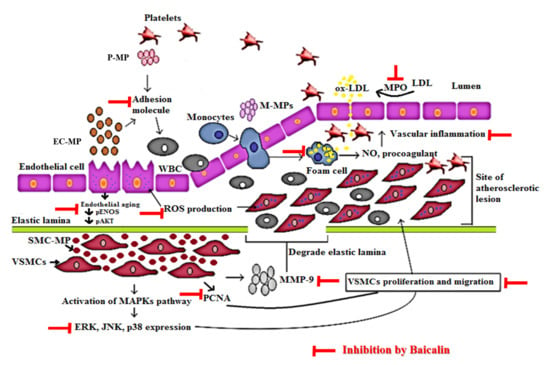
Microparticles-Mediated Vascular Inflammation and its Amelioration by Antioxidant Activity of Baicalin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell-Free In Vitro Experiments
2.1.1. Chemicals and Reagents
2.1.2. Antioxidant Assay
2.2. Cell-Based In Vitro Experiments
2.2.1. Cell Culture and Reagents
2.2.2. Isolation, Characterization, and Quantification of Microparticles.
2.2.3. Cell Viability Assay
2.2.4. NO Production
2.2.5. Foam Cell Assay
2.2.6. Western Blot
2.2.7. ROS Formation
2.2.8. Apoptosis Assay
2.2.9. VSMC Proliferation Assay
2.2.10. Immunocytochemistry (PCNA Expression in VSMC and β-Galactosidase Staining in EC)
2.2.11. Gelatin Zymography
2.2.12. Boyden Chamber Assay
2.2.13. Wound Healing Assay
2.3. Statistical Analysis
3. Results
3.1. Characterization of Microparticles
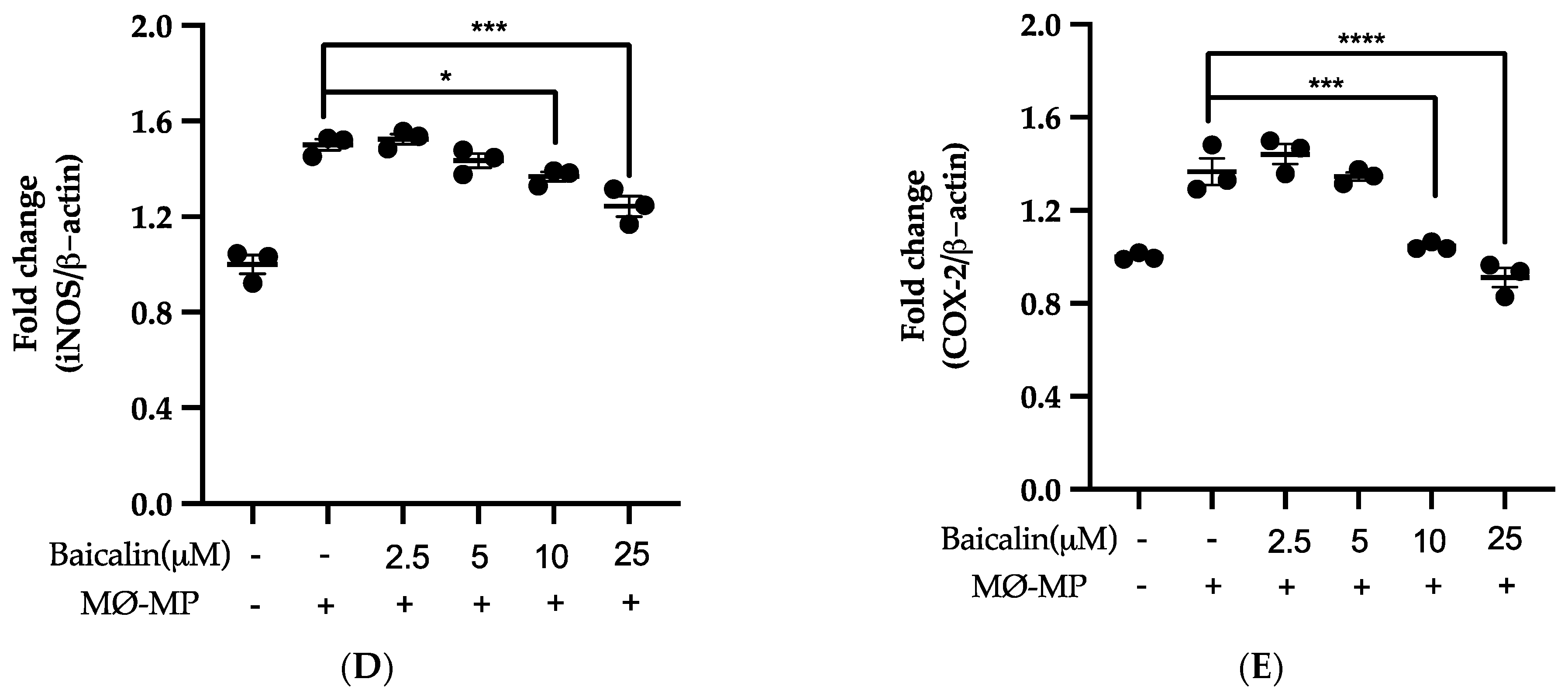
3.2. Baicalin Inhibits NO Production, iNOS, and COX-2 Protein Expression in MØ-MP Induced RAW264.7 Cell
3.3. Baicalin Inhibits MØ-MP Induced ROS Production, Apoptosis and Foam Cell Formation in RAW264.7 Cell
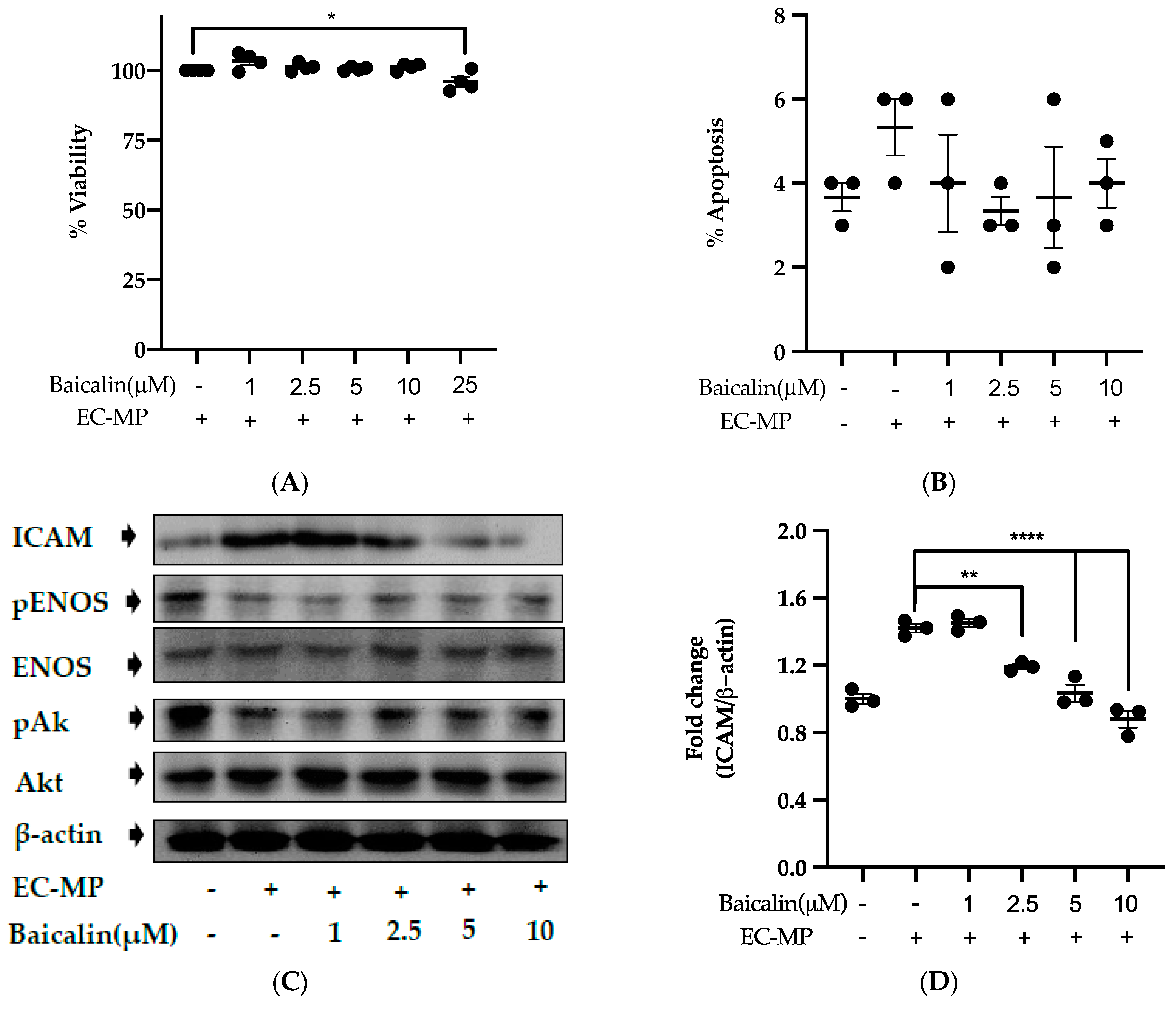
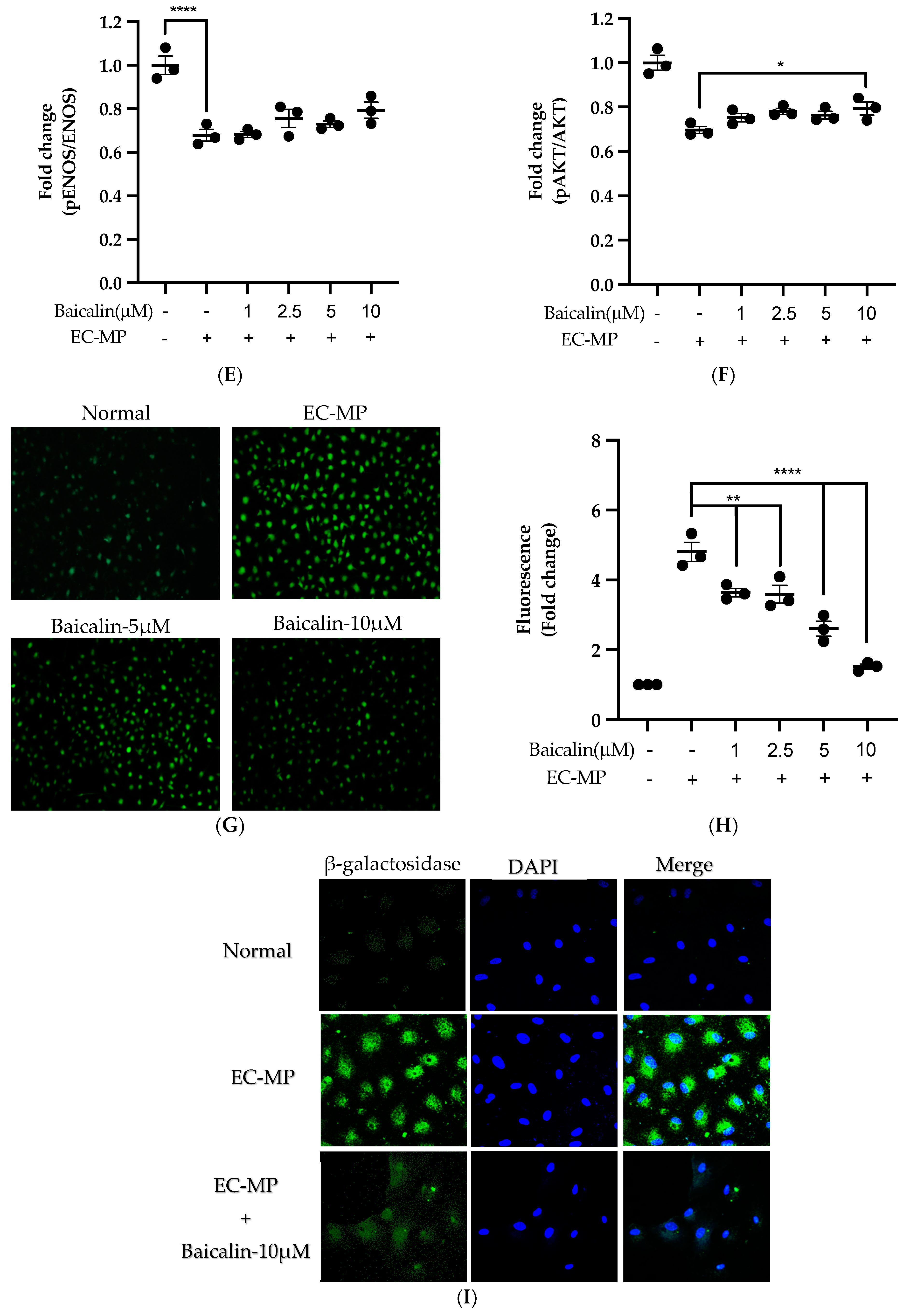
3.4. Baicalin Target the Endothelial Dysfunction Marker to Restore EC Function
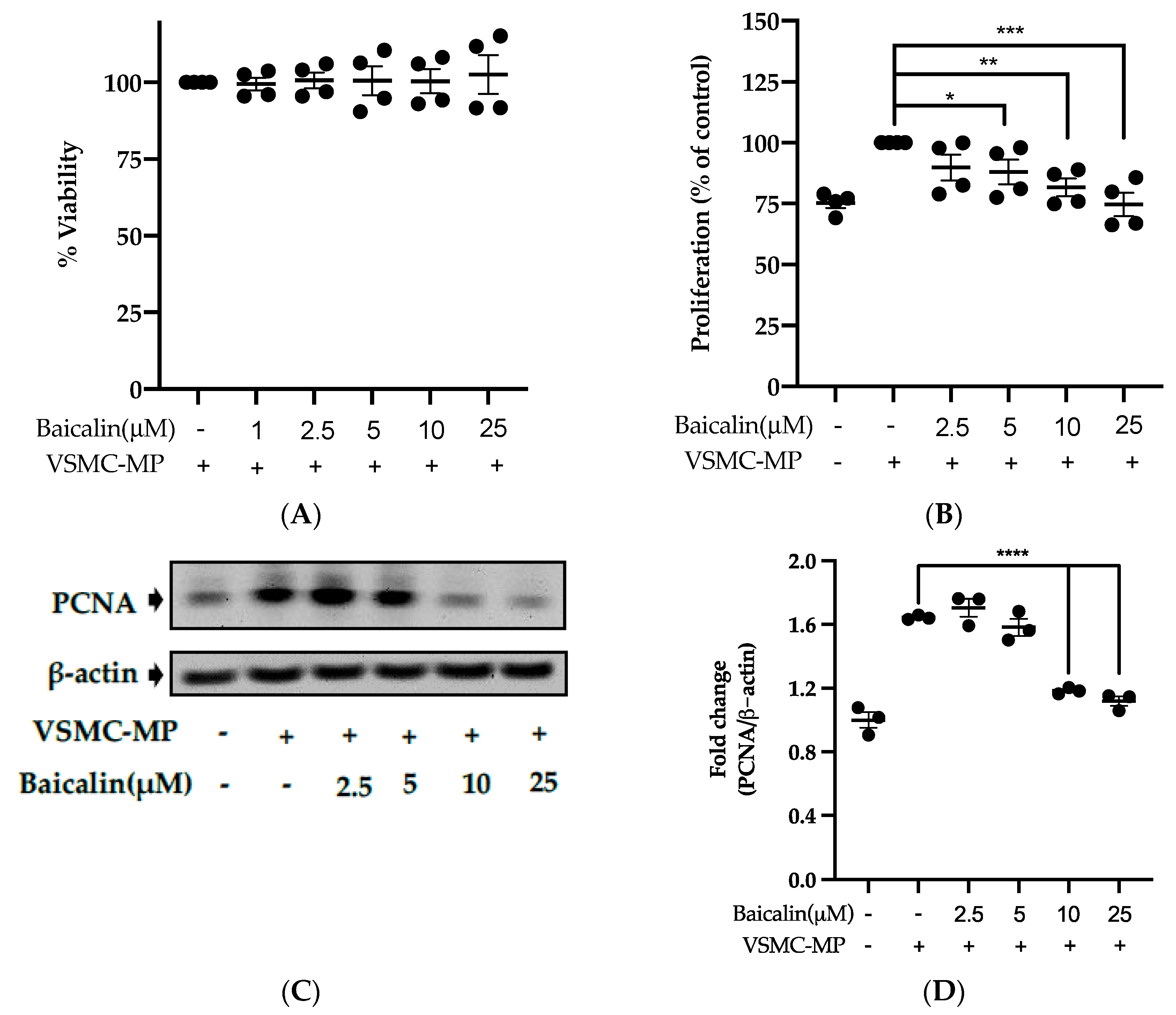
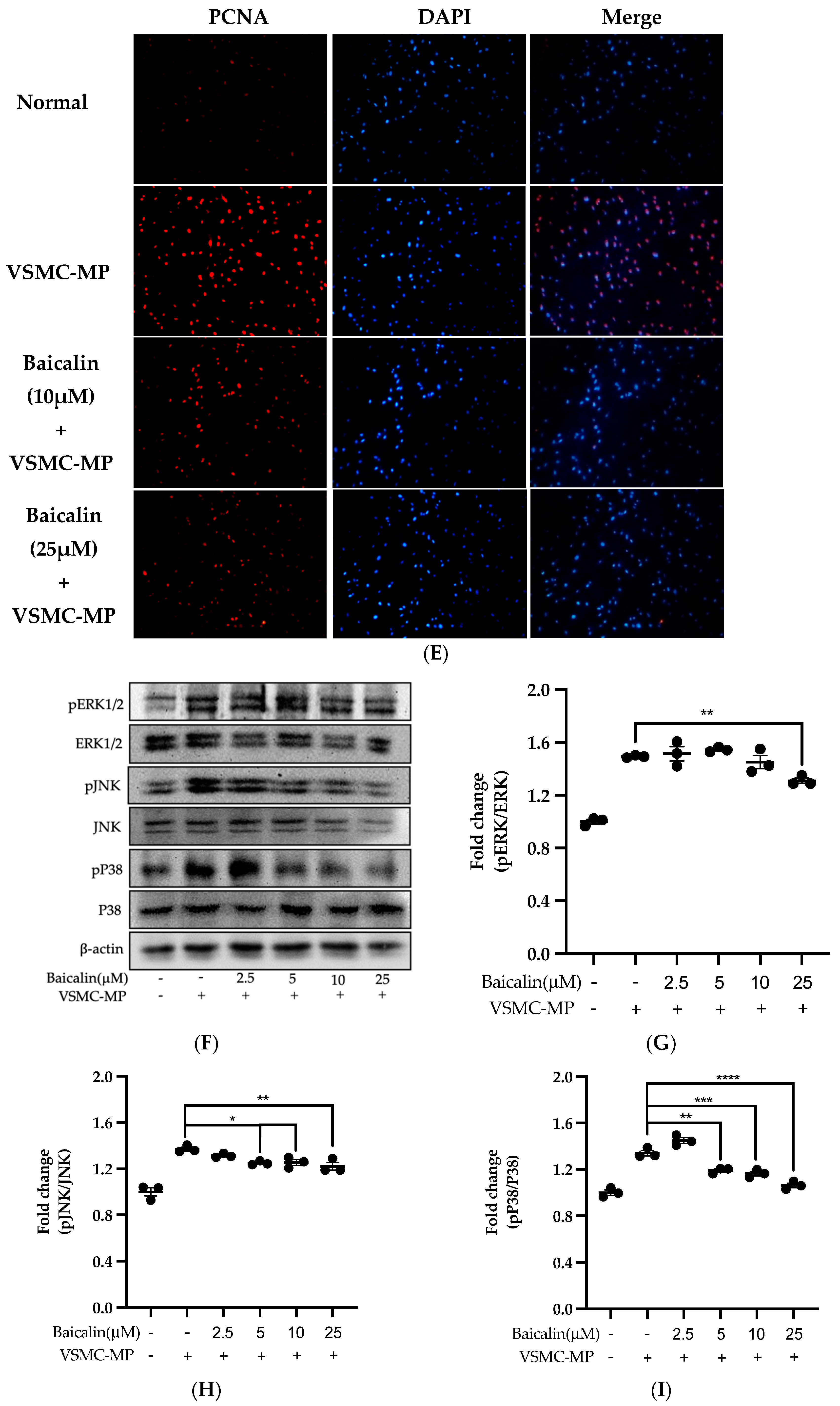
3.5. Baicalin Inhibits Proliferation of VSMC Induced by VSMC-MP
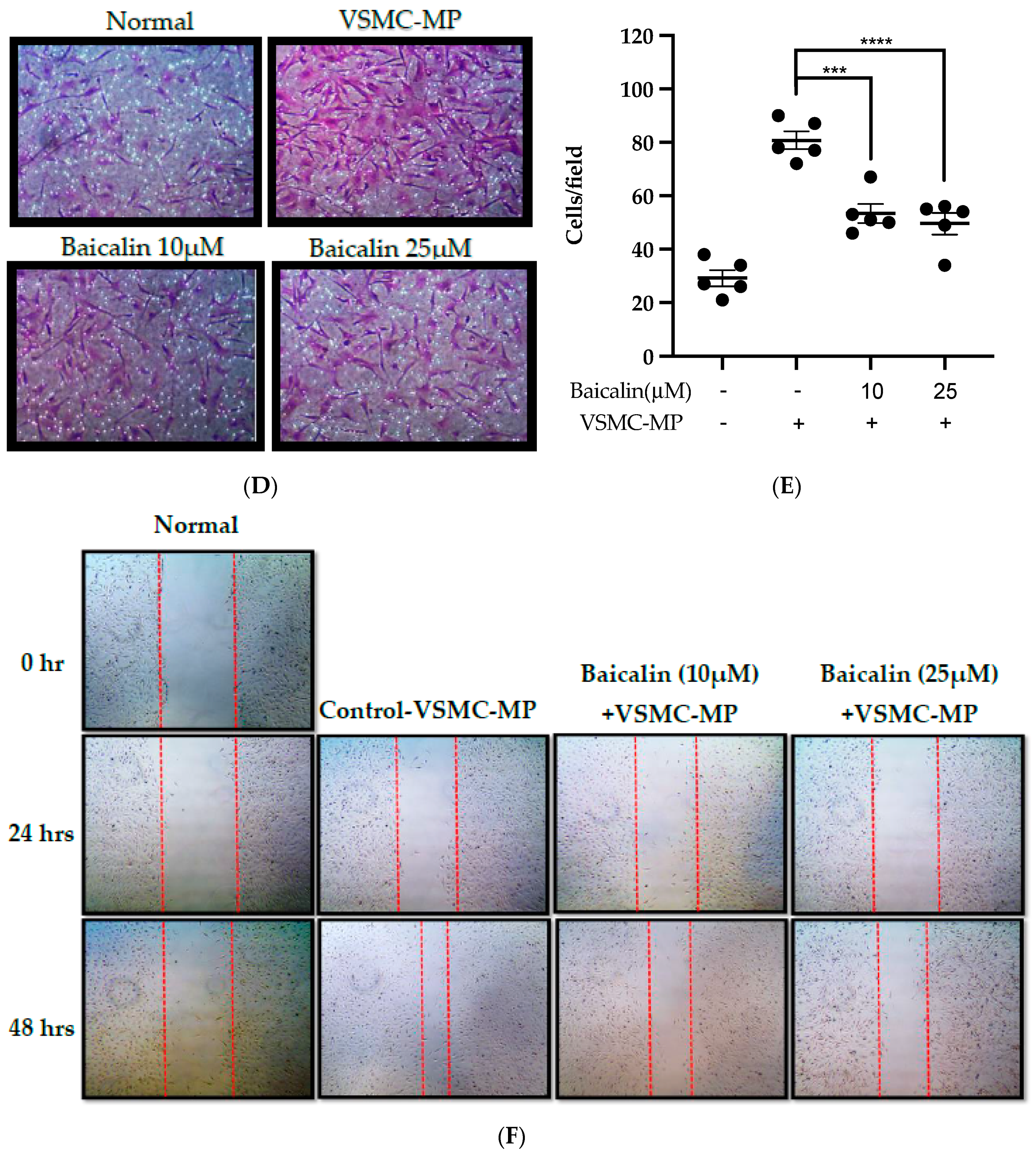
3.6. Baicalin Inhibits VSMC Migration Induced by EC-MP and VSMC-MP
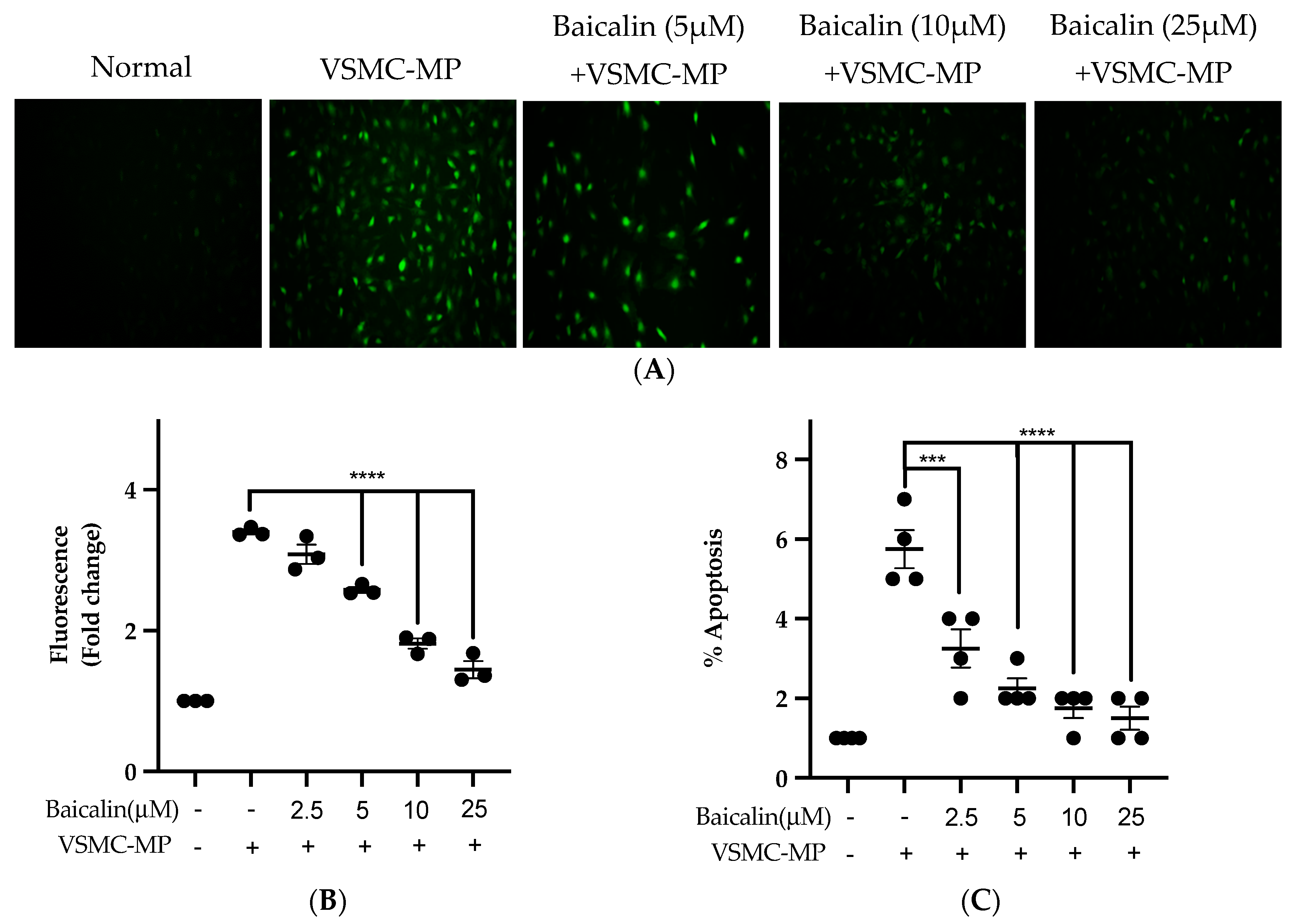
3.7. Baicalin Inhibits ROS Production and Apoptosis in VSMC-MP Induced VSMC
3.8. Antioxidant Activity of Baicalin
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- VanWijk, M.J.; VanBavel, E.; Sturk, A.; Nieuwland, R. Microparticles in cardiovascular diseases. Cardiovasc. Res. 2003, 59, 277–287. [Google Scholar] [CrossRef]
- Franca, C.N.; Izar, M.C.; Amaral, J.B.; Tegani, D.M.; Fonseca, F.A. Microparticles as potential biomarkers of cardiovascular disease. Arq. Bras. Cardiol. 2015, 104, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Patz, S.; Trattnig, C.; Grunbacher, G.; Ebner, B.; Gully, C.; Novak, A.; Rinner, B.; Leitinger, G.; Absenger, M.; Tomescu, O.A.; et al. More than cell dust: Microparticles isolated from cerebrospinal fluid of brain injured patients are messengers carrying mRNAs, miRNAs, and proteins. J. Neurotrauma 2013, 30, 1232–1242. [Google Scholar] [CrossRef] [PubMed]
- Connor, D.E.; Exner, T.; Ma, D.D.; Joseph, J.E. The majority of circulating platelet-derived microparticles fail to bind annexin V, lack phospholipid-dependent procoagulant activity and demonstrate greater expression of glycoprotein Ib. Thromb Haemost 2010, 103, 1044–1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schindler, S.M.; Little, J.P.; Klegeris, A. Microparticles: A new perspective in central nervous system disorders. Biomed Res. Int. 2014, 2014, 756327. [Google Scholar] [CrossRef] [Green Version]
- Tramontano, A.F.; Lyubarova, R.; Tsiakos, J.; Palaia, T.; Deleon, J.R.; Ragolia, L. Circulating endothelial microparticles in diabetes mellitus. Mediat. Inflamm. 2010, 2010, 250476. [Google Scholar] [CrossRef]
- Roos, M.A.; Gennero, L.; Denysenko, T.; Reguzzi, S.; Cavallo, G.; Pescarmona, G.P.; Ponzetto, A. Microparticles in physiological and in pathological conditions. Cell Biochem. Funct. 2010, 28, 539–548. [Google Scholar] [CrossRef]
- Distler, J.H.; Pisetsky, D.S.; Huber, L.C.; Kalden, J.R.; Gay, S.; Distler, O. Microparticles as regulators of inflammation: novel players of cellular crosstalk in the rheumatic diseases. Arthritis Rheum 2005, 52, 3337–3348. [Google Scholar] [CrossRef]
- Perez-Hernandez, J.; Cortes, R. Extracellular vesicles as biomarkers of systemic lupus erythematosus. Dis. Markers 2015, 2015, 613536. [Google Scholar] [CrossRef] [Green Version]
- Pitanga, T.N.; de Aragao Franca, L.; Rocha, V.C.; Meirelles, T.; Borges, V.M.; Goncalves, M.S.; Pontes-de-Carvalho, L.C.; Noronha-Dutra, A.A.; dos-Santos, W.L. Neutrophil-derived microparticles induce myeloperoxidase-mediated damage of vascular endothelial cells. BMC Cell Biol. 2014, 15, 21. [Google Scholar] [CrossRef] [Green Version]
- Mallat, Z.; Hugel, B.; Ohan, J.; Leseche, G.; Freyssinet, J.M.; Tedgui, A. Shed membrane microparticles with procoagulant potential in human atherosclerotic plaques: a role for apoptosis in plaque thrombogenicity. Circulation 1999, 99, 348–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezentsev, A.; Merks, R.M.; O’Riordan, E.; Chen, J.; Mendelev, N.; Goligorsky, M.S.; Brodsky, S.V. Endothelial microparticles affect angiogenesis in vitro: role of oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1106–H1114. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Sugiyama, S.; Kugiyama, K.; Watanabe, K.; Fukushima, H.; Tanaka, T.; Sakamoto, T.; Yoshimura, M.; Jinnouchi, H.; Ogawa, H. Elevated levels of VE-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J. Am. Coll Cardiol. 2005, 45, 1622–1630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauley, J.; Pisetsky, D.S. The release of microparticles by RAW 264.7 macrophage cells stimulated with TLR ligands. J. Leukoc. Biol. 2010, 87, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Milbank, E.; Soleti, R.; Martinez, E.; Lahouel, B.; Hilairet, G.; Martinez, M.C.; Andriantsitohaina, R.; Noireaud, J. Microparticles from apoptotic RAW 264.7 macrophage cells carry tumour necrosis factor-alpha functionally active on cardiomyocytes from adult mice. J. Extracell Vesicles 2015, 4, 28621. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pertierra, E.; Benavente, L.; Blanco-Gelaz, M.Á.; Fernández-Martín, J.L.; Lahoz, C.H.; Calleja, S.; López-Larrea, C.; Cernuda-Morollón, E.J.J. Lysophosphatidylcholine Induces vascular smooth muscle cell membrane vesiculation: Potential role in atherosclerosis through Caveolin-1 Regulation. JPB 2014, 7, 332. [Google Scholar]
- Chiva-Blanch, G.; Suades, R.; Padró, T.; Vilahur, G.; Peña, E.; Ybarra, J.; Pou, J.M.; Badimon, L.J.R.E.d.C. Microparticle shedding by erythrocytes, monocytes and vascular smooth muscular cells is reduced by aspirin in diabetic patients. Rev. Española Cardiol. 2016, 69, 672–680. [Google Scholar] [CrossRef]
- Essayagh, S.; Brisset, A.C.; Terrisse, A.D.; Dupouy, D.; Tellier, L.; Navarro, C.; Arnal, J.F.; Sie, P. Microparticles from apoptotic vascular smooth muscle cells induce endothelial dysfunction, a phenomenon prevented by beta3-integrin antagonists. Thromb. Haemost. 2005, 94, 853–858. [Google Scholar] [CrossRef]
- Paudel, K.R.; Oak, M.H.; Kim, D.W. Smooth muscle cell derived microparticles acts as autocrine activation of smooth muscle cell proliferation by mitogen associated protein kinase upregulation. J. Nanosci. Nanotechnol. 2020, 20, 5746–5750. [Google Scholar] [CrossRef]
- Paudel, K.R.; Panth, N.; Kim, D.W. Circulating endothelial microparticles: A key hallmark of atherosclerosis progression. Scientifica (Cairo) 2016, 2016, 8514056. [Google Scholar] [CrossRef] [Green Version]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.D.; Guan, J.P.; Tang, R.C.; Qiao, Y.F. Application of natural flavonoids to impart antioxidant and antibacterial activities to polyamide fiber for health care applications. Antioxidants (Basel) 2019, 8, 301. [Google Scholar] [CrossRef] [Green Version]
- Jun, M.Y.; Karki, R.; Paudel, K.R.; Sharma, B.R.; Adhikari, D.; Kim, D.W. Alkaloid rich fraction from Nelumbo nucifera targets VSMC proliferation and migration to suppress restenosis in balloon-injured rat carotid artery. Atherosclerosis 2016, 248, 179–189. [Google Scholar] [CrossRef]
- Paudel, K.R.; Karki, R.; Kim, D.W. Cepharanthine inhibits in vitro VSMC proliferation and migration and vascular inflammatory responses mediated by RAW264.7. Toxicol In Vitro 2016, 34, 16–25. [Google Scholar] [CrossRef]
- Park, S.H.; Shim, B.S.; Yoon, J.S.; Lee, H.H.; Lee, H.W.; Yoo, S.B.; Wi, A.J.; Park, W.S.; Kim, H.J.; Kim, D.W.; et al. Vascular protective effect of an ethanol extract of camellia japonica fruit: Endothelium-Dependent relaxation of coronary artery and reduction of smooth muscle cell migration. Oxid Med. Cell. Longev. 2015, 2015, 6309565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulanger, C.M.; Scoazec, A.; Ebrahimian, T.; Henry, P.; Mathieu, E.; Tedgui, A.; Mallat, Z. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation 2001, 104, 2649–2652. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.; Yang, X.; Baumann, K.; Przybilla, D.; Schmitz, T.; Flender, A.; Paul, K.; Alhusseiny, A.; Nickenig, G.; Werner, N. Endothelial microparticles reduce ICAM-1 expression in a microRNA-222-dependent mechanism. J. Cell. Mol. Med. 2015, 19, 2202–2214. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, J.K.; Jang, H.D. Genistein inhibits osteoclastic differentiation of RAW 264.7 cells via regulation of ROS production and scavenging. Int. J. Mol. Sci. 2014, 15, 10605–10621. [Google Scholar] [CrossRef] [Green Version]
- Li, A.E.; Ito, H.; Rovira, I.I.; Kim, K.S.; Takeda, K.; Yu, Z.Y.; Ferrans, V.J.; Finkel, T. A role for reactive oxygen species in endothelial cell anoikis. Circ. Res. 1999, 85, 304–310. [Google Scholar] [CrossRef]
- Burger, D.; Montezano, A.C.; Nishigaki, N.; He, Y.; Carter, A.; Touyz, R.M. Endothelial microparticle formation by angiotensin II is mediated via Ang II receptor type I/NADPH oxidase/ Rho kinase pathways targeted to lipid rafts. Arter. Thromb. Vasc. Biol. 2011, 31, 1898–1907. [Google Scholar] [CrossRef] [Green Version]
- Abbas, M.; Jesel, L.; Auger, C.; Amoura, L.; Messas, N.; Manin, G.; Rumig, C.; Leon-Gonzalez, A.J.; Ribeiro, T.P.; Silva, G.C.; et al. Endothelial microparticles from acute coronary syndrome patients induce premature coronary artery endothelial cell aging and thrombogenicity: Role of the ang II/AT1 receptor/NADPH oxidase-mediated activation of MAPKs and PI3-Kinase pathways. Circulation 2017, 135, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Ashino, T.; Yamamoto, M.; Numazawa, S. Nrf2/Keap1 system regulates vascular smooth muscle cell apoptosis for vascular homeostasis: role in neointimal formation after vascular injury. Sci. Rep. 2016, 6, 26291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.H.; Huang, L.; Zhang, X.; Zhang, P.; Zhang, S.M.; Guan, H.; Zhang, Y.; Zhu, X.Y.; Tian, S.; Deng, K.; et al. Mindin regulates vascular smooth muscle cell phenotype and prevents neointima formation. Clin. Sci. (Lond.) 2015, 129, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Lee, H.H.; Gong, D.S.; Park, S.H.; Yi, E.; Schini-Kerth, V.; Oak, M.H. Fine air pollution particles induce endothelial senescence via redox-sensitive activation of local angiotensin system. Environ. Pollut. 2019, 252, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.W.; Lee, S.J.; Kim, Y.H.; Bae, J.U.; Park, S.Y.; Bae, S.S.; Kim, C.D. Mechanical stretch increases MMP-2 production in vascular smooth muscle cells via activation of PDGFR-beta/Akt signaling pathway. PLoS ONE 2013, 8, e70437. [Google Scholar] [CrossRef]
- Lee, H.H.; Paudel, K.R.; Kim, D.W. Terminalia chebula Fructus Inhibits Migration and Proliferation of Vascular Smooth Muscle Cells and Production of Inflammatory Mediators in RAW 264.7. Evid. Based Complement. Altern. Med. 2015, 2015, 502182. [Google Scholar] [CrossRef] [Green Version]
- Karki, R.; Kim, S.B.; Kim, D.W. Magnolol inhibits migration of vascular smooth muscle cells via cytoskeletal remodeling pathway to attenuate neointima formation. Exp. Cell Res. 2013, 319, 3238–3250. [Google Scholar] [CrossRef]
- Paudel, K.R.; Lee, U.W.; Kim, D.W. Chungtaejeon, a Korean fermented tea, prevents the risk of atherosclerosis in rats fed a high-fat atherogenic diet. J. Integr. Med. 2016, 14, 134–142. [Google Scholar] [CrossRef]
- Karki, R.; Sahi, N.; Jeon, E.R.; Park, Y.S.; Kim, D.W. Chungtaejeon, a Korean fermented tea, scavenges oxidation and inhibits cytokine induced proliferation and migration of human aortic smooth muscle cells. Plant Foods Hum. Nutr. 2011, 66, 27–33. [Google Scholar] [CrossRef]
- Karki, R.; Jeon, E.R.; Kim, D.W. Magnoliae Cortex inhibits intimal thickening of carotid artery through modulation of proliferation and migration of vascular smooth muscle cells. Food Chem. Toxicol. 2012, 50, 634–640. [Google Scholar] [CrossRef]
- Karki, R.; Jeon, E.R.; Kim, D.W. Nelumbo nucifera leaf extract inhibits neointimal hyperplasia through modulation of smooth muscle cell proliferation and migration. Nutrition 2013, 29, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Panth, N.; Paudel, K.R.; Gong, D.S.; Oak, M.H. Vascular protection by ethanol extract of morus alba root bark: Endothelium-Dependent relaxation of rat aorta and decrease of smooth muscle cell migration and proliferation. Evid. Based Complement. Altern. Med. 2018, 2018, 7905763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.B.; Paudel, K.R.; Kim, D.W. Preventive Effect of traditional korean formulations on intimal thickening of rat carotid artery injured by balloon catheter. Korean J. Plant Resour. 2013, 26, 678–685. [Google Scholar] [CrossRef]
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.B.; Lee, S.M.Y.; Wang, R. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020, 11, 2622. [Google Scholar] [CrossRef]
- Lee, H.H.; Paudel, K.R.; Jeong, J.; Wi, A.J.; Park, W.S.; Kim, D.W.; Oak, M.H. Antiatherogenic effect of camellia japonica fruit extract in high fat diet-fed rats. Evid. Based Complement. Altern. Med. 2016, 2016, 9679867. [Google Scholar] [CrossRef] [Green Version]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive oxygen species: A key hallmark of cardiovascular disease. Adv. Med. 2016, 2016, 9152732. [Google Scholar] [CrossRef] [Green Version]
- Panth, N.; Park, S.H.; Kim, H.J.; Kim, D.H.; Oak, M.H. Protective effect of salicornia europaea extracts on high salt intake-induced vascular dysfunction and hypertension. Int. J. Mol. Sci. 2016, 17, 1176. [Google Scholar] [CrossRef]
- Dimmeler, S.; Fleming, I.; Fisslthaler, B.; Hermann, C.; Busse, R.; Zeiher, A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 1999, 399, 601–605. [Google Scholar] [CrossRef]
- Horn, P.; Amabile, N.; Angeli, F.S.; Sansone, R.; Stegemann, B.; Kelm, M.; Springer, M.L.; Yeghiazarians, Y.; Schroeter, H.; Heiss, C. Dietary flavanol intervention lowers the levels of endothelial microparticles in coronary artery disease patients. Br. J. Nutr. 2014, 111, 1245–1252. [Google Scholar] [CrossRef]
- Kam, A.; Li, K.M.; Razmovski-Naumovski, V.; Nammi, S.; Chan, K.; Grau, G.E.; Li, G.Q. Curcumin reduces tumour necrosis factor-enhanced annexin V-Positive microparticle release in human vascular endothelial cells. J. Pharm. Pharm. Sci. 2015, 18, 424–433. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, V.; Bachelier, K.; Schirmer, S.H.; Werner, C.; Laufs, U.; Bohm, M. Red wine prevents the acute negative vascular effects of smoking. Am. J. Med. 2017, 130, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paudel, K.R.; Kim, D.-W. Microparticles-Mediated Vascular Inflammation and its Amelioration by Antioxidant Activity of Baicalin. Antioxidants 2020, 9, 890. https://doi.org/10.3390/antiox9090890
Paudel KR, Kim D-W. Microparticles-Mediated Vascular Inflammation and its Amelioration by Antioxidant Activity of Baicalin. Antioxidants. 2020; 9(9):890. https://doi.org/10.3390/antiox9090890
Chicago/Turabian StylePaudel, Keshav Raj, and Dong-Wook Kim. 2020. "Microparticles-Mediated Vascular Inflammation and its Amelioration by Antioxidant Activity of Baicalin" Antioxidants 9, no. 9: 890. https://doi.org/10.3390/antiox9090890