.7) | 526.0 (2.0) | |||
| Primary school/Junior high school | 8768 (33.5) | 2598 (42.2) | 6170 (30.8) | 2598 (44.9) | 2616 (45.2) | 8757.8 (32.0) | 8727.4 (33.7) | |||
| Senior high school | 5769 (22.0) | 1330 (21.6) | 4439 (22.2) | 1330 (23.0) | 1431 (24.7) | 5541.4 (20.2) | 5913.9 (22.8) | |||
| College graduate or above | 4114 (15.7) | 899 (14.6) | 3215 (16.1) | 899 (15.5) | 919 (15.9) | 4620.7 (16.9) | 4082.2 (15.8) | |||
| Missing | 7015 (26.8) | 1198 (19.5) | 5817 (29.1) | 831 (14.3) | 710 (12.3) | 7981.0 (29.2) | 6638.0 (25.6) | |||
| Employment condition | 0.388 | 0.030 | 0.082 | |||||||
| Working | 9025 (34.5) | 1803 (29.3) | 7222 (36.1) | 1803 (31.1) | 2138 (36.9) | 6850.9 (25.0) | 9614.7 (37.1) | |||
| Retired | 9017 (34.4) | 3160 (51.3) | 5857 (29.3) | 2793 (48.2) | 2572 (44.4) | 10,373.2 (37.9) | 8240.2 (31.8) | |||
| Unemployed | 1582 (6.0) | 487 (7.9) | 1095 (5.5) | 487 (8.4) | 371 (6.4) | 2720.6 (9.9) | 1363.3 (5.3) | |||
| Student | 33 (0.1) | 4 (0.1) | 29 (0.1) | 4 (0.1) | 2 (0.0) | 8.6 (0.0) | 31.3 (0.1) | |||
| Missing | 6523 (24.9) | 706 (11.5) | 5817 (29.1) | 706 (12.2) | 710 (12.3) | 7423.6 (27.1) | 6638.0 (25.6) | |||
| Economic level | 0.229 | 0.062 | 0.077 | |||||||
| Far below average | 1107 (4.2) | 277 (4.5) | 830 (4.2) | 277 (4.8) | 308 (5.3) | 1049.5 (3.8) | 1170.0 (4.5) | |||
| Below average | 3719 (14.2) | 1090 (17.7) | 2629 (13.1) | 1090 (18.8) | 1065 (18.4) | 4171.4 (15.2) | 3641.8 (14.1) | |||
| Average | 13,805 (52.7) | 3467 (56.3) | 10,338 (51.6) | 3467 (59.9) | 3593 (62.2) | 13,609.2 (49.7) | 13,910.4 (53.7) | |||
| Above average | 468 (1.8) | 108 (1.8) | 360 (1.8) | 108 (1.9) | 105 (1.8) | 469.5 (1.7) | 468.1 (1.8) | |||
| Far above average | 61 (0.2) | 15 (0.2) | 46 (0.2) | 15 (0.3) | 12 (0.2) | 50.9 (0.2) | 59.1 (0.2) | |||
| Missing | 7020 (26.8) | 1203 (19.5) | 5817 (29.1) | 836 (14.4) | 710 (12.3) | 8026.4 (29.3) | 6638.0 (25.6) | |||
| Self-rated socioeconomic status | 0.217 | 0.065 | 0.082 | |||||||
| Higher | 584 (2.2) | 154 (2.5) | 430 (2.2) | 154 (2.7) | 127 (2.2) | 767.5 (2.8) | 558.5 (2.2) | |||
| Equality | 14,914 (57.0) | 3784 (61.4) | 11,130 (55.6) | 3784 (65.3) | 3970 (68.5) | 14,658.0 (53.5) | 15,027.8 (58.1) | |||
| Lower | 3662 (14.0) | 1019 (16.5) | 2643 (13.2) | 1019 (17.6) | 986 (17.0) | 3925.0 (14.3) | 3663.2 (14.2) | |||
| Missing | 7020 (26.8) | 1203 (19.5) | 5817 (29.1) | 836 (14.4) | 710 (12.3) | 8026.4 (29.3) | 6638.0 (25.6) | |||
| Living area | 0.433 | 0.002 | 0.020 | |||||||
| Urban | 11,149 (42.6) | 1660 (27.0) | 9489 (47.4) | 1660 (28.7) | 1654 (28.6) | 12,029.2 (43.9) | 11,124.0 (43.0) | |||
| Rural | 15,031 (57.4) | 4500 (73.1) | 10531 (52.6) | 4133 (71.3) | 4139 (71.5) | 15,347.6 (56.1) | 14,763.5 (57.0) | |||
| Multimorbidity | 0.065 | 0.084 | ||||||||
| Yes | 4654 (17.8) | 1639 (26.6) | 3015 (15.1) | 0.198 | 1639 (28.3) | 1655 (28.6) | 5011.4 (18.3) | 4738.4 (18.3) | ||
| No | 14,506 (55.4) | 3318 (53.4) | 11,188 (55.9) | 3318 (57.3) | 3428 (59.2) | 14,339.1 (52.4) | 14,511.0 (56.1) | |||
| Missing | 7020 (26.8) | 1203 (19.5) | 5817 (29.1) | 836 (14.4) | 710 (12.3) | 8026.4 (29.3) | 6638.0 (25.6) | |||
| Self-rated health condition | 0.250 | 0.063 | 0.072 | |||||||
| Very unhealthy | 496 (1.9) | 136 (2.2) | 360 (1.8) | 136 (2.4) | 132 (2.3) | 461.8 (1.7) | 514.7 (2.0) | |||
| Unhealthy | 1405 (5.4) | 424 (6.9) | 981 (4.9) | 424 (7.3) | 420 (7.3) | 1539.8 (5.6) | 1373.2 (5.3) | |||
| Fair healthy | 5995 (22.9) | 1771 (28.8) | 4224 (21.1) | 1771 (30.6) | 1860 (32.1) | 6533.3 (23.9) | 6013.6 (23.2) | |||
| Healthy | 8995 (34.4) | 2117 (34.4) | 6878 (34.4) | 2117 (36.5) | 2209 (38.1) | 8391.2 (30.7) | 9154.8 (35.4) | |||
| Very healthy | 2269 (8.7) | 509 (8.3) | 1760 (8.8) | 509 (8.8) | 462 (8.0) | 2424.3 (8.9) | 2193.2 (8.5) | |||
| Missing | 7020 (26.8) | 1203 (19.5) | 5817 (29.1) | 836 (14.4) | 710 (12.3) | 8026.4 (29.3) | 6638.0 (25.6) | |||
| Hepatitis B Vaccination (≥1 Dose) | Hepatitis B Vaccine Series Completion (≥3 Doses) | Vaccine-Mediated Immunity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Group | Control Group | OR (95% CI) | p Value | Treatment Group | Control Group | OR (95% CI) | p Value | Treatment Group | Control Group | OR (95% CI) | p Value | |
| Original sample unadjusted model | 1708 (27.7) | 3779 (18.9) | 1.649 (1.543, 1.761) | <0.0001 | 1300 (21.1) | 2839 (14.2) | 1.619 (1.505, 1.741) | <0.0001 | 2339 (38.0) | 6210 (31.0) | 1.361 (1.283, 1.445) | <0.0001 |
| Original sample adjusted model a | 1708 (27.7) | 3779 (18.9) | 1.958 (1.816, 2.111) | <0.0001 | 1300 (21.1) | 2839 (14.2) | 1.912 (1.761, 2.077) | <0.0001 | 2339 (38.0) | 6210 (31.0) | 1.672 (1.565, 1.785) | <0.0001 |
| Propensity 1:1 Matching | 1681 (29.0) | 1033 (17.8) | 1.884 (1.725, 2.057) | <0.0001 | 1278 (22.1) | 761 (13.1) | 1.872 (1.696, 2.065) | <0.0001 | 2211 (38.2) | 1596 (27.6) | 1.623 (1.501, 1.755) | <0.0001 |
| IPTW sample | 7227.7 (26.4) | 4781.6 (18.5) | 1.583 (1.519, 1.650) | <0.0001 | 5515.2 (20.2) | 3580.4 (13.8) | 1.572 (1.501, 1.646) | <0.0001 | 11328.6 (41.4) | 7801.6 (30.1) | 1.636 (1.539, 1.696) | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Qiu, W.; Liang, Y.; Zhang, W.; Qiu, Q.; Bai, X.; Dai, G.; Ma, H.; Hu, H.; Zhao, W.; et al. Effect of a Community-Based Hepatitis B Virus Infection Detection Combined with Vaccination Program in China. Vaccines 2022, 10, 19. https://doi.org/10.3390/vaccines10010019
Liu X, Qiu W, Liang Y, Zhang W, Qiu Q, Bai X, Dai G, Ma H, Hu H, Zhao W, et al. Effect of a Community-Based Hepatitis B Virus Infection Detection Combined with Vaccination Program in China. Vaccines. 2022; 10(1):19. https://doi.org/10.3390/vaccines10010019
Chicago/Turabian StyleLiu, Xinyao, Wuqi Qiu, Yan Liang, Wei Zhang, Qian Qiu, Xinxin Bai, Guolin Dai, Hao Ma, Hongpu Hu, Wei Zhao, and et al. 2022. "Effect of a Community-Based Hepatitis B Virus Infection Detection Combined with Vaccination Program in China" Vaccines 10, no. 1: 19. https://doi.org/10.3390/vaccines10010019
APA StyleLiu, X., Qiu, W., Liang, Y., Zhang, W., Qiu, Q., Bai, X., Dai, G., Ma, H., Hu, H., Zhao, W., & Hu, G. (2022). Effect of a Community-Based Hepatitis B Virus Infection Detection Combined with Vaccination Program in China. Vaccines, 10(1), 19. https://doi.org/10.3390/vaccines10010019






