The Sorbus spp.—Underutilised Plants for Foods and Nutraceuticals: Review on Polyphenolic Phytochemicals and Antioxidant Potential
Abstract
:1. Introduction
2. Botanical Classification and General Uses
3. Nutritional Composition
4. Total Phenolic Content and Quantitative Composition of Phytochemical Antioxidants in Sorbus spp.
4.1. Total Phenolic Content
4.2. Phenolic Acids
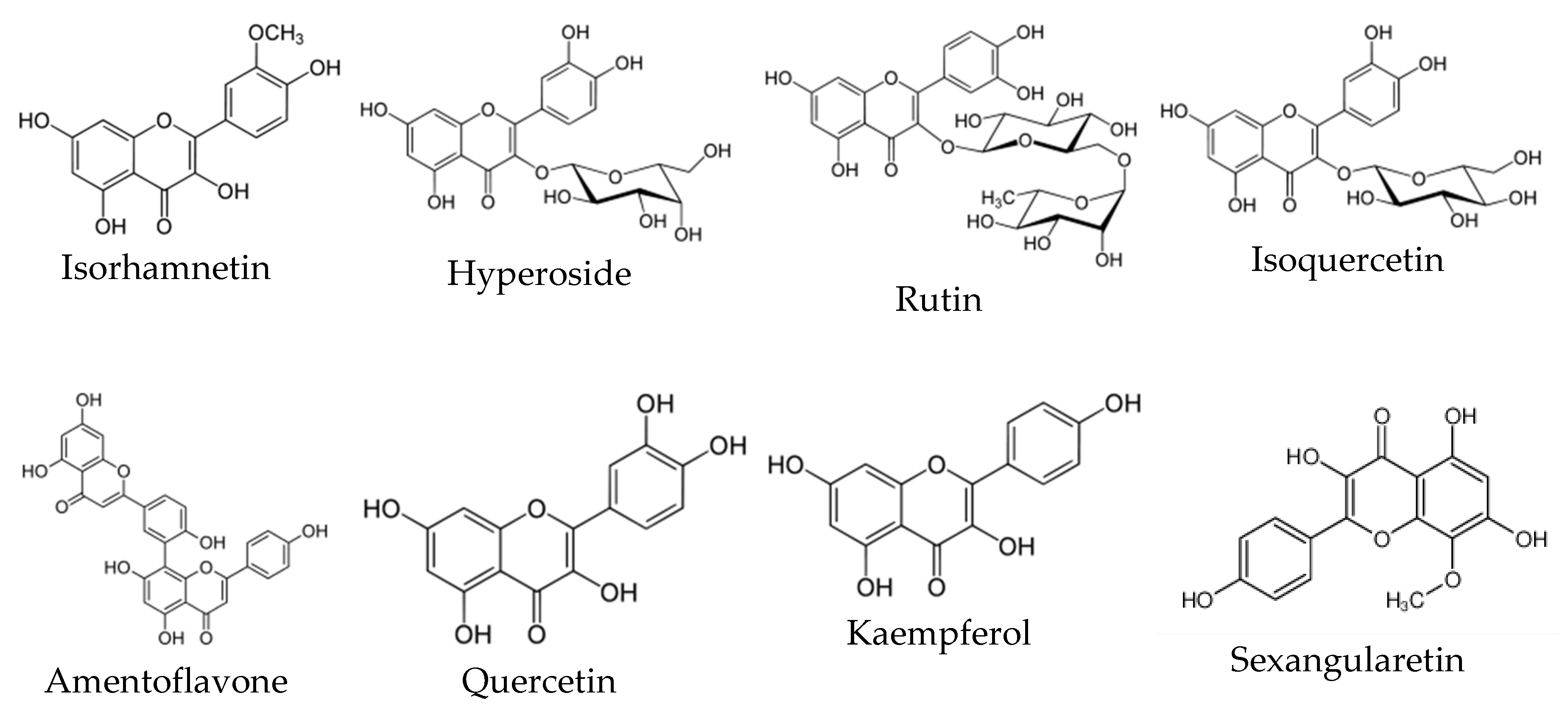
4.3. Flavonoids
5. Antioxidant Potential of Sorbus spp.
6. Toxic Constituents of Rowanberries
7. Promising Health Benefits and Related Applications in Foods, Nutraceuticals and Pharmaceuticals
8. Conclusions and Further Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vazquez-Flores, A.A.; Martinez-Gonzalez, A.I.; Alvarez-Parrilla, E.; Díaz-Sánchez, Á.G.; Rosa, L.A.; González-Aguilar, G.A.; Aguilar, C.N. Proanthocyanidins with a low degree of polymerization are good inhibitors of digestive enzymes because of their ability to form specific interactions: A hypothesis. J. Food Sci. 2018, 83, 2895–2902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.; Metrani, R.; Shivanagoudra, S.R.; Jayaprakasha, G.K.; Patil, B.S. Review on bile acids: Effects of the gut microbiome, interactions with dietary fiber, and alterations in the bioaccessibility of bioactive compounds. J. Agric. Food Chem. 2019, 67, 9124–9138. [Google Scholar] [CrossRef] [PubMed]
- Robertson, K.R.; Phipps, J.B.; Rohrer, J.R.; Smith, P.G. A synopsis of genera in Maloideae (Rosaceae). Syst. Bot. 1991, 16, 376–394. [Google Scholar] [CrossRef]
- Sołtys, A.; Galanty, A.; Podolak, I. Ethnopharmacologically important but underestimated genus Sorbus: A comprehensive review. Phytochem. Rev. 2020, 19, 491–526. [Google Scholar] [CrossRef]
- Poyrazoǧlu, E.S. Changes in ascorbic acid and sugar content of rowanberries during ripening. J. Food Qual. 2004, 27, 366–370. [Google Scholar] [CrossRef]
- Miletic, R.; Paunovic, S.M. Research into service tree (Sorbus domestica L.) population in eastern Serbia. Genetika 2012, 44, 483–490. [Google Scholar] [CrossRef]
- Fomenko, S.E.; Kushnerova, N.F.; Sprygin, V.G.; Drugova, E.S.; Momot, T.V. Chemical composition and biological action of rowanberry extract. Russ. J. Bioorg. Chem. 2016, 42, 764–769. [Google Scholar] [CrossRef]
- Hejcman, M.; Hejcmanová, P.; Pavlů, V.; Thorhallsdottirl, A.G. Nutritive value of leaf fodder from the main woody species in Iceland. In Grassland Science in Europe, Vol. 19—EGF at 50: The Future of European Grasslands; Hopkins, A., Collins, R.P., Fraser, M.D., King, V.R., Lloyd, D.C., Moorby, J.M., Robson, P.R.H., Eds.; European Grassland Federation EGF: Zürich, Switzerland, 2014; pp. 566–568. [Google Scholar]
- USDA National Rersources Conservation Service. Available online: http://plants.usda.gov (accessed on 27 August 2020).
- Ayupbek, A.; Hu, K.-L.; Aisa, H.A. Chemical constituents from the leaves of Sorbus tianschanica. Chem. Nat. Compd. 2012, 48, 133–134. [Google Scholar] [CrossRef]
- Bailie, A.; Renaut, S.; Ubalijoro, E.; Guerrero-Analco, J.A.; Saleem, A.; Haddad, P.; Arnason, J.T.; Johns, T.; Cuerrier, A. Phytogeographic and genetic variation in Sorbus, a traditional antidiabetic medicine—Adaptation in action in both a plant and a discipline. PeerJ 2016, 2, 1–22. [Google Scholar] [CrossRef]
- Mccune, L.M.; Johns, T. Antioxidant activity in medicinal plants associated with the symptoms of diabetes mellitus used by the indigenous peoples of the North American boreal forest. J. Ethnopharmacol. 2002, 82, 197–205. [Google Scholar] [CrossRef]
- Moon, S.C.; Choi, H.J.; Chung, T.W.; Lee, J.H.; Lee, S.O.; Jung, M.H.; Kim, B.J.; Choi, J.Y.; Ha, K.T. Sorbus commixta water extract induces apoptotic cell death via a ROS-dependent pathway. Oncol. Lett. 2018, 16, 4193–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, L.R.; Bae, M.S.; Kim, B.M.; Oh, G.S.; Chai, K.Y. A chalcone glycoside from the fruits of Sorbus commixta Hedl. Molecules 2009, 14, 5323–5327. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Fatima, I.; Kazmi, M.H.; Malik, A.; Afza, N.; Iqbal, L.; Latif, M. Cashmins A and B, potent antioxidant coumarins from Sorbus cashmiriana. Chem. Nat. Compd. 2015, 51, 626–629. [Google Scholar] [CrossRef]
- Li, H.; Matsuura, M.; Li, W.; Li, Q.; Zhang, Q.; Koike, K. Chemical constituents from the fruits of Sorbus pohuashanensis. Biochem. Syst. Ecol. 2012, 43, 166–168. [Google Scholar] [CrossRef]
- Francis, J.K. (Ed.) Wildland Shrubs of the United States and Its Territories: Thamnic Descriptions: Volume 1; Gen. Tech. Rep. IITF-GTR-26; U.S. Department of Agriculture, Forest Service, International Institute of Tropical Forestry: San Juan, PR, USA; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2004; 830p.
- Olszewska, M. Separation of quercetin, sexangularetin, kaempferol and isorhamnetin for simultaneous HPLC determination of flavonoid aglycones in inflorescences, leaves and fruits of three Sorbus species. J. Pharm. Biomed. Anal. 2008, 48, 629–635. [Google Scholar] [CrossRef]
- Gil-Izquierdo, A.; Mellenthin, A. Identification and quantitation of flavonols in rowanberry (Sorbus aucuparia L.) juice. Eur. Food Res. Technol. 2001, 213, 12–17. [Google Scholar] [CrossRef]
- Vogl, S.; Picker, P.; Mihaly-Bison, J.; Fakhrudin, N.; Atanasov, A.G.; Heiss, E.H.; Wawrosch, C.; Reznicek, G.; Dirsch, V.M.; Saukel, J.; et al. Ethnopharmacological in vitro studies on Austria’s folk medicine—An unexplored lore in vitro anti-inflammatory activities of 71 Austrian traditional herbal drugs. J. Ethnopharmacol. 2013, 149, 750–771. [Google Scholar] [CrossRef] [Green Version]
- Räty, M.; Caudullo, G.; de Rigo, D. Sorbus aucuparia in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-MiguelAyanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publ. Off. EU: Luxembourg, 2016; pp. 176–177. [Google Scholar]
- Plants for A Future. Available online: https://pfaf.org/user/DatabaseSearhResult.aspx (accessed on 23 July 2020).
- Kalle, R.; Sõukand, R. Historical ethnobotanical review of wild edible plants of Estonia (1770s-1960s). Acta Soc. Bot. Pol. 2012, 81, 271–281. [Google Scholar] [CrossRef]
- Broholm, S.L.; Gramsbergen, S.M.; Nyberg, N.T.; Jäger, A.K.; Staerk, D. Potential of Sorbus berry extracts for management of type 2 diabetes: Metabolomics investigation of 1H NMR spectra, α-amylase and α-glucosidase inhibitory activities, and in vivo anti-hyperglycaemic activity of S. norvegica. J. Ethnopharmacol. 2019, 242. [Google Scholar] [CrossRef]
- Kovanda, M.; Challice, J. The genus Micromeles revisited. Folia Geobot. Phytotaxon. 1981, 16, 181–193. [Google Scholar] [CrossRef]
- Cheon, S.M.; Jang, I.; Lee, M.H.; Kim, D.K.; Jeon, H.; Cha, D.S. Sorbus alnifolia protects dopaminergic neurodegeneration in Caenorhabditis elegans. Pharm. Biol. 2017, 55, 481–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aldasoro, J.J.; Aedo, C.; Garmendia, F.M. Revision of Sorbus subgenera Aria and Torminaria (Rosaceae-Maloideae). Syst. Bot. Monogr. 2004, 69, 1–148. [Google Scholar] [CrossRef]
- Mrkonjić, Z.O.; Nađpal, J.D.; Beara, I.N.; Sabo, V.S.A.; Četojević-Simin, D.D.; Mimica-Dukić, N.M.; Lesjak, M.M. Phenolic profiling and bioactivities of fresh fruits and jam of Sorbus species. J. Serbian Chem. Soc. 2017, 82, 651–664. [Google Scholar] [CrossRef] [Green Version]
- Sulusoglu, M. In vitro pollen viability and pollen germination of service tree (Sorbus domestica L.). Int. J. Biosci. 2014, 5, 108–114. [Google Scholar] [CrossRef]
- Majić, B.; Šola, I.; Likić, S.; Cindrić, I.J.; Rusak, G. Characterisation of Sorbus domestica L. bark, fruits and seeds: Nutrient composition and antioxidant activity. Food Technol. Biotechnol. 2015, 53, 463–471. [Google Scholar] [CrossRef]
- Hukkanen, A.T.; Pölönen, S.S.; Kärenlampi, S.O.; Kokko, H.I. Antioxidant capacity and phenolic content of sweet rowanberries. J. Agric. Food Chem. 2006, 54, 112–119. [Google Scholar] [CrossRef]
- Sokolov, V.V.; Savel’ev, N.I.; Goncharov, N.P. I. V. Michurin’s work on expansion of the plant horticulture assortment and improvement of food quality. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2015, 69, 190–197. [Google Scholar] [CrossRef] [Green Version]
- Rengarten, G.A.; Sorokopudov, V.N. Selection of rows as a decorative culture in Russia and in European countries. Vestn. KrasGAU Agron. 2019, 6, 9–15. (In Russian) [Google Scholar]
- Mlcek, J.; Rop, O.; Jurikova, T.; Sochor, J.; Fisera, M.; Balla, S.; Baron, M.; Hrabe, J. Bioactive compounds in sweet rowanberry fruits of interspecific rowan crosses. Cent. Eur. J. Biol. 2014, 9, 1078–1086. [Google Scholar] [CrossRef]
- Berna, E.; Kampuse, S.; Straumite, E. The suitability of different rowanberry cultivars for production of fruit marmalade. In Proceedings of the Annual 18th International Scientific Conference Research for Rural Development, Jelgava, Latvia, 16–18 May 2012; Treija, S., Skuja, I., Eds.; Latvia University of Agriculture: Jelgava, Latvia, 2012; Volume 1, pp. 109–116. [Google Scholar]
- Acuña, U.M.; Atha, D.E.; Ma, J.; Nee, M.H.; Kennelly, E.J. Antioxidant capacities of ten edible North American plants. Phyther. Res. 2002, 16, 63–65. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Presler, A.; Michel, P. Profiling of phenolic compounds and antioxidant activity of dry extracts from the selected Sorbus species. Molecules 2012, 17, 3093–3113. [Google Scholar] [CrossRef] [PubMed]
- Im, N.K.; Lee, D.S.; Lee, S.R.; Jeong, G.S. Lupeol isolated from Sorbus commixta suppresses 1α,25-(OH)2D3-mediated osteoclast differentiation and bone loss in vitro and in vivo. J. Nat. Prod. 2016, 79, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Oh, J.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Kang, R.L. Two new phenolic glycosides from Sorbus commixta. Chem. Pharm. Bull. 2018, 66, 839–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikulic-Petkovsek, M.; Krska, B.; Kiprovski, B.; Veberic, R. Bioactive components and antioxidant capacity of fruits from nine Sorbus genotypes. J. Food Sci. 2017, 82, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Termentzi, A.; Kefalas, P.; Kokkalou, E. LC-DAD-MS (ESI+) analysis of the phenolic content of Sorbus domestica fruits in relation to their maturity stage. Food Chem. 2008, 106, 1234–1245. [Google Scholar] [CrossRef]
- Connolly, B.A. × Sorbaronia fallax (Rosaceae): A new record of an intergeneric hybrid in Connecticut. Rhodora 2009, 111, 123–125. [Google Scholar] [CrossRef]
- Pasko, P. South Siberian fruits: Their selected chemical constituents, biological activity, and traditional use in folk medicine and daily nutrition. J. Med. Plants Res. 2012, 6, 4698–4706. [Google Scholar] [CrossRef]
- Velebil, J.; Businský, R. Sorbus × thuringiaca, the correct name for the diploid hybrid between Sorbus aria and S. aucuparia (Rosaceae). Taxon 2016, 65, 352–360. [Google Scholar] [CrossRef]
- Termentzi, A.; Alexiou, P.; Demopoulos, V.J.; Kokkalou, E. The aldose reductase inhibitory capacity of Sorbus domestica fruit extracts depends on their phenolic content and may be useful for the control of diabetic complications. Pharmazie 2008, 63, 693–696. [Google Scholar] [CrossRef]
- Yang, B.; Ahotupa, M.; Maeaettae, P.; Kallio, H. Composition and antioxidative activities of supercritical CO2-extracted oils from seeds and soft parts of northern berries. Food Res. Int. 2011, 44, 2009–2017. [Google Scholar] [CrossRef]
- Klavins, L.; Kviesis, J.; Steinberga, I.; Klavina, L.; Klavins, M. Gas chromatography-mass spectrometry study of lipids in northern berries. Agron. Res. 2016, 14, 1328–1346. [Google Scholar]
- Niki, E.; Noguchi, N.; Tsuchihashi, H.; Gotoh, N. Interaction among vitamin C, vitamin E, and β-carotene. Am. J. Clin. Nutr. 1995, 995, 13225–13265. [Google Scholar] [CrossRef] [PubMed]
- B Berņa, E.; Kampuse, S. The marmalades of sweet rowanberries as an example of a functional food. In Proceedings of the 7th International Congress of Food Technologists, Biotechnologists and Nutritionists, Opatija, Croatia, 20–23 September 2011; pp. 112–120. [Google Scholar]
- Koushik, A.; Hunter, D.J.; Spiegelman, D.; Anderson, K.E.; Buring, J.E.; Freudenheim, J.L.; Goldbohm, R.A.; Hankinson, S.E.; Larsson, S.C.; Leitzmann, M.; et al. Intake of the major carotenoids and the risk of epithelial ovarian cancer in a pooled analysis of 10 cohort studies. Int. J. Cancer 2006, 119, 2148–2154. [Google Scholar] [CrossRef] [PubMed]
- Aslantas, R.; Pirlak, L.; Güleryüz, M. The nutritional value of wild fruits from the North eastern Anatolia region of Turkey. Asian J. Chem. 2007, 19, 3072–3078. [Google Scholar]
- Ivakhnov, A.D.; Sadkova, K.S.; Sobashnikova, A.S.; Skrebets, T.E. Optimization of oil extraction from rowanberry waste in alcoholic beverage production. Russ. J. Phys. Chem. B 2019, 13, 1135–1138. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Nowak, S.; Michel, P.; Banaszczak, P.; Kicel, A. Assessment of the content of phenolics and antioxidant action of inflorescences and leaves of selected species from the genus Sorbus sensu stricto. Molecules 2010, 15, 8769–8783. [Google Scholar] [CrossRef] [Green Version]
- Olszewska, M.A.; Michel, P. Antioxidant activity of inflorescences, leaves and fruits of three Sorbus species in relation to their polyphenolic composition. Nat. Prod. Res. 2009, 23, 1507–1521. [Google Scholar] [CrossRef]
- Becerra-Herrera, M.; Lazzoi, M.R.; Sayago, A.; Beltrán, R.; Del Sole, R.; Vasapollo, G. Extraction and determination of phenolic compounds in the berries of Sorbus americana Marsh and Lonicera oblongifolia (Goldie) Hook. Food Anal. Methods 2015, 8, 2554–2559. [Google Scholar] [CrossRef]
- Gaivelyte, K.; Jakstas, V.; Razukas, A.; Janulis, V. Variation in the contents of neochlorogenic acid, chlorogenic acid and three quercetin glycosides in leaves and fruits of rowan (Sorbus) species and varieties from collections in Lithuania. Nat. Prod. Commun. 2013, 8, 1105–1110. [Google Scholar] [CrossRef] [Green Version]
- Šavikin, K.P.; Zdunić, G.M.; Krstić-Milošević, D.B.; Šircelj, H.J.; Stešević, D.D. Sorbus aucuparia and Sorbus aria as a source of antioxidant phenolics, tocopherols, and pigments. Chem. Biodivers. 2017, 14, 1–11. [Google Scholar] [CrossRef]
- Termentzi, A.; Kefalas, P.; Kokkalou, E. Antioxidant activities of various extracts and fractions of Sorbus domestica fruits at different maturity stages. Food Chem. 2006, 98, 599–608. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Grootaert, C.; Van Camp, J.; Šarkinas, A.; Liaudanskas, M.; Žvikas, V.; Viškelis, P.; Venskutonis, P.R. Chemical composition, antioxidant, antimicrobial and antiproliferative activities of the extracts isolated from the pomace of rowanberry (Sorbus aucuparia L.). Food Res. Int. 2020, 109310. [Google Scholar] [CrossRef]
- Kylli, P.; Nohynek, L.; Puupponen-Pimiä, R.; Westerlund-Wikström, B.; McDougall, G.; Stewart, D.; Heinonen, M. Rowanberry phenolics: Compositional analysis and bioactivities. J. Agric. Food Chem. 2010, 58, 11985–11992. [Google Scholar] [CrossRef] [PubMed]
- Ekin, H.N.; Gokbulut, A.; Aydin, Z.U.; Donmez, A.A.; Orhan, I.E. Insight into anticholinesterase and antioxidant potential of thirty-four Rosaceae samples and phenolic characterization of the active extracts by HPLC. Ind. Crops Prod. 2016, 91, 104–113. [Google Scholar] [CrossRef]
- Kim, M.B.; Park, J.S.; Lim, S. Bin Antioxidant activity and cell toxicity of pressurised liquid extracts from 20 selected plant species in Jeju, Korea. Food Chem. 2010, 122, 546–552. [Google Scholar] [CrossRef]
- Rutkowska, M.; Olszewska, M.A.; Kolodziejczyk-Czepas, J.; Nowak, P.; Owczarek, A. Sorbus domestica leaf extracts and their activity markers: Antioxidant potential and synergy effects in scavenging assays of multiple oxidants. Molecules 2019, 24, 2289. [Google Scholar] [CrossRef] [Green Version]
- Isaikina, N.V.; Kalinkina, G.I.; Razina, T.G.; Zueva, E.P.; Rybalkina, O.Y.; Ulirich, A.V.; Fedorova, E.P.; Shilova, A.B. Sorbus aucuparia L. fruit is a source of the drug for increasing the efficiency of tumor chemotherapy. Russ. J. Bioorg. Chem. 2018, 44, 899–905. [Google Scholar] [CrossRef]
- Olszewska, M.A.; Michel, P. Activity-guided isolation and identification of free radical-scavenging components from various leaf extracts of Sorbus aria (L.) Crantz. Nat. Prod. Res. 2012, 26, 243–254. [Google Scholar] [CrossRef]
- Denev, P.; Kratchanova, M.; Ciz, M.; Lojek, A.; Vasicek, O.; Nedelcheva, P.; Blazheva, D.; Toshkova, R.; Gardeva, E.; Yossifova, L.; et al. Biological activities of selected polyphenol-rich fruits related to immunity and gastrointestinal health. Food Chem. 2014, 157, 37–44. [Google Scholar] [CrossRef]
- Gu, H.; Chen, F.; Zhang, Q.; Zang, J. Application of ionic liquids in vacuum microwave-assisted extraction followed by macroporous resin isolation of three flavonoids rutin, hyperoside and hesperidin from Sorbus tianschanica leaves. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1014, 45–55. [Google Scholar] [CrossRef]
- Yoo, J.-H.; Doh, E.-S.; Chang, J.-P.; Kil, K.-J. Antioxidant activities of hot water and ethanol extracts from the stem of Sorbus commixta Heal. Korea J. Herbol. 2017, 32, 29–36. [Google Scholar] [CrossRef]
- Bae, J.-T.; Sim, G.-S.; Kim, J.-H.; Pyo, H.-B.; Yun, J.-W.; Lee, B.-C. Antioxidative activity of the hydrolytic enzyme treated Sorbus commixta Hedl. and its inhibitory effect on matrix metalloproteinase-1 in UV irradiated human dermal fibroblasts. Arch. Pharm. Res. 2007, 30, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, Y.; Kawaii, S.; Urashima, M.; Fukase, T.; Sato, T.; Murofushi, N.; Nishimura, H. Differentiation-inducing effects of small fruit juices on HL-60 leukemic cells. J. Agric. Food Chem. 2000, 48, 3177–3182. [Google Scholar] [CrossRef] [PubMed]
- Raudone, L.; Raudonis, R.; Gaivelyte, K.; Pukalskas, A.; Viškelis, P.; Venskutonis, P.R.; Janulis, V. Phytochemical and antioxidant profiles of leaves from different Sorbus L. species. Nat. Prod. Res. 2015, 29, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, M.; Owczarek, A.; Kolodziejczyk-Czepas, J.; Michel, P.; Piotrowska, D.G.; Kapusta, P.; Nowak, P.; Olszewska, M.A. Identification of bioactivity markers of Sorbus domestica leaves in chromatographic, spectroscopic and biological capacity tests: Application for the quality control. Phytochem. Lett. 2019, 30, 278–287. [Google Scholar] [CrossRef]
- Ullah, H.; Wilfred, C.D.; Shaharun, M.S. Ionic liquid-based extraction and separation trends of bioactive compounds from plant biomass. Sep. Sci. Technol. 2019, 54, 559–579. [Google Scholar] [CrossRef]
- Guerrero-Analco, J.A.; Martineau, L.; Saleem, A.; Madiraju, P.; Muhammad, A.; Durst, T.; Haddad, P.; Arnason, J.T. Bioassay-guided isolation of the antidiabetic principle from Sorbus decora (Rosaceae) used traditionally by the Eeyou Istchee Cree First Nations. J. Nat. Prod. 2010, 73, 1519–1523. [Google Scholar] [CrossRef]
- Magnus, S.; Gazdik, F.; Anjum, N.A.; Kadlecova, E.; Lackova, Z.; Cernei, N.; Brtnicky, M.; Kynicky, J.; Klejdus, B.; Necas, T.; et al. Assessment of antioxidants in selected plant rootstocks. Antioxidants 2020, 9, 209. [Google Scholar] [CrossRef] [Green Version]
- Kähkönen, M.P.; Hopia, A.I.; Heinonen, M. Berry phenolics and their antioxidant activity. J. Agric. Food Chem. 2001, 49, 4076–4082. [Google Scholar] [CrossRef]
- Saftner, R.; Polashock, J.; Ehlenfeldt, M.; Vinyard, B. Biochemical composition and antiradical activity of rowanberry (Sorbus L.) cultivars and hybrids with different Rosaceae L. cultivars. Eur. J. Hortic. Sci. 2009, 59, 195–201. [Google Scholar]
- Baltacioǧlu, C.; Velioǧlu, S.; Karacabey, E. Changes in total phenolic and flavonoid contents of rowanberry fruit during postharvest storage. J. Food Qual. 2011, 34, 278–283. [Google Scholar] [CrossRef]
- Raspé, O.; Findlay, C.; Jacquemart, A.L. Sorbus aucuparia L. J. Ecol. 2000, 88, 910–930. [Google Scholar] [CrossRef]
- EFSA. Opinion of the scientific panel on contaminants in the food chain on a request from the Commission related to cyanogenic compounds as undesirable substances in animal feed. EFSA J. 2007, 434, 1–67. [Google Scholar]
- Aladedunye, F.; Matthäus, B. Phenolic extracts from Sorbus aucuparia (L.) and Malus baccata (L.) berries: Antioxidant activity and performance in rapeseed oil during frying and storage. Food Chem. 2014, 159, 273–281. [Google Scholar] [CrossRef]
- Vlavcheski, F.; Young, M.; Tsiani, E. Antidiabetic effects of hydroxytyrosol: In vitro and in vivo evidence. Antioxidants 2019, 8, 188. [Google Scholar] [CrossRef] [Green Version]
- Zymone, K.; Raudone, L.; Raudonis, R.; Marksa, M.; Ivanauskas, L.; Janulis, V. Phytochemical profiling of fruit powders of twenty Sorbus L. Cultivars. Molecules 2018, 23, 2593. [Google Scholar] [CrossRef] [Green Version]
- Yu, T.; Lee, Y.J.; Jang, H.J.; Kim, A.R.; Hong, S.; Kim, T.W.; Kim, M.Y.; Lee, J.; Lee, Y.G.; Cho, J.Y. Anti-inflammatory activity of Sorbus commixta water extract and its molecular inhibitory mechanism. J. Ethnopharmacol. 2011, 134, 493–500. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant phenolics and phenolic-enriched extracts as antimicrobial agents against food-contaminating microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J.; Gaforio, J.J. Dietary flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef] [Green Version]
- Kristo, A.S.; Klimis-Zacas, D.; Sikalidis, A.K. Protective role of dietary berries in cancer. Antioxidants 2016, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Razina, T.G.; Zueva, E.P.; Ulrich, A.V.; Rybalkina, O.Y.; Chaikovskii, A.V.; Isaikina, N.V.; Kalinkina, G.I.; Zhdanov, V.V.; Zyuz’kov, G.N. Antitumor effects of Sorbus aucuparia L. extract highly saturated with anthocyans and their mechanisms. Bull. Exp. Biol. Med. 2016, 162, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Deligiannidou, G.E.; Papadopoulos, R.E.; Kontogiorgis, C.; Detsi, A.; Bezirtzoglou, E.; Constantinides, T. Unraveling natural products’ role in osteoarthritis management—An overview. Antioxidants 2020, 9, 348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varela-López, A.; Bullón, P.; Giampieri, F.; Quiles, J.L. Non-nutrient, naturally occurring phenolic compounds with antioxidant activity for the prevention and treatment of periodontal diseases. Antioxidants 2015, 4, 447–481. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent advances on the anti-inflammatory and antioxidant properties of red grape polyphenols: In vitro and in vivo studies. Antioxidants 2020, 9, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grodzicki, W.; Dziendzikowska, K. The role of selected bioactive compounds in the prevention of Alzheimer’s disease. Antioxidants 2020, 9, 229. [Google Scholar] [CrossRef] [Green Version]
- Laube, M.; Kniess, T.; Pietzsch, J. Development of antioxidant COX-2 inhibitors as radioprotective agents for radiation therapy—A hypothesis-driven review. Antioxidants 2016, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Bernini, R.; Carastro, I.; Palmini, G.; Tanini, A.; Zonefrati, R.; Pinelli, P.; Brandi, M.L.; Romani, A. Lipophilization of hydroxytyrosol-enriched fractions from Olea europaea L. byproducts and evaluation of the in vitro effects on a model of colorectal cancer cells. J. Agric. Food Chem. 2017, 65, 6506–6512. [Google Scholar] [CrossRef] [PubMed]
- Aladedunye, F.; Niehaus, K.; Bednarz, H.; Thiyam-Hollander, U.; Fehling, E.; Matthäus, B. Enzymatic lipophilization of phenolic extract from rowanberry (Sorbus aucuparia) and evaluation of antioxidative activity in edible oil. LWT Food Sci. Technol. 2015, 60, 56–62. [Google Scholar] [CrossRef]
- Zhong, Y.; Shahidi, F. Lipophilized epigallocatechin gallate (EGCG) derivatives as novel antioxidants. J. Agric. Food Chem. 2011, 59, 6526–6533. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Kraujalis, P.; Tamkutė, L.; Urbonavičienė, D.; Viškelis, P.; Venskutonis, P.R. Recovery of bioactive substances from rowanberry pomace by consecutive extraction with supercritical carbon dioxide and pressurized solvents. J. Ind. Eng. Chem. 2020, 85, 152–160. [Google Scholar] [CrossRef]
- Clifford, M.N.; Kerimi, A.; Williamson, G. Bioavailability and metabolism of chlorogenic acids (acyl-quinic acids) in humans. Compr. Rev. Food Sci. Food Saf. 2020, 1–54. [Google Scholar] [CrossRef]
- Kim, T.H. Chlorogenic acid isomers from Sorbus commixta of ulleung island origin and their inhibitory effects against advanced glycation end product (AGE) formation and radical scavenging activity. J. Korean Soc. Food Sci. Nutr. 2016, 45, 1208–1213. [Google Scholar] [CrossRef]
- Boath, A.S.; Stewart, D.; McDougall, G.J. Berry components inhibit α-glucosidase in vitro: Synergies between acarbose and polyphenols from black currant and rowanberry. Food Chem. 2012, 135, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Küpeli Akkol, E.; Gürağaç Dereli, F.T.; Taştan, H.; Sobarzo-Sánchez, E.; Khan, H. Effect of Sorbus domestica and its active constituents in an experimental model of colitis rats induced by acetic acid. J. Ethnopharmacol. 2020, 251. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.K.; Roh, H.S.; Yu, J.S.; Kwon, D.J.; Kim, S.Y.; Baek, K.H.; Kim, K.H. A novel cytotoxic activity of the fruit of Sorbus commixta against human lung cancer cells and isolation of the major constituents. J. Funct. Foods 2017, 30, 1–7. [Google Scholar] [CrossRef]
- Kim, M.S.; Sohn, H.Y. Anti-coagulation and anti-platelet aggregation activity of the mature fruit of Sorbus commixta. Korean J. Microbiol. Biotechnol. 2015, 43, 373–377. [Google Scholar] [CrossRef]
- Kavak, D.D.; Akdeniz, B. Sorbus umbellata (Desf.) Fritsch var. umbellata leaves: Optimization of extraction conditions and investigation antimicrobial, cytotoxic, and β-glucuronidase inhibitory potential. Plant Foods Hum. Nutr. 2019, 74, 364–369. [Google Scholar] [CrossRef]
- Choi, H.J. In vitro antiviral activity of sakuranetin against human rhinovirus 3. Osong Public Heal. Res. Perspect. 2017, 8, 415–420. [Google Scholar] [CrossRef]
- Kim, M.J.; Jung, T.K.; Kim, M.-H.; Yoon, K.-S. In vitro screening of Jeju island plants for cosmetic ingredients. KSBB J. 2018, 33, 76–82. [Google Scholar] [CrossRef]
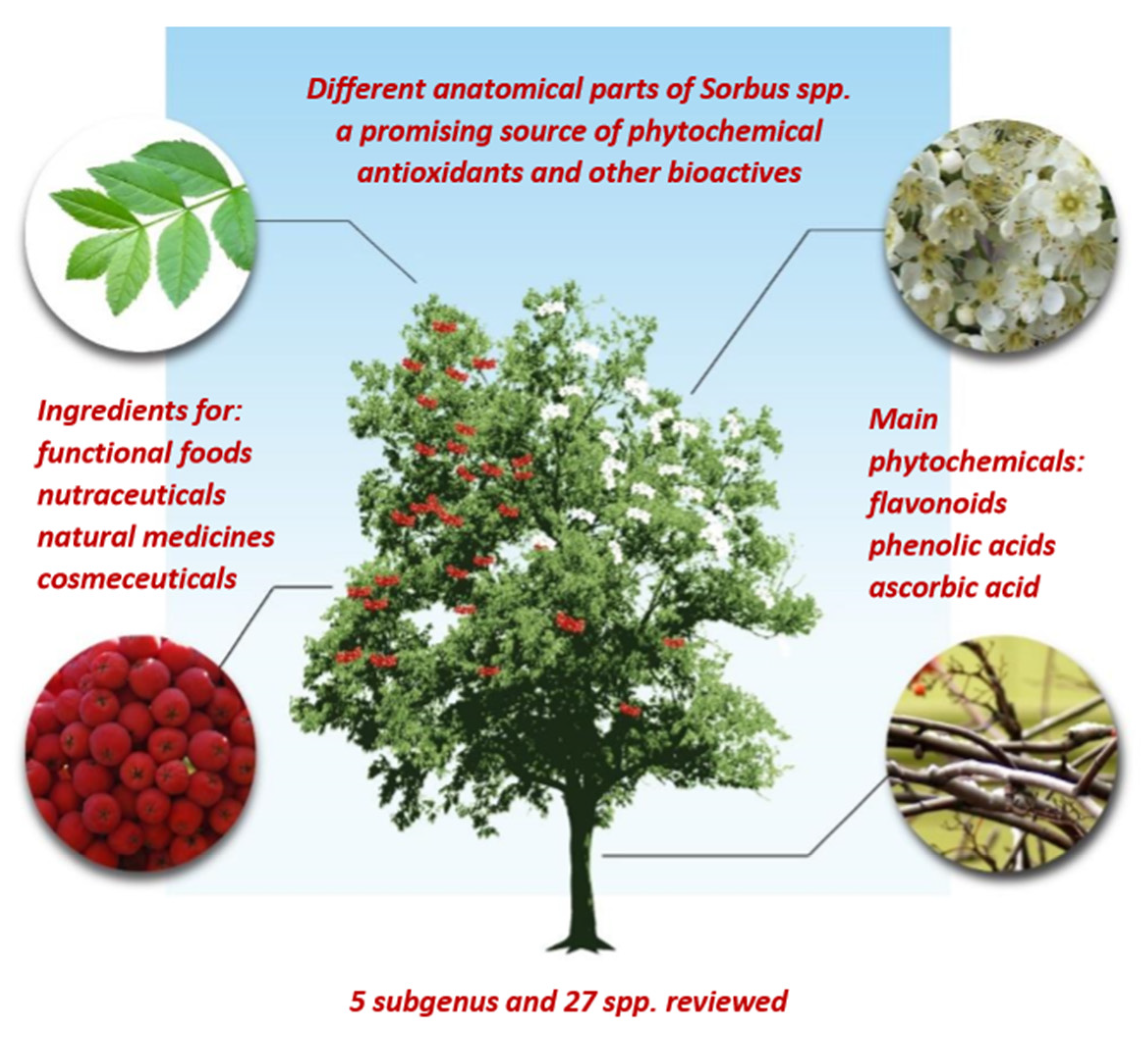
| Species and Varieties (Subgenus) | Food Uses of Fruits | Anatomical Part: Medicinal and Other Uses | Ref. |
|---|---|---|---|
| S. alnifolia (Sieb. & Zucc.) K. Koch. (Aria) | Twigs: treatment of neurological disorders as a traditional medicine in Korea | [26] | |
| S. americana Marshall— American mountain ash (Sorbus; informal group Commixtae) | Bark: treatment diabetes hypo-glycaemic, vaso-relaxant, antitussive and tonic agent | [36] | |
| S. aria L. Crantz—chess-apple (Aria) | Jellies, jams, brandy, liqueurs, conserves and vinegar, traditional bread flour extender | Fruit: diuretic, anti-inflammatory, anti-diarrhoeal, vasodilatory and vitamin agent; leaves: ethnomedical antidiarrheal ingredients; inflorescences and fruit: diuretic, laxative and emmenagogue; treatment of painful menstruation, constipation and kidney disorders | [11] [18] [37] |
| S. aucuparia L.—European mountain ash (Sorbus) | Alcohol beverages, jams, jellies, honey (floured dried fruit) | Traditional diuretic, anti-inflammatory, antidiarrheal (dried fruits), vasodilatory and an appetite- improving agent, source of vitamins, mild laxative | [18] [22] [7] |
| S. cashmiriana Hedl. (Sorbus series Multijugae) | Bark: tea made from its bark—to treat nausea, the bark preparation- to treat heart diseases; berries: to cure scurvy | [15] | |
| S. commixta Hedl. (Sorbus; informal group Commixtae) | Stembark: for treating asthma, bronchitis, gastritis and oedema, anti-inflammatory, -atherosclerotic, -alcoholic, and vascular-relaxant effects, anti-atherogenic, for treating arthritis, hypoglycaemic, antitussive and tonic agent | [38] [14] [39] [13] | |
| S. decora (Sarg.) C.K. Schneid—northern mountain ash (Sorbus; informal group Commixtae) | Leaves and bark- an antidiabetic medicine | [11] | |
| S. domestica L. (Cormus) | Food ingredients | Traditional diuretic, anti-inflammatory, antidiarrheal (dried fruits), vasodilatory, antidiabetic and vitamin agents | [18] [40] [41] |
| S. hybrida L.—oakleaf mountain ash (Aria sect. Aria × Sorbus) | An ornamental tree in northern Europe | [42] | |
| S. pohuashanensis (Hance) Hedl. (Sorbus) | Fruits, stems and bark: traditional Chinese medicine for the treatment of chronic tracheitis, tuberculosis and oedema | [16] | |
| S. sambucifolia (Cham. & Schlecht.) M. Roem.—Siberian mountain ash (Sorbus Lucidae Kom.) | Alcohol beverages, jams, jellies, honey (floured dried fruit) | In avitaminosis, arteriosclerosis, as antipyretic or diuretic agent. | [43] |
| S. scopulina Greene—Greene’s mountain ash (Sorbus; informal group Commixtae) | Sometimes used in pies, preserves, or wine-making | [17] | |
| Sorbus × thuringiaca (Ilse) Fritsch—mountain ash (Aria sect. Aria × Sorbus) | An ornamental tree | [44] | |
| S. tianschanica Rupr. (Sorbus series Tianshanicae Kom.) | Leaves: asthma, ventricular myocytes, dyspnoea, tuberculosis and gastritis | [10] | |
| S. torminalis (L.) Crantz var. torminalis (Torminaria) | Jams and ingredients for food and fodder | Traditional diuretic, anti-inflammatory, antidiarrheal (dried fruits), vasodilatory and vitamin agents | [18] [28] [40] |
| S. torminalis var. semitorminalis (Torminaria) | Traditional diuretic, anti-inflammatory, antidiarrheal (dried fruits), vasodilatory and vitamin agents | [18] |
| No. | Species, Tested Material and Its Isolation Method | Phenolic Acids: Total Amount (To) in GAE (%) or as Specified | Flavonoids/Proanthocyanidins in CyE (%) or as Specified | Ref. |
|---|---|---|---|---|
| 1. | S. americana; 5 mL ME 10 mL ME 20 mL ME of F | All in mg/g dwe: To 3.599; 5-CQA 0.662; 3-CQA 2.837; QG 0.101 To 4.432; 5-CQA 0.714; 3-CQA 3.599; QG 0.119 To 5.388; 5-CQA 0.905; 3-CQA 0.417; QG 0.066 | [55] | |
| S. americana; 70% ME of L | To 6.47; 5-CQA 0.04; 3-CQA 1.85 | Fl: QU 0.46; KA 0.04; PAC 3.66 | [53] | |
| 2. | S. aria; 70% ME of I, F & L | I: To 6.58; 5-CQA 1.18; 3-CQA 1.78 L: To 6.06; 5-CQA 0.99; 3-CQA 0.74 F: To 2.98; 5-CQA 0.32; 3-CQA 0.30 | Fl in I: QU 0.277; SX 0.050; KA 0.041; IS 0.284 Fl in L: QU 0.493; SX 0.014; KA 0.242; IS 0.095 Fl in F: QU 0.009; KA 0.002; IS 0.007 PAC: I 2.75; L 3.53; F 1.80 | [54] |
| S. aria; ME of I, F & L | Fl, mg/100 g; F: Ag 20.3; Gl 31.1; QU 9.4; KA 2.4; IS 8.5 I: Ag 687.2; Gl 1049.0; QU 291.6; SX 52.6; KA 43.7; IS−299.3 L: Ag 888.1; Gl 1371.9; IS 99.8; QU 518.9; SX 14.8; KA 254.6 | [18] | ||
| S. aria; EtE of F | In mg/g dw; To: 3.91−10.81; 5-CQA: 0.18−4.00; 3-CQA: 0.22−2.30 | Fl, µg/g dw; RU: 138.4–892.0; HY: 2.3–27.6; IQ: 10.9–108.6; QU: 2.1–35.2 PAC, mg/g dw: avr. 1.11 | [57] | |
| 3. | S. aucuparia; 70% ME of I | To 21.17; DEF 37.61; EtAF 54.34; BF 48.71; WR 9.05 | [43] | |
| S. aucuparia; AE of F | To 190 mg/100 g dw | Fl-To 68.1 mg/100 g dw | [49] | |
| S. aucuparia; 80% AE of F | To 0.2148; 5-CQA 0.0427; 3-CQA 0.0705 | PAC 0.0005 | [66] | |
| S. aucuparia; 70% AE of F | mg/g dw; wild F: 5-CQA 5.36; 3-CQA 8.59; other 1.84; HB 0.11. Cultivars: 5-CQA 2.23−7.31; 3-CQA 3.20−9.22; other 0.61−1.84; HB: 0.16−0.70 | mg/g dw; wild: flavonols 1.84; flavanols 0.97; PAC 0.12. Cultivars: Fl 0.94–1.88; flavonols 0.95–1.89; PAC 0.36–6.04 | [60] | |
| S. aucuparia; ME of F | Cultivars 4.35−8.19 | Fl, g/kg fm: wild 3.11 | [34] | |
| S. aucuparia; WE of F, ME of F & jam | µg/g dwe: WE-F: 3-CQA 5.69x103; FA 7.8 ME-F: 3-CQA 5.80x103; FA 9.59 Jam: PCA 12.5; 3-CQA 2.60x103; FA 11.4 | Fl, µg/ g dwe; WE-F: AF 10.7; KA-3-O-gl 9.0; QU-3-O-gl 49.3; HY 36.6; RU 82.3 ME-F: AF 11.9; KA-3-O-gl 8.56; QU-3-O-gl 55.8; HY 39.6; RU 80.4 Jam: AF 8.4; KA-3-O-gl 3.99; QU-3-O-gl 17.9; HY 9.68 | [28] | |
| S. aucuparia; 70% AE of cultivars | In mg/ 100 g fw: To 550–1014; 5-CQA 34–104; 3-CQA 29–160 | PAC 6–80 mg/100 g fw | [31] | |
| S. aucuparia; 70% ME of I, F & L | I: To 11.83; 5-CQA 1.37; 3-CQA 2.98 L: To 9.09; 5-CQA 1.15; 3-CQA 2.75 F: To 2.68; 5-CQA 0.29; 3-CQA 0.64 | I: (Fl) QU 1.054; SX 0.151; KA 0.071. PAC 5.01 L: (Fl) QU 0.835; KA 0.188. PAC 3.84 F: (Fl) QU 0.051; KA 0.006. PAC 1.07 | [54] | |
| S. aucuparia EtE of F | In mg/g dw: To 5.25–15.91; 5-CQ 0.67–7.03; 3-CQA 0.3510.01 | Fl, µg/g dw: RU 40.1–598.3; HY 2.4–559.9; IQ 6.1–252.8; QU 2.8–83.5. PAC (avr.) 0.92 mg/g dw | [57] | |
| S. aucuparia; ME of I, F & L | Fl, mg/100 g: (L) Ag 1078; Gl 1666; QU 881.1; KA 196.9. (F) Ag 60.2; Gl 92.9; QU 53.8; KA 6.4. (I) Ag 1344.1; Gl 2067.4; QU 1110.7; SX 0.1582; KA 75.2 | [18] | ||
| S. aucuparia; 70% ME of I & L | I: To 10.02; 5-CQA 0.74; 3-CQA 2.27 L: To 8.23; 5-CQA 0.51; 3-CQA 1.90 | Fl: (I) QU 1.048; SX 0.190; KA 0.084 (L) QU 0.903; KA 0.157. PAC: I 5.94; L 3.59 | [53] | |
| 4. | S. cashmiriana; 70% ME of L | L: To 5.78; 5-CQA 0.37; 3-CQA 1.25 | Fl: QU 0.532; KA 0.113. PAC 4.02 | [53] |
| 5. | S. tianschanica; 50% EtE of F& L | F, mg/g: 5-CQA 3.7; 3-CQA 2.6. L.: 5-CQA 6.0; 3-CQA 7.0 | Fl, mg/g: (F) RU 0.15; HY 0.08; IQ 0.32. (L) RU 1.5; HY 1.4; IQ 5.1 | [56] |
| S. tianschanica; WE of L | Fl, mg/g: RU 0.71; HY 1.18; HE 0.48 | [67] | ||
| 6. | S. commixta; 50% EtE of L & F | L, mg/g: To 35.74; 5-CQ-.1.10; 3-CQA-21.91. F: To-11.19; 5-CQA- 1.8; 3-CQA-7.5 | Fl, mg/g: L: HY-7.5; IQ-5.3. F: HY-1.20; IQ-0.65; RU-0.02 | [56] |
| S. commixta; hot-WE and 70% EtE of S | To in µg/mg: We 364.64; EtE 504.39 | To-Fl, µg/mg: WE 124.59; EtE 160.09 | [68] | |
| S. commixta; 70% EtE of C | To, µg/mg: Without enzyme 447.3; treated with: amylase 501.6; amyloglucosidase 461.2; glucosidase 510.7; glucanase 493.3; cellulase 449.6 | Fl, µg/mg: without enzyme 35.1; treated with: amylase 55.1; amyloglucosidase 41.4; glucosidase 51.3; glucanase 63.0; cellulase 36.8 | [69] | |
| S. commixta; 70% ME of I and fractions (f) | ME 21.17; DEf 37.61; EtAf 54.34; Buf 48.71; WR 9.05 | [37] | ||
| S. commixta; 70% ME of I & L | I: To 9.29; 5-CQ 0.76; 3-CQA 3.92 L: To 8.08; 5-CQ 0.05; 3-CQA 0.79 | Fl: (I) QU 0.422; KA 0.050; SX 0.045 (L) QU 0.470; KA 0.011. PAC: I 5.98; L 3.58 | [53] | |
| 7. | S. decora; 70% ME of I & L | I: To 11.67; 5-CQA 1.26; 3-CQA 3.85 L: To 8.10; 5-CQA 0.19; 3-CQA 2.10 | Fl: (I) QU 0.839; KA 0.059; SX 0.07. (L) QU 0.474; KA 0.035. PAC: I 6.40; L 4.03 | [53] |
| S. decora; 70% ME of I | ME 24.61; DEf 34.50; EtAf 55.16; Buf 53.75; WR 10.06 | [37] | ||
| 8. | S. domestica; ME of (1), (2), (3), (4), (5) | To, µg/mg: R: 13.6 (1) → 25.4 (2) → 20.5 (3) → 32.1 (4) → 30.2 (5); DCMF: 74.5 (1) → 27.0 (2) → 97.0 (3) → 66.5 (4); DEf: 245(1) →151(2) → 324 (3) → 148(4) → 143 (5); EtAf: 285 (1) → 137 (2) → 198 (3) → 64(4) → 341(5); Buf: 94.0 (1) → 16.1 (2) → 25.1 (3) → 12.5 (4) →140 (5); Wf: 14.8 (1) → 3.03 (2) → 11.3 (3) → 2.27 (4) → 34.4 (5); ME: 32.5 (1) → 10.3 (2) → 26.3 (3) → 5.58 (4) → 28.1 (5) | [58] | |
| S. domestica; ME of (1), (2), (3), (4), (5) | (1): To 14.72; CiA 10.55; BA 4.17. (2): To 18.85; CiA 9.91; BA 8.94. (3): To 18.18; CiA 14.24; BA 4.57. (4): To 19.28; CiA 12.19; BA 7.09. (5): To 4.86; CiA 2.55; BA 2.31 | Fl: (1) To 8.68; Ag 1.22; Gl 7.46. (2) To 3.08; Ag 0.36; Gl 2.72. (3) To 10.59; Ag 1.46; Gl 8.83. (4): To 2.45; Ag 0.46; Gl 1.99. (5) To 7.9; Ag 0.73; Gl 7.17 | [41] | |
| 9. | S. gracilis; 70% ME of I & L | I: To 11.06; 5-CQA 0.19; 3-CQA 3.31 L: To 10.72; 5-CQA 0.03; 3-CQA 0.93 | Fl: (I) QU 0.194; KA 0.012; SX 0.072 (L) QU 0.113; KA 0.008. PAC: I 6.54; L 6.56 | [53] |
| S. gracilis; 70% ME of I & L | I: ME 24.63; DEf-36.87; EtAf 54.09; Buf 57.09; WR 8.21. L: ME 30.62; DEf 34.90; EtAf 52.37; Buf 48.62; WR 11.45 | [37] | ||
| 10. | S. intermedia; ME of I, F & L | Fl, mg/100g: (I) Ag 1514.8; Gl 2320.7; QU 1053.4; SX 117.3; KA 29.3; IS 314.8. (L) Ag 424.1; Gl 652.6; QU 303.6; KA 52.0; IS 68.5 (F) Ag 44.4; Gl 68.2; QU 32.5; IS 9.5; KA 2.4 | [18] | |
| S. intermedia 70% ME of I, F & L | I: To 9.25; 5-CQA 0.68; 3-CQA 2.35 L: To 8.74; 5-CQA 0.65; 3-CQA 1.26 F: To 2.24; 5-CQA 0.27; 3-CQA 0.23 | Fl: (I) QU 0.277; SX 0.05; KA 0.041; IS 0.284. PAC 5.52 (L) QU 0.493; SX 0.014; KA 0.242; IS 0.095. PAC 5.45 (F) QU 0.009, KA 0.002; IS 0.007. PAC 0.82 | [54] | |
| 11. | S. koehneana; ME of I & L | I: To 11.67; 5-CQA 1.98; 3-CQA 2.05 L: To 9.87; 5 CQA-0.53; 3-CQA 1.97 | Fl: (I) QU 0.27; KA 0.02; SX 0.05. PAC 6.86 L: QU 0.25; KA 0.11. PAC 5.81 | [53] |
| S. koehneana; 70% ME of I & L | To: ME 26.38; DEf32.10; EtAf50.51; Buf 58.17; WR 10.51 | [37] | ||
| 12. | S. pohuashanensis; 70% ME of I & L | I: To 11.32; 5-CQA 0.7; 3-CQA 2.48 L: To 6.26; 5-CQA 0.12; 3-CQA 0.67 | Fl: I: QU-0.4; KA-0.04; SX-0.02. L: QU-0.12; KA-0.03. PAC: I-7.67; L-3.93 | [53] |
| 13. | S. pogonopetala; 70% ME of L | To 10.9; 5-CQA 0.22; 3-CQA 1.63 | Fl: QU 0.38; KA 0.26. PAC 5.89 | [53] |
| S. pogonopetala; 70% ME of L | To: ME 24.03; Def 42.85; EtAf 53.29. Buf 39.56; WR 10.38 | [37] | ||
| 14. | S. sambucifolia; 70% ME of I & L | I: To 8.2; 5-CQA 0.42; 3-CQA 4.17. L: To 5.07; 5-CQA 0.1; 3-CQA 1.02 | Fl: (I) QU 0.81; KA 0.06; SX 0.13. PAC 3.79 (L) QU 0.16; KA 0.01. PAC 1.96 | [53] |
| S. sambucifolia; EtE of F | To 0.733 | Fl: To 0.002 | [70] | |
| 15. | S. scalaris; 70% ME of I & L | I: To 8.47; 5-CQA 0.6; 3-CQA 2.36 L: To 4.23; 5-CQA 0.36; 3-CQA 1.24 | Fl: (I) QU 0.34; KA 0.06; SX 0.15. PAC 5.68 (L) QU 0.22; KA 0.13. PAC 1.47 | [53] |
| 16. | S. setschwanensis; 70% ME of L | To 10.18; 5-CQA 0.22; 3-CQA 2.61 | Fl: QU 0.57; KA 0.31. PAC 5.56 | [53] |
| 17. | S. sitchensis; 70% ME of I & L | I: To 10.08; 5-CQA 0.45; 3-CQA 3.13 L: To-4.89; 5-CQA- 0.05; 3-CQA-0.56 | Fl: (I) QU 0.38; KA 0.02; SX 0.05 L: QU-0.27; KA-0.02. PAC: I-7.14; L-1.48 | [53] |
| 18. | S. torminalis var. torminalis; WE of F, ME of F & jam | In µg/g dwe; WE-F: PCA 13.7; FA 27.8 ME-F: PCA 23.2; FA 62.6 Jam: PCA 5.92; FA 13.3 | Fl, µg/g dwe; WE-F: AF 15.8 ME-F: AF 19.3; QU-3-O-gl 13.6; HY 10.4 Jam: AF 16.8; QU-3-O-gl 2.53; HY 1.61 | [28] |
| S. torminalis var. semitorminalis; WE of F, ME of F & jam | WE-F: GA 5.69; FA 43.3; PCA 4.61 ME-F: FA 38.3; PCA 3.44 Jam: FA 18.4; PCA 2.11 | Fl, µg/g dwe; WE-F: AF 362; KA-3-O-gl 2.34; QU 6.53; QU-3-O-gl 3.33; Cat 10.6 ME-F: AF 974; KA-3-O-gl 2.43; QU 11; QU-3-O-gl 2.06 Jam: AF 195; QU 3.76; QU-3-O-gl 1.60 | [28] | |
| 19. | S. wilfordii; 70% ME of L | To 12.31; 5-CQA 0.13; 3-CQA 2.58 | Fl: QU-0.88; KA-0.05. PAC: 5.31 | [53] |
| S. wilfordii; 70% ME of L | ME 29.93; DEf 53.13; EtAf 54.34; Buf 48.37; WR 15.27 | [37] |
| No. | Species, Tested Material and Its Isolation Method | Antioxidant Activity EC50 (µg/mL) or as Specified | TEAA, mmol/g or LPO% | FRAP, mmol Fe2+/g or as Specified | Ref. |
|---|---|---|---|---|---|
| 1. | S. alnifolia; 75% EtE of L | DPPH• 30.6 | [39] | ||
| 2. | S. americana; 70% ME of L | DPPH• 38.76 | TEAA-0.34; LPO-54.29 | [53] | |
| S. americana; EtAE of F | DPPH• 113.9 | [12] | |||
| S. americana ME of B | DPPH• 15.8 | [36] | |||
| 3. | S. aria; 70% ME of I, L & F | DPPH•: I 42.05; L 50.17; F 95.31 | TEAA: I 0.41; L 0.344; F 0.18 | I 1.394; L 1.119; F 0.498 | [54] |
| S. aria EtE of F | DPPH•, mg/mL: 0.49−2.50 | [57] | |||
| 4. | S. aucuparia; 70% ME | DPPH•: ME 8.93; DEf 5.53; EtAf 3.37; Buf 3.52; WR 9.96 | TEAA: ME 1.72; DEf 2.14; EtAf 3.22; Buf 3.58; WR 0.94 | ME 4.43; DEf 9.30; EtAf 12.77; Buf 10.84; WR 2.58 | [37] |
| S. aucuparia; ME of F and cultivars | ME-F, DPPH•, g/kg fm: 6.73; % of inhibition: HO• 16.33; O2• 26.74; •NO 24.75. Cultivars: DPPH• 6.58−9.62; % of inhib.: HO• 16.12–24.73; O2• 27.19–34.02; •NO 25.03–31.39 | ME-F LPO, % of inhibition: 8.21 Cultivars: 7.93–13.12 | [34] | ||
| S. aucuparia; AE of F | DPPH•, mmol/kg dw: 357 | mmol Fe2+/ kg dw: 315.5 | [49] | ||
| S. aucuparia; WE of F, ME of F & jam | WE: DPPH• 70; •NO 1430; O2• 20.16 × 106; HO• 160 ME: DPPH• 80; •NO 430; O2• 20.5 × 106; HO• 240 Jam: DPPH• 130; •NO 2260; O2• 67.8 × 106; HO• 610 | LPO mg mL: WE-F - 6.40; ME-F - 7.38; jam - 4.08 | mg of AAE/g: WE-F: 10.6. ME-F: 11.2. Jam: 4.22 | [28] | |
| S. aucuparia (sweet cultivars); 70% AE | DPPH•, g/g: 21.3−9.7 | 0.061−0.105 | [31] | ||
| S. aucuparia; 70% ME of I, L, & F | DPPH•: I 18.05; L 27.47; F 163.63 | TEAA: I 0.956; L 0.628; F 0.106 | I 2.454; L 2.148; F 0.442 | [54] | |
| S. aucuparia; 70% ME of I & L | DPPH•: I 16.69; L 24.10 | TEAA: I 0.78; L 0.54 LPO: I 68.34; L 58.69 | [53] | ||
| S. aucuparia EtE of F | DPPH• 340−4260 | [57] | |||
| 5. | S. cashmiriana | In µmol/mL; DPPH• 7.6−12.5; H2O2 15.4−18.6; ABTS•+ 18.3−24.4 | µmol/mL:11.3−23.8 | [15] | |
| S. cashmiriana; 70% ME of L | DPPH• 48.59 | TEAA 0.27; LPO 53.59 | [53] | ||
| 6. | S. commixta; hot-WE of S | In % of inhibition: (50 µg/L) •OH 10.37; •NO 92.63 (pH1.2), 66.82 (pH3). (25 µg/L) •OH 10.08; •NO 65.36 (pH1.2); 41.06 (pH3). (12.5 µg/L) •OH 7.63; •NO 42.59(pH1.2), 26.78 (pH3). (10 µg/L): DPPH• 21.39; ABTS•+ 43.21. (5 µg/L) DPPH• 12.75; ABTS•+ 24.96. (1 µg/L) DPPH• 5.27; ABTS•+ 8.77 | In %: 50 µg/L 19.28; 25 µg/L 9.28 12.5 µg/L 6.83 | [68] | |
| S. commixta; 70% EtE of S | In % of inhibition: (50 µg/L) •OH 23.61; •NO 96.64 (pH1.2), 82.51 (pH3). (25 µg/L) •OH 22.15; •NO 91.97 (pH1.2), 80.02 (pH3). (12.5 µg/L) •OH 18.42; •NO 86.55 (pH1.2), 72.44 (pH3). (10 µg/L) DPPH• 26.36; ABTS•+ 59.64. (5 µg/L) DPPH• 15.96; ABTS•+ 37.01. (1 µg/L) DPPH• 6.93; ABTS•+ 12.14. | In %: 50 µg/L 13.06 25 µg/L 10.31; 12.5 µg/L 9.30 | [68] | ||
| S. commixta; 70% EtE of C | Without enzyme: O2• 14.2; DPPH• 18.0. Amylase: O2• 14.8; DPPH• 15.4. Amyloglucosidase: O2• 14.2, DPPH• 15.8. Glucosidase: O2• 13.8, DPPH• 15.7. Glucanase: O2• 13.6, DPPH• 15.2. Cellulase: O2• 14.6, DPPH• 18.2 | [69] | |||
| S. commixta; 70% ME, f and R | DPPH•: ME 7.16; DEf 5.72; EtAf 3.52; Buf 3.53; WR 9.66 | TEAA: ME 1.70; DEf 2.14; EtAf 2.62; Buf-3.40; WR 1.26 | ME 5.04; DEf 7.58; EtAf 12.23; Buf 11.01; WR 2.70 | [37] | |
| S. commixta; 70% ME of I & L | DPPH•: I 23.22; L 28.56 | TEAA: I 0.56, L 0.46 LPO: I 78.21, L 58.65 | [53] | ||
| 7. | S. decora; 70% ME, Fs and R of I | DPPH•: ME 7.76; DEf 5.57; EtAf 3.44; Buf 3.17; WR 9.84 | TEAA: ME 1.79; DEf 2.67; EtAf 3.98; Buf 3.55; WR 1.21 | ME 5.42; DEf 8.5; EtAf 13.74; Buf 11.47; WR 2.77 | [37] |
| S. decora; 70% ME of I & L | DPPH•: I 16.20; L 27.21 | TEAA: I 0.81; L 0.48 LPO: I 70.99; L 59.99 | [53] | ||
| 8. | S. domestica; ME of (1), (2), (3), (4), (5) | DPPH•: (R) 4829(1)→6290(2)→3720(3)→ 2730(4)→1810(5). DCMf: 3600(1)→ 9880(2)→3820(3)→6010(4). DEf: 997(1) →1740(2)→825(3)→3280(4)→ 2970(5). (EtAf) 1780(1)→1750(2)→1840(3)→3170 (4)→899(5). (Buf) 588(1)→8000(2)→3750 (3)→13200(4)→341(5). Wf: 4950(1)→ 39100(2)→5570(3)→39500(4)→2170(5). (ME) 2550(1)→10600(2)→1890(3)→ 20000(4)→1450(5) | [58] | ||
| 9. | S. gracilis; 70% ME of I & L | DPPH•: (I) ME 7.93; DEf 5.39; EtAf 3.71; Buf 3.25; WR 10.12. (L) ME 6.60; DEf 5.29; EtAf 3.70; Buf 3.83; WR 9.54 | TEAA: (I) ME 1.99; DEf 2.71; EtAf 3.65; Buf 3.68; WR 1.15. (L) ME 2.12; DEf 2.14; EtAf 3.72; Buf 3.33; WR 1.31 | I: ME 5.36; DEf 9.34; EtAf 13.06; Buf-9.92; WR 2.26. (L) ME 6.2; DEf 8.72; EtAf 12.94; Buf 11.05; WR 2.98 | [37] |
| S. gracilis; 70% ME of I & L | DPPH•: I 19.09; L 20.71 | TEAA: I 0.68; L 0.63 LPO: I 73.01; L 70.72 | [53] | ||
| 10. | S. intermedia; 70% ME of I, L & F | DPPH•: I 25.41; L 30.71; F 198.69 | TEAA: I 0.679; L 0.572; F 0.087 | I 2.123; L 1.512; F 0.298 | [54] |
| 11. | S. koehneana; 70% ME of I & L | DPPH•: I 16.20; L 24.74 | TEAA: I 0.81; L 0.53 LPO: I 73.34; L 54.15 | [53] | |
| S. koehneana; 70% ME of I | DPPH•: ME 6.74; DEf 5.70; EtAf 3.46; Buf 3.15; WR 9.71 | TEAA: ME 2.08; DEf 2.60; EtAf 3.56; Buf 3.94; WR 1.29 | ME 5.44; DEf 8.38; EtAf 12.87; Buf 9.81; WR 2.54 | [37] | |
| 12. | S. pohuashanensis; 70% ME of I & L | DPPH•: I 17.89; L 43.86 | TEAA: I 0.73; L 0.30 LPO: I 68.69; L 50.21 | [53] | |
| 13. | S. pogonopetala; 70% ME of L | DPPH•: ME 6.84; DEf 4.89; EtAf 3.8; Buf 5.18; WR 9.83 | TEAA: ME 1.81; DEf 2.28; EtAf 3.44; Buf 2.96; WR 1.03 | ME 5.54; DEf 10.92; EtAf 11.42; Buf 8.67; WR 2.92 | [37] |
| S. pogonopetala; 70% ME of L | DPPH• 19.87 | TEAA 0.66; LPO 74.73 | [53] | ||
| 14. | S. sambucifolia; 70% ME of I & L | DPPH•: I 28.03; L 52.63 | TEAA: I 0.47; L 0.25 LPO: I 58.12; L 54.03 | [53] | |
| 15. | S. scalaris; 70% ME of I & L | DPPH•: I 27.65; L 57.86 | TEAA: I 0.47; L 0.23 LPO: I 55.23; L 41.70 | [53] | |
| 16. | S. setschwanensis; 70% ME L | DPPH• 23.30 | TEAA 0.56; LPO 63.77 | [53] | |
| 17. | S. sitchensis; 70% ME of I & L | DPPH•: I 20.75; L 54.23 | TEAA: I 0.63; L 0.24 LPO: I 68.26; L 53.13 | [53] | |
| 18. | S. torminalis (L.) Crantz var. torminalis; WE of F, ME of F & jam | WE-F: DPPH• 1380; O2• 7.09×106; HO• 300. ME-F: DPPH• 570; O2• 12.2 × 106, •NO 2820; HO• 260. Jam: DPPH• 440; •NO 640; O2• 36.9 × 106; HO• 1110 | mg AAE/g: WE-F 1.11; ME-F 2.12; jam: 3.1 | [28] | |
| 19. | S. torminalis var. semitorminalis; WE of F, ME of F & jam | WE-F: DPPH• 1270; O2• 12.8 × 106; HO• 430. ME-F: DPPH• 420; •NO 3.12; O2• 12.5 × 106; HO• 270. Jam: DPPH• 180; •NO 2.45; O2• 50.3 × 106; HO• 290 | LPO, mg mL: jam 3.02 | mg AAE/g: WE-F 2.12; ME-F 3.81; jam 6.41 | [28] |
| 20. | S. wilfordii; 70% ME of L | DPPH•: ME 6.01; DEf 3.67; EtAf 3.45; Buf 3.28; WR 9.04 | TEAA: ME 2.24; DEf 2.97; EtAf 3.41; Buf 2.83; WR 1.51 | ME 6.78; DEf 11.60; EtAf 12.55; Buf 10.99; WR 4.03 | [37] |
| S. wilfordii; 70% ME of L | DPPH• 15.23 | TEAA: L-0.86. LPO-86.92 | [53] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarv, V.; Venskutonis, P.R.; Bhat, R. The Sorbus spp.—Underutilised Plants for Foods and Nutraceuticals: Review on Polyphenolic Phytochemicals and Antioxidant Potential. Antioxidants 2020, 9, 813. https://doi.org/10.3390/antiox9090813
Sarv V, Venskutonis PR, Bhat R. The Sorbus spp.—Underutilised Plants for Foods and Nutraceuticals: Review on Polyphenolic Phytochemicals and Antioxidant Potential. Antioxidants. 2020; 9(9):813. https://doi.org/10.3390/antiox9090813
Chicago/Turabian StyleSarv, Viive, Petras Rimantas Venskutonis, and Rajeev Bhat. 2020. "The Sorbus spp.—Underutilised Plants for Foods and Nutraceuticals: Review on Polyphenolic Phytochemicals and Antioxidant Potential" Antioxidants 9, no. 9: 813. https://doi.org/10.3390/antiox9090813
APA StyleSarv, V., Venskutonis, P. R., & Bhat, R. (2020). The Sorbus spp.—Underutilised Plants for Foods and Nutraceuticals: Review on Polyphenolic Phytochemicals and Antioxidant Potential. Antioxidants, 9(9), 813. https://doi.org/10.3390/antiox9090813








