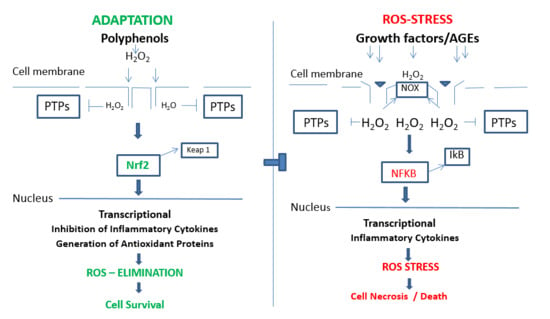
Polyphenols by Generating H2O2, Affect Cell Redox Signaling, Inhibit PTPs and Activate Nrf2 Axis for Adaptation and Cell Surviving: In Vitro, In Vivo and Human Health
Abstract
1. Introduction
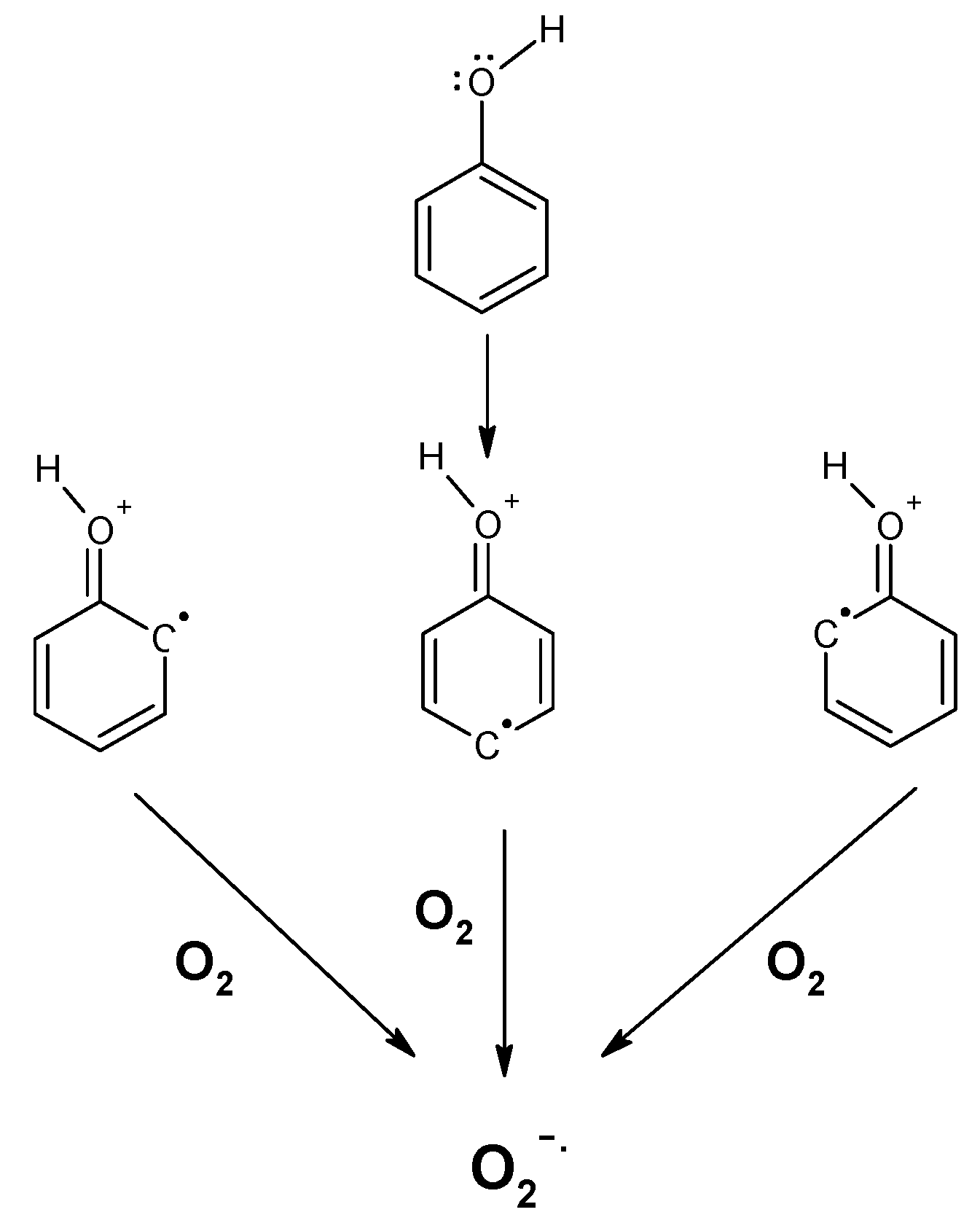
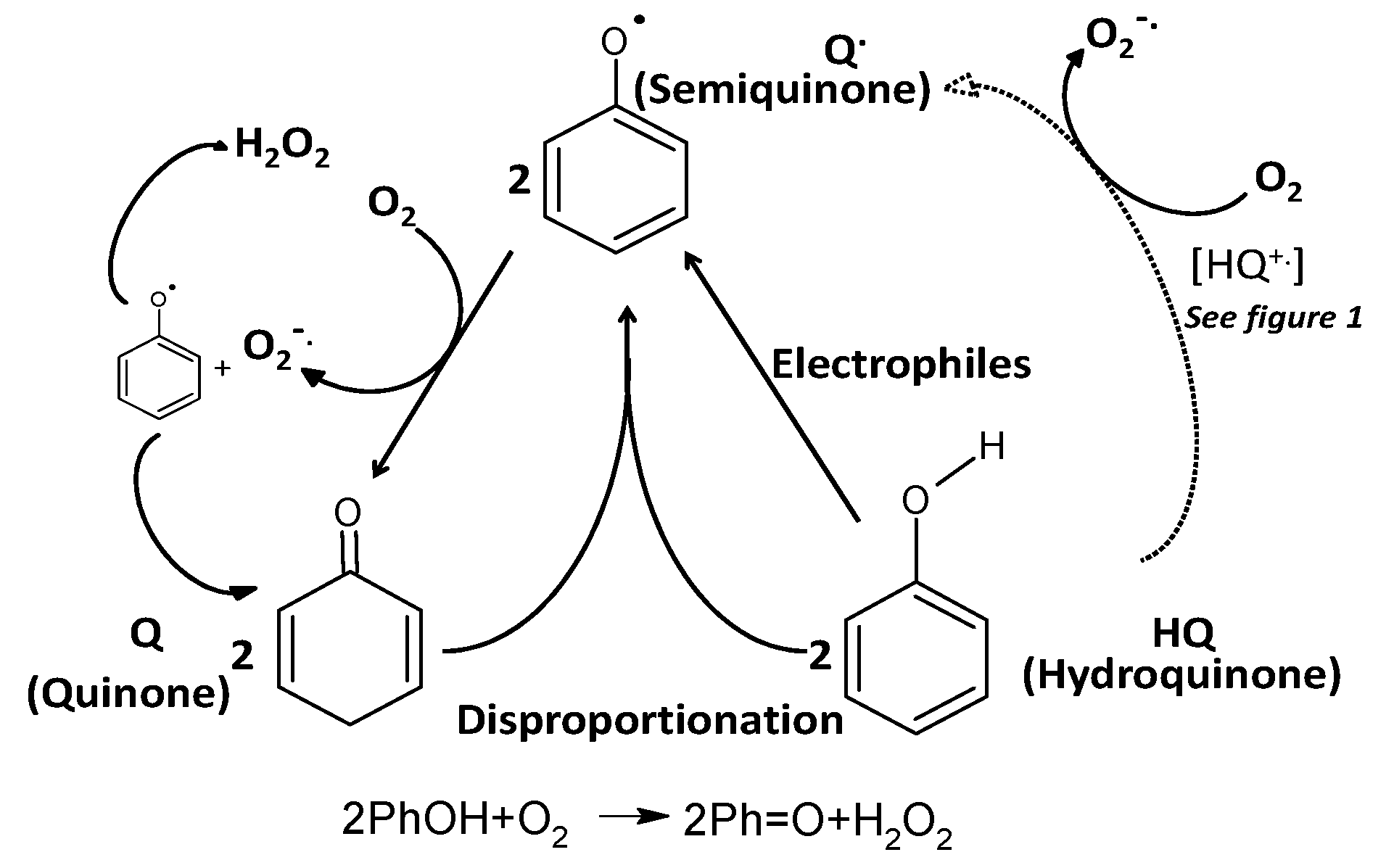
2. Polyphenols as Reducing Agents and Pro-Oxidants
3. Polyphenol Auto-Oxidation without the Involvment of Metal Ions
4. The Pro-Oxidant Action of Polyphenol in the Cardiovascular System and Organs
5. Cell Proliferation, Inhibition and Progression by Polyphenols
6. Adaptation, Protection and Cell Survival; In Vitro
7. Adaptation, Protection and Cell Survival; In Vivo
8. Polyphenols and Cardiovascular System, Ex Vivo
9. Several Other Effects of Polyphenols/H2O2 in Animal and Human Organisms
10. Polyphenols and Brain Function
11. Hormesis/Eustress and Distress by Polyphenols
12. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073s–2085s. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Jimenez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Cassidy, A.; Comte, B.; Heinonen, M.; Richelle, M.; Richling, E.; Serafini, M.; Scalbert, A.; Sies, H.; Vidry, S. The biological relevance of direct antioxidant effects of polyphenols for cardiovascular health in humans is not established. J. Nutr. 2011, 141, 989S–1009S. [Google Scholar] [CrossRef] [PubMed]
- Pourcel, L.; Routaboul, J.M.; Cheynier, V.; Lepiniec, L.; Debeaujon, I. Flavonoid oxidation in plants: From biochemical properties to physiological functions. Trends Plant Sci. 2007, 12, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Tresserra-Rimbau, A.; Medina-Remon, A.; Perez-Jimenez, J.; Martinez-Gonzalez, M.A.; Covas, M.I.; Corella, D.; Salas-Salvado, J.; Gomez-Gracia, E.; Lapetra, J.; Aros, F.; et al. Dietary intake and major food sources of polyphenols in a Spanish population at high cardiovascular risk: The PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Steffen, Y.; Gruber, C.; Schewe, T.; Sies, H. Mono-O-methylated flavanols and other flavonoids as inhibitors of endothelial NADPH oxidase. Arch. Biochem. Biophys. 2008, 469, 209–219. [Google Scholar] [CrossRef]
- Heumuller, S.; Wind, S.; Barbosa-Sicard, E.; Schmidt, H.H.H.W.; Busse, R.; Schroder, K.; Brandes, R.P. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 2008, 51, 211–217. [Google Scholar] [CrossRef]
- Mora-Pale, M.; Kwon, S.J.; Linhardt, R.J.; Dordick, J.S. Trimer hydroxylated quinone derived from apocynin targets cysteine residues of p47(phox) preventing the activation of human vascular NADPH oxidase. Free Radic. Biol. Med. 2012, 52, 962–969. [Google Scholar] [CrossRef][Green Version]
- Basheer, L.; Schultz, K.; Fichman, M.; Kerem, Z. Use of In Vitro and Predictive In Silico Models to Study the Inhibition of Cytochrome P4503A by Stilbenes. PLoS ONE 2015, 10, e0141061. [Google Scholar] [CrossRef]
- Vauzour, D.; Corsini, S.; Muller, M.; Spencer, J.P.E. Inhibition of PP2A by hesperetin may contribute to Akt and ERK1/2 activation status in cortical neurons. Arch. Biochem. Biophys. 2018, 650, 14–21. [Google Scholar] [CrossRef]
- Oak, M.H.; Auger, C.; Belcastro, E.; Park, S.H.; Lee, H.H.; Schini-Kerth, V.B. Potential mechanisms underlying cardiovascular protection by polyphenols: Role of the endothelium. Free Radic. Biol. Med. 2018, 122, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Elbling, L.; Herbacek, I.; Weiss, R.M.; Jantschitsch, C.; Micksche, M.; Gerner, C.; Pangratz, H.; Grusch, M.; Knasmuller, S.; Berger, W. Hydrogen peroxide mediates EGCG-induced antioxidant protection in human keratinocytes. Free Radic. Biol. Med. 2010, 49, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Erlank, H.; Elmann, A.; Kohen, R.; Kanner, J. Polyphenols activate Nrf2 in astrocytes via H2O2, semiquinones, and quinones. Free Radic. Biol. Med. 2011, 51, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef]
- Bertolotti, M.; Bestetti, S.; Garcia-Manteiga, J.M.; Medrano-Fernandez, I.; Dal Mas, A.; Malosio, M.L.; Sitia, R. Tyrosine Kinase Signal Modulation: A Matter of H2O2 Membrane Permeability? Antioxid. Redox Signal. 2013, 19, 1447–1451. [Google Scholar] [CrossRef]
- Kanner, J.; Gorelik, S.; Roman, S.; Kohen, R. Protection by polyphenols of postprandial human plasma and low-density lipoprotein modification: The stomach as a bioreactor. J. Agric. Food Chem. 2012, 60, 8790–8796. [Google Scholar] [CrossRef]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Asp. Med. 2018, 61, 41–49. [Google Scholar] [CrossRef]
- Galati, G.; O′Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef]
- Lapidot, T.; Walker, M.D.; Kanner, J. Can apple antioxidants inhibit tumor cell proliferation? Generation of H2O2 during interaction of phenolic compounds with cell culture media. J. Agric. Food Chem. 2002, 50, 3156–3160. [Google Scholar] [CrossRef]
- Zhang, K.; Dong, R.; Sun, K.; Wang, X.; Wang, J.; Yang, C.S.; Zhang, J. Synergistic toxicity of epigallocatechin-3-gallate and diethyldithiocarbamate, a lethal encounter involving redox-active copper. Free Radic. Biol. Med. 2017, 113, 143–156. [Google Scholar] [CrossRef]
- Wright, J.S.; Johnson, E.R.; DiLabio, G.A. Predicting the activity of phenolic antioxidants: Theoretical method, analysis of substituent effects, and application to major families of antioxidants. J. Am. Chem. Soc. 2001, 123, 1173–1183. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C. Antioxidant properties of phenols. J. Pharm. Pharmacol. 2007, 59, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Galleano, M.; Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Arch. Biochem. Biophys. 2010, 501, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.; Ingold, K.U.; Lusztyk, J. The Surprisingly High Reactivity of Phenoxyl Radicals. J. Am. Chem. Soc. 1994, 116, 9440–9447. [Google Scholar] [CrossRef]
- Kanner, J.; German, J.B.; Kinsella, J.E. Initiation of lipid peroxidation in biological systems. Crit. Rev. Food Sci. Nutr. 1987, 25, 317–364. [Google Scholar] [CrossRef]
- Kanner, J. Oxidative Processes in Meat and Meat-Products—Quality Implications. Meat Sci. 1994, 36, 169–189. [Google Scholar] [CrossRef]
- Satoh, T.; McKercher, S.R.; Lipton, S.A. Reprint of: Nrf2/ARE-mediated antioxidant actions of pro-electrophilic drugs. Free Radic. Biol. Med. 2014, 66, 45–57. [Google Scholar] [CrossRef]
- Rhodin, J.A.G. Ultrastructure of Mammalian Arterioles and Precapillary Sphincters. J. Ultra Mol. Struct. R 1967, 18, 181–223. [Google Scholar] [CrossRef]
- Lotito, S.; Zhang, W.; Yang, C.; Crozier, A.; Frei, B. Metabolic conversion of dietary flavonoids alters their anti-inflammatory and antioxidant properties. Free Radic. Biol. Med. 2011, 51, 454–463. [Google Scholar] [CrossRef]
- Long, L.H.; Halliwell, B. Coffee drinking increases levels of urinary hydrogen peroxide detected in healthy human volunteers. Free Radic. Res. 2000, 32, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, K.; Kida, T.; Kikugawa, K. Increased urinary hydrogen peroxide levels caused by coffee drinking. Biol. Pharm. Bull. 2002, 25, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Moller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Moller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific aquaporins facilitate the diffusion of hydrogen peroxide across membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.R.; Yang, S.P. Hydrogen peroxide: A signaling messenger. Antioxid. Redox Signal. 2006, 8, 243–270. [Google Scholar] [CrossRef]
- Rhee, S.G. H2O2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef]
- Koren, E.; Kohen, R.; Ginsburg, I. Polyphenols enhance total oxidant-scavenging capacities of human blood by binding to red blood cells. Exp. Biol. Med. 2010, 235, 689–699. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Lapidot, T.; Walker, M.D.; Kanner, J. Antioxidant and prooxidant effects of phenolics on pancreatic beta-cells in vitro. J. Agric. Food Chem. 2002, 50, 7220–7225. [Google Scholar] [CrossRef]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef]
- Hsu, S.; Bollag, W.B.; Lewis, J.; Huang, Q.; Singh, B.; Sharawy, M.; Yamamoto, T.; Schuster, G. Green tea polyphenols induce differentiation and proliferation in epidermal keratinocytes. J. Pharmacol. Exp. Ther. 2003, 306, 29–34. [Google Scholar] [CrossRef]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Araki, M.; Kusunoki, M.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol induces proliferation and cytoprotection against oxidative injury in vascular endothelial cells: Role of Nrf2 activation and HO-1 induction. J. Agric. Food Chem. 2011, 59, 4473–4482. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Artefacts with ascorbate and other redox-active compounds in cell culture: Epigenetic modifications, and cell killing due to hydrogen peroxide generation in cell culture media. Free Radic. Res. 2018, 52, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.W.; Yoon, C.H.; Park, K.M.; Han, H.S.; Park, Y.K. Hexane fraction of Zingiberis Rhizoma Crudus extract inhibits the production of nitric oxide and proinflammatory cytokines in LPS-stimulated BV2 microglial cells via the NF-kappaB pathway. Food Chem. Toxicol. 2009, 47, 1190–1197. [Google Scholar] [CrossRef]
- Podlogar, J.A.; Verspohl, E.J. Antiinflammatory Effects of Ginger and Some of its Components in Human Bronchial Epithelial (BEAS-2B) Cells. Phytother. Res. 2012, 26, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Woo, A.Y.H.; Cheng, C.H.K.; Waye, M.M.Y. Baicalein protects rat cardiomyocytes from hypoxia/reoxygenation damage via a prooxidant mechanism. Cardiovasc. Res. 2005, 65, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Pinkus, R.; Weiner, L.M.; Daniel, V. Role of oxidants and antioxidants in the induction of AP-1, NF-kappa B, and glutathione S-transferase gene expression. J. Biol. Chem. 1996, 271, 13422–13429. [Google Scholar] [CrossRef]
- Collins, Q.F.; Liu, H.Y.; Pi, J.; Liu, Z.; Quon, M.J.; Cao, W. Epigallocatechin-3-gallate (EGCG), a green tea polyphenol, suppresses hepatic gluconeogenesis through 5′-AMP-activated protein kinase. J. Biol. Chem. 2007, 282, 30143–30149. [Google Scholar] [CrossRef]
- Alhosin, M.; Anselm, E.; Rashid, S.; Kim, J.H.; Madeira, S.V.; Bronner, C.; Schini-Kerth, V.B. Redox-sensitive up-regulation of eNOS by purple grape juice in endothelial cells: Role of PI3-kinase/Akt, p38 MAPK, JNK, FoxO1 and FoxO3a. PLoS ONE 2013, 8, e57883. [Google Scholar] [CrossRef]
- Bartholome, A.; Kampkotter, A.; Tanner, S.; Sies, H.; Klotz, L.O. Epigallocatechin gallate-induced modulation of FoxO signaling in mammalian cells and C. elegans: FoxO stimulation is masked via PI3K/Akt activation by hydrogen peroxide formed in cell culture. Arch. Biochem. Biophys. 2010, 501, 58–64. [Google Scholar] [CrossRef]
- Yang, G.Y.; Liao, J.; Kim, K.; Yurkow, E.J.; Yang, C.S. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis 1998, 19, 611–616. [Google Scholar] [CrossRef]
- Bauman, B.M.; Jeong, C.; Savage, M.; Briker, A.L.; Janigian, N.G.; Nguyen, L.L.; Kemmerer, Z.A.; Eggler, A.L. Dr. Jekyll and Mr. Hyde: Oxidizable phenol-generated reactive oxygen species enhance sulforaphane’s antioxidant response element activation, even as they suppress Nrf2 protein accumulation. Free Radic. Biol. Med. 2018, 124, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Sirota, R.; Gibson, D.; Kohen, R. The role of the catecholic and the electrophilic moieties of caffeic acid in Nrf2/Keapl pathway activation in ovarian carcinoma cell lines. Redox Biol. 2015, 4, 48–59. [Google Scholar] [CrossRef]
- Kohen, R.; Kakunda, A.; Rubinstein, A. The role of cationized catalase and cationized glucose oxidase in mucosal oxidative damage induced in the rat jejunum. J. Biol. Chem. 1992, 267, 21349–21354. [Google Scholar] [PubMed]
- Nishimoto, T.; Matsumoto, A.; Kihara, T.; Akaike, A.; Sugimoto, H. Protective effect of H2O2 against subsequent H2O2-induced cytotoxicity involves activation of the PI3K-Akt signaling pathway. J. Pharmacol. Sci. 2011, 115, 169p. [Google Scholar]
- Angeloni, C.; Motori, E.; Fabbri, D.; Malaguti, M.; Leoncini, E.; Lorenzini, A.; Hrelia, S. H2O2 preconditioning modulates phase II enzymes through p38 MAPK and PI3K/Akt activation. Am. J. Physiol.-Heart Circ. Physiol. 2011, 300, H2196–H2205. [Google Scholar] [CrossRef]
- Mo, L.; Yang, C.T.; Gu, M.F.; Zheng, D.D.; Lin, L.; Wang, X.Y.; Lan, A.P.; Hu, F.; Feng, J.Q. PI3K/Akt signaling pathway-induced heme oxygenase-1 upregulation mediates the adaptive cytoprotection of hydrogen peroxide preconditioning against oxidative injury in PC12 cells. Int. J. Mol. Med. 2012, 30, 314–320. [Google Scholar] [CrossRef][Green Version]
- Plauth, A.; Geikowski, A.; Cichon, S.; Wowro, S.J.; Liedgens, L.; Rousseau, M.; Weidner, C.; Fuhr, L.; Kliem, M.; Jenkins, G.; et al. Hormetic shifting of redox environment by pro-oxidative resveratrol protects cells against stress. Free Radic. Biol. Med. 2016, 99, 608–622. [Google Scholar] [CrossRef]
- Gao, Z.H.; Huang, K.X.; Xu, H.B. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol. Res. 2001, 43, 173–178. [Google Scholar] [CrossRef]
- Yu, W.; Fu, Y.C.; Zhou, X.H.; Chen, C.J.; Wang, X.; Lin, R.B.; Wang, W. Effects of resveratrol on H(2)O(2)-induced apoptosis and expression of SIRTs in H9c2 cells. J. Cell. Biochem. 2009, 107, 741–747. [Google Scholar] [CrossRef]
- Quincozes-Santos, A.; Bobermin, L.D.; Latini, A.; Wajner, M.; Souza, D.O.; Goncalves, C.A.; Gottfried, C. Resveratrol protects C6 astrocyte cell line against hydrogen peroxide-induced oxidative stress through heme oxygenase 1. PLoS ONE 2013, 8, e64372. [Google Scholar] [CrossRef]
- Hwang, S.L.; Yen, G.C. Neuroprotective effects of the citrus flavanones against H2O2-induced cytotoxicity in PC12 cells. J. Agric. Food Chem. 2008, 56, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol reduces intracellular reactive oxygen species levels in vascular endothelial cells by upregulating catalase expression through the AMPK-FOXO3a pathway. Eur. J. Pharmacol. 2011, 660, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, F.; Echeverry, C.; Abin-Carriquiry, J.A.; Blasina, F.; Antunez, K.; Jones, D.P.; Go, Y.M.; Liang, Y.L.; Dajas, F. After cellular internalization, quercetin causes Nrf2 nuclear translocation, increases glutathione levels, and prevents neuronal death against an oxidative insult. Free Radic. Biol. Med. 2010, 49, 738–747. [Google Scholar] [CrossRef]
- Milbury, P.E.; Graf, B.; Curran-Celentano, J.M.; Blumberg, J.B. Bilberry (Vaccinium myrtillus) anthocyanins modulate heme oxygenase-1 and glutathione S-transferase-pi expression in ARPE-19 cells. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2343–2349. [Google Scholar] [CrossRef] [PubMed]
- Vuong, T.; Matar, C.; Ramassamy, C.; Haddad, P.S. Biotransformed blueberry juice protects neurons from hydrogen peroxide-induced oxidative stress and mitogen-activated protein kinase pathway alterations. Br. J. Nutr. 2010, 104, 656–663. [Google Scholar] [CrossRef][Green Version]
- Johnson, J.; Maher, P.; Hanneken, A. The flavonoid, eriodictyol, induces long-term protection in ARPE-19 cells through its effects on Nrf2 activation and phase 2 gene expression. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2398–2406. [Google Scholar] [CrossRef]
- Chen, C.J.; Yu, W.; Fu, Y.C.; Wang, X.; Li, J.L.; Wang, W. Resveratrol protects cardiomyocytes from hypoxia-induced apoptosis through the SIRT1-FoxO1 pathway. Biochem. Biophys. Res. Commun. 2009, 378, 389–393. [Google Scholar] [CrossRef]
- Schaffer, S.; Halliwell, B. Comment on Hydroxytyrosol Induces Proliferation and Cytoprotection against Oxidative Injury in Vascular Endothelial Cells: Role of Nrf2 Activation and HO-1 Induction. J. Agric. Food Chem. 2011, 59, 10770–10771. [Google Scholar] [CrossRef]
- Walter, A.; Etienne-Selloum, N.; Sarr, M.; Kane, M.O.; Beretz, A.; Schini-Kerth, V.B. Angiotensin II induces the vascular expression of VEGF and MMP-2 in vivo: Preventive effect of red wine polyphenols. J. Vascualr Res. 2008, 45, 386–394. [Google Scholar] [CrossRef]
- Yang, J.Y.; Li, Y.; Wang, F.; Wu, C.F. Hepatoprotective Effects of Apple Polyphenols on CCI(4)-Induced Acute Liver Damage in Mice. J. Agric. Food Chem. 2010, 58, 6525–6531. [Google Scholar] [CrossRef]
- Tipoe, G.L.; Leung, T.M.; Liong, E.C.; Lau, T.Y.H.; Fung, M.L.; Nanji, A.A. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCl4)-induced liver injury in mice. Toxicology 2010, 273, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.Q.; Li, Z.; Xie, W.R.; Liu, C.M.; Liu, S.S. Quercetin protects mouse liver against CCl4-induced inflammation by the TLR2/4 and MAPK/NF-kappa B pathway. Int. Immunopharmacol. 2015, 28, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Wang, Y.J.; Zhang, Q.Y.; Liu, B.; Wang, F.Y.; Li, J.J.; Zhu, R.Z. Hepatoprotective effects of baicalein against CCl4-induced acute liver injury in mice. World J. Gastroenterol. 2012, 18, 6605–6613. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.C.; Kang, S.M.; Ahn, G.; Kim, K.N.; Kang, N.; Samarakoon, K.W.; Oh, M.C.; Lee, J.S.; Jeon, Y.J. Protective effect of a marine polyphenol, dieckol against carbon tetrachloride-induced acute liver damage in mouse. Environ. Toxicol. Pharmacol. 2013, 35, 517–523. [Google Scholar] [CrossRef]
- Zou, J.F.; Qi, F.J.; Ye, L.P.; Yao, S.Y. Protective Role of Grape Seed Proanthocyanidins Against Ccl(4) Induced Acute Liver Injury in Mice. Med. Sci. Monit. 2016, 22, 880–889. [Google Scholar] [CrossRef]
- Xie, X.X.; Sun, S.C.; Zhong, W.T.; Soromou, L.W.; Zhou, X.; Wei, M.M.; Ren, Y.L.; Ding, Y. Zingerone attenuates lipopolysaccharide-induced acute lung injury in mice. Int. Immunopharmacol. 2014, 19, 103–109. [Google Scholar] [CrossRef]
- Ma, F.Y.; Liu, F.; Ding, L.; You, M.; Yue, H.M.; Zhou, Y.J.; Hou, Y.Y. Anti-inflammatory effects of curcumin are associated with down regulating microRNA-155 in LPS-treated macrophages and mice. Pharm. Biol. 2017, 55, 1263–1273. [Google Scholar] [CrossRef]
- Wu, S.S.; Yano, S.; Chen, J.H.; Hisanaga, A.; Sakao, K.; He, X.; He, J.H.; Hou, D.X. Polyphenols from Lonicera caerulea L. Berry Inhibit LPS-Induced Inflammation through Dual Modulation of Inflammatory and Antioxidant Mediators. J. Agric. Food Chem. 2017, 65, 5133–5141. [Google Scholar] [CrossRef]
- Ha, S.K.; Lee, P.; Park, J.A.; Oh, H.R.; Lee, S.Y.; Park, J.H.; Lee, E.H.; Ryu, J.H.; Lee, K.R.; Kim, S.Y. Apigenin inhibits the production of NO and PGE(2) in microglia and inhibits neuronal cell death in a middle cerebral artery occlusion-induced focal ischemia mice model. Neurochem. Int. 2008, 52, 878–886. [Google Scholar] [CrossRef]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Xu, Z.L.; Zhao, H.W.; Wang, X.; Pang, J.; Li, Q.; Yang, Y.; Ling, W.H. Anthocyanin supplementation improves anti-oxidative and anti-inflammatory capacity in a dose-response manner in subjects with dyslipidemia. Redox Biol. 2020, 32, 101474. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.; Mente, A.; Dehghan, M.; Rangarajan, S.; Zhang, X.; Swaminathan, S.; Dagenais, G.; Gupta, R.; Mohan, V.; Lear, S.; et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): A prospective cohort study. Lancet 2017, 390, 2037–2049. [Google Scholar] [CrossRef]
- Shimokawa, H. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pflug. Arch. Eur. J. Physiol. 2010, 459, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Ushio-Fukai, M.; Alexander, R.W.; Akers, M.; Yin, Q.; Fujio, Y.; Walsh, K.; Griendling, K.K. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 1999, 274, 22699–22704. [Google Scholar] [CrossRef]
- Covas, G.; Marinho, H.S.; Cyrne, L.; Antunes, F. Activation of Nrf2 by H2O2: De novo synthesis versus nuclear translocation. Methods Enzymol. 2013, 528, 157–171. [Google Scholar] [CrossRef]
- Sies, H. Role of metabolic H2O2 generation: Redox signaling and oxidative stress. J. Biol. Chem. 2014, 289, 8735–8741. [Google Scholar] [CrossRef]
- Fratantonio, D.; Speciale, A.; Canali, R.; Natarelli, L.; Ferrari, D.; Saija, A.; Virgili, F.; Cimino, F. Low nanomolar caffeic acid attenuates high glucose-induced endothelial dysfunction in primary human umbilical-vein endothelial cells by affecting NF-kappaB and Nrf2 pathways. BioFactors 2017, 43, 54–62. [Google Scholar] [CrossRef]
- Cao, G.H.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Denu, J.M.; Tanner, K.G. Specific and reversible inactivation of protein tyrosine phosphatases by hydrogen peroxide: Evidence for a sulfenic acid intermediate and implications for redox regulation. Biochemistry 1998, 37, 5633–5642. [Google Scholar] [CrossRef]
- Lee, K.; Esselman, W.J. Inhibition of PTPs by H(2)O(2) regulates the activation of distinct MAPK pathways. Free Radic. Biol. Med. 2002, 33, 1121–1132. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Truong, T.H.; Garcia, F.J.; Homann, A.; Gupta, V.; Leonard, S.E.; Carroll, K.S. Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 2011, 8, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of (-)-epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Martin, S.; Andriambeloson, E.; Takeda, K.; Andriantsitohaina, R. Red wine polyphenols increase calcium in bovine aortic endothelial cells: A basis to elucidate signalling pathways leading to nitric oxide production. Br. J. Pharmacol. 2002, 135, 1579–1587. [Google Scholar] [CrossRef]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Kane, M.O.; Anselm, E.; Rattmann, Y.D.; Auger, C.; Schini-Kerth, V.B. Role of gender and estrogen receptors in the rat aorta endothelium-dependent relaxation to red wine polyphenols. Vascul. Pharmacol. 2009, 51, 140–146. [Google Scholar] [CrossRef]
- Kane, M.O.; Etienne-Selloum, N.; Madeira, S.V.; Sarr, M.; Walter, A.; Dal-Ros, S.; Schott, C.; Chataigneau, T.; Schini-Kerth, V.B. Endothelium-derived contracting factors mediate the Ang II-induced endothelial dysfunction in the rat aorta: Preventive effect of red wine polyphenols. Pflug. Arch. Eur. J. Physiol. 2010, 459, 671–679. [Google Scholar] [CrossRef]
- Cui, X.; Liu, X.; Feng, H.; Zhao, S.; Gao, H. Grape seed proanthocyanidin extracts enhance endothelial nitric oxide synthase expression through 5′-AMP activated protein kinase/Surtuin 1-Krupple like factor 2 pathway and modulate blood pressure in ouabain induced hypertensive rats. Biol. Pharm. Bull. 2012, 35, 2192–2197. [Google Scholar] [CrossRef]
- Cassidy, A. Berry anthocyanin intake and cardiovascular health. Mol. Asp. Med. 2018, 61, 76–82. [Google Scholar] [CrossRef]
- Guo, X.; Yang, B.; Tan, J.; Jiang, J.; Li, D. Associations of dietary intakes of anthocyanins and berry fruits with risk of type 2 diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2016, 70, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Cremonini, E.; Fraga, C.G.; Oteiza, P.I. (-)-Epicatechin in the control of glucose homeostasis: Involvement of redox-regulated mechanisms. Free Radic. Biol. Med. 2018, 130, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Daveri, E.; Cremonini, E.; Mastaloudis, A.; Hester, S.N.; Wood, S.M.; Waterhouse, A.L.; Anderson, M.; Fraga, C.G.; Oteiza, P.I. Cyanidin and delphinidin modulate inflammation and altered redox signaling improving insulin resistance in high fat-fed mice. Redox Biol. 2018, 18, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Zhang, W.; Tian, H.; Li, R.; Huang, S.; Li, X.; Qi, G.; Liu, X. EGCG evokes Nrf2 nuclear translocation and dampens PTP1B expression to ameliorate metabolic misalignment under insulin resistance condition. Food Funct. 2018, 9, 1510–1523. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Qi, G.; Fan, R.; Qiao, Q.; Sun, Y.; Gao, Y.; Liu, X. EGCG ameliorates high-fat- and high-fructose-induced cognitive defects by regulating the IRS/AKT and ERK/CREB/BDNF signaling pathways in the CNS. FASEB J. 2017, 31, 4998–5011. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.; Qi, G.; Fan, R.; Ji, X.; Liu, Z.; Liu, X. EGCG ameliorates diet-induced metabolic syndrome associating with the circadian clock. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1575–1589. [Google Scholar] [CrossRef]
- Hoek-van den Hil, E.F.; van Schothorst, E.M.; van der Stelt, I.; Swarts, H.J.M.; van Vliet, M.; Amolo, T.; Vervoort, J.J.M.; Venema, D.; Hollman, P.C.H.; Rietjens, I.M.C.M.; et al. Direct comparison of metabolic health effects of the flavonoids quercetin, hesperetin, epicatechin, apigenin and anthocyanins in high-fat-diet-fed mice. Genes Nutr. 2015, 10, 469. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Tav, S.I.; Rothschild, D.; Eijer, M.T.M.; Levy, M.; Moresi, C.; Dohnalova, L.; Braverman, S.; Rozin, S.; Malitsky, S.; et al. Persistent microbiome alterations modulate the rate of post-dieting weight regain. Nature 2016, 540, 544–551. [Google Scholar] [CrossRef]
- Zou, T.D.; Chen, D.W.; Yang, Q.Y.; Wang, B.; Zhu, M.J.; Nathanielsz, P.W.; Du, M. Resveratrol supplementation of high-fat diet-fed pregnant mice promotes brown and beige adipocyte development and prevents obesity in male offspring. J. Physiol. 2017, 595, 1547–1562. [Google Scholar] [CrossRef]
- Goldwasser, J.; Cohen, P.Y.; Yang, E.; Balaguer, P.; Yarmush, M.L.; Nahmias, Y. Transcriptional Regulation of Human and Rat Hepatic Lipid Metabolism by the Grapefruit Flavonoid Naringenin: Role of PPAR alpha, PPAR gamma and LXR alpha. PLoS ONE 2010, 5, e12399. [Google Scholar] [CrossRef]
- Kudo, N.; Arai, Y.; Suhara, Y.; Ishii, T.; Nakayama, T.; Osakabe, N. A Single Oral Administration of Theaflavins Increases Energy Expenditure and the Expression of Metabolic Genes. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Yun, J.W. Chrysin induces brown fat-like phenotype and enhances lipid metabolism in 3T3-L1 adipocytes. Nutrition 2016, 32, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Kamble, P.; Litvinov, D.; Narasimhulu, C.A.; Jiang, X.T.; Parthasarathy, S. Aspirin may influence cellular energy status. Eur. J. Pharmacol. 2015, 749, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Kamble, P.; Selvarajan, K.; Aluganti Narasimhulu, C.; Nandave, M.; Parthasarathy, S. Aspirin may promote mitochondrial biogenesis via the production of hydrogen peroxide and the induction of Sirtuin1/PGC-1alpha genes. Eur. J. Pharmacol. 2013, 699, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Fratantonio, D.; Cimino, F.; Molonia, M.S.; Ferrari, D.; Saija, A.; Virgili, F.; Speciale, A. Cyanidin-3-O-glucoside ameliorates palmitate-induced insulin resistance by modulating IRS-1 phosphorylation and release of endothelial derived vasoactive factors. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 351–357. [Google Scholar] [CrossRef]
- Fratantonio, D.; Speciale, A.; Ferrari, D.; Cristani, M.; Saija, A.; Cimino, F. Palmitate-induced endothelial dysfunction is attenuated by cyanidin-3-O-glucoside through modulation of Nrf2/Bach1 and NF-kappaB pathways. Toxicol. Lett. 2015, 239, 152–160. [Google Scholar] [CrossRef]
- Cremonini, E.; Wang, Z.; Bettaieb, A.; Adamo, A.M.; Daveri, E.; Mills, D.A.; Kalanetra, K.M.; Haj, F.G.; Karakas, S.; Oteiza, P.I. (-)-Epicatechin protects the intestinal barrier from high fat diet-induced permeabilization: Implications for steatosis and insulin resistance. Redox Biol. 2018, 14, 588–599. [Google Scholar] [CrossRef]
- Rhee, S.G.; Bae, Y.S.; Lee, S.R.; Kwon, J. Hydrogen peroxide: A key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. STKE 2000, 2000, pe1. [Google Scholar] [CrossRef]
- Prince, P.D.; Rodriguez Lanzi, C.; Fraga, C.G.; Galleano, M. Dietary (-)-epicatechin affects NF-kappaB activation and NADPH oxidases in the kidney cortex of high-fructose-fed rats. Food Funct. 2019, 10, 26–32. [Google Scholar] [CrossRef]
- Rendeiro, C.; Rhodes, J.S.; Spencer, J.P. The mechanisms of action of flavonoids in the brain: Direct versus indirect effects. Neurochem. Int. 2015, 89, 126–139. [Google Scholar] [CrossRef]
- Hong, Y.; An, Z. Hesperidin attenuates learning and memory deficits in APP/PS1 mice through activation of Akt/Nrf2 signaling and inhibition of RAGE/NF-kappaB signaling. Arch. Pharm. Res. 2018, 41, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Nouhi, F.; Tusi, S.K.; Abdi, A.; Khodagholi, F. Dietary supplementation with tBHQ, an Nrf2 stabilizer molecule, confers neuroprotection against apoptosis in amyloid beta-injected rat. Neurochem. Res. 2011, 36, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.L.; Yu, S.; Pan, Z.; Ma, J.; Wu, T.Y.; Kong, A.N. tBHQ-induced HO-1 expression is mediated by calcium through regulation of Nrf2 binding to enhancer and polymerase II to promoter region of HO-1. Chem. Res. Toxicol. 2011, 24, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, L.; Wang, F.; Shi, Y.; Ren, Y.; Liu, Q.; Cao, Y.; Duan, H. Attenuation of glomerular injury in diabetic mice with tert-butylhydroquinone through nuclear factor erythroid 2-related factor 2-dependent antioxidant gene activation. Am. J. Nephrol. 2011, 33, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.Y.; Li, P.; Murphy, T.H. A small-molecule-inducible Nrf2-mediated antioxidant response provides effective prophylaxis against cerebral ischemia in vivo. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 10321–10335. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Wang, H.; Fang, J.; Zhu, Y.; Zhou, J.; Wang, X.; Zhou, Y.; Zhou, M. Curcumin provides neuroprotection in model of traumatic brain injury via the Nrf2-ARE signaling pathway. Brain Res. Bull. 2018, 140, 65–71. [Google Scholar] [CrossRef]
- Huang, L.; Chen, C.; Zhang, X.; Li, X.; Chen, Z.; Yang, C.; Liang, X.; Zhu, G.; Xu, Z. Neuroprotective Effect of Curcumin Against Cerebral Ischemia-Reperfusion Via Mediating Autophagy and Inflammation. J. Mol. Neurosci. 2018, 64, 129–139. [Google Scholar] [CrossRef]
- Allen, E.N.; Potdar, S.; Tapias, V.; Parmar, M.; Mizuno, C.S.; Rimando, A.; Cavanaugh, J.E. Resveratrol and pinostilbene confer neuroprotection against aging-related deficits through an ERK1/2-dependent mechanism. J. Nutr. Biochem. 2018, 54, 77–86. [Google Scholar] [CrossRef]
- Hervera, A.; De Virgiliis, F.; Palmisano, I.; Zhou, L.; Tantardini, E.; Kong, G.; Hutson, T.; Danzi, M.C.; Perry, R.B.; Santos, C.X.C.; et al. Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol. 2018, 20, 307–319. [Google Scholar] [CrossRef]
- Krishnamoorthy, L.; Chang, C.J. Exosomal NADPH Oxidase: Delivering Redox Signaling for Healing. Biochemistry 2018, 57, 3993–3994. [Google Scholar] [CrossRef]
- Kanner, J.; Lapidot, T. The stomach as a bioreactor: Dietary lipid peroxidation in the gastric fluid and the effects of plant-derived antioxidants. Free Radic. Biol. Med. 2001, 31, 1388–1395. [Google Scholar] [CrossRef]
- Kanner, J.; Selhub, J.; Shpaizer, A.; Rabkin, B.; Shacham, I.; Tirosh, O. Redox homeostasis in stomach medium by foods: The Postprandial Oxidative Stress Index (POSI) for balancing nutrition and human health. Redox Biol. 2017, 12, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Nieva-Echevarria, B.; Goicoechea, E.; Guillen, M.D. Food lipid oxidation under gastrointestinal digestion conditions: A review. Crit. Rev. Food Sci. Nutr. 2018, 60, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Frei, B.; Yamamoto, Y.; Niclas, D.; Ames, B.N. Evaluation of an Isoluminol Chemi-Luminescence Assay for the Detection of Hydroperoxides in Human-Blood Plasma. Anal. Biochem. 1988, 175, 120–130. [Google Scholar] [CrossRef]
- Tong, S.J.; Li, Z.Z.; Qiu, B.L.; Zhao, Y.N.; Zhang, Z.H. Biphasic nickel phosphide nanosheets: Self-supported electrocatalyst for sensitive and selective electrochemical H2O2 detection and its practical applications in blood and living cells. Sens. Actuators B-Chem. 2018, 258, 789–795. [Google Scholar] [CrossRef]
- Ju, J.; Chen, W. In Situ Growth of Surfactant-Free Gold Nanoparticles on Nitrogen-Doped Graphene Quantum Dots for Electrochemical Detection of Hydrogen Peroxide in Biological Environments. Anal. Chem. 2015, 87, 1903–1910. [Google Scholar] [CrossRef]
- Forman, H.J.; Bernardo, A.; Davies, K.J.A. What is the concentration of hydrogen peroxide in blood and plasma? Arch. Biochem. Biophys. 2016, 603, 48–53. [Google Scholar] [CrossRef]
- Surh, Y.J.; Na, H.K. NF-kappaB and Nrf2 as prime molecular targets for chemoprevention and cytoprotection with anti-inflammatory and antioxidant phytochemicals. Genes Nutr. 2008, 2, 313–317. [Google Scholar] [CrossRef]
- Kweon, M.H.; Adhami, V.M.; Lee, J.S.; Mukhtar, H. Constitutive overexpression of Nrf2-dependent heme oxygenase-1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3-gallate. J. Biol. Chem. 2006, 281, 33761–33772. [Google Scholar] [CrossRef]
- Nakamura, Y.; Kumagai, T.; Yoshida, C.; Naito, Y.; Miyamoto, M.; Ohigashi, H.; Osawa, T.; Uchida, K. Pivotal role of electrophilicity in glutathione S-transferase induction by tert-butylhydroquinone. Biochemistry 2003, 42, 4300–4309. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Tsatsakis, A.; Agathokleous, E.; Giordano, J.; Calabrese, V. Does Green Tea Induce Hormesis? Dose-Response Publ. Int. Hormesis Soc. 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, O.; Shpaizer, A.; Kanner, J. Lipid Peroxidation in a Stomach Medium Is Affected by Dietary Oils (Olive/Fish) and Antioxidants: The Mediterranean versus Western Diet. J. Agric. Food Chem. 2015, 63, 7016–7023. [Google Scholar] [CrossRef] [PubMed]
- Kanner, J. Dietary advanced lipid oxidation endproducts are risk factors to human health. Mol. Nutr. Food Res. 2007, 51, 1094–1101. [Google Scholar] [CrossRef]


| Preconditioning with | Stress Compound | Model | Molecular Target | References |
|---|---|---|---|---|
| Plant/Compound | ||||
| H2O2 (10 µM) | H2O2 (6 mM) | COS cells | ↑PI3K/Akt | [54] |
| H2O2 (100 µM, 10 min) | H2O2 (100 µM, 30 min) | Cardiomyocyte | ↑PI3K/Akt/Nrf2 | [55] |
| H2O2 (100 µM, 1.5 h) | H2O2 (100 µM, 30 h) | PC12 cells | ↑PI3K/Akt/HO-1 | [56] |
| Resveratrol (20 µM) | H2O2 (50 µM) | H9c2 cells | ↑SirT1/SirT7, ↓caspase3 | [59] |
| Resveratrol (100 µM) | H2O2 (1 mM) | C6 astrocytes | ↑GSH/SOD/HO1, ↓ROS/iNOS | [60] |
| Resveratrol (100 µM) | Ethanol (0.78%) | Keratinocytes | ↑GSH, ↓ROS | [57] |
| Baicalein (10 µM) | H2O2 (1 mM) | SH-SYSY cells | ↑Nrf2/NQO1/SirT1,↓ necrosis | [58] |
| Hesperedin (1–50 µM) | H2O2 (0.4 mM) | PC12 cells | ↑GSH-Px/cat,↓ LDH | [61] |
| Hydroxytyrosol (50 µM) | H2O2 (250 µM) | Endothelial cells | ↑cat/AMPK/FOXO3,↓ ROS | [62] |
| Quercetin (25 µM) | H2O2 (60 µM) | Neurons cells | ↑GSH/Nrf2/survival | [63] |
| Berry anthocy. (1 mg/mL) | H2O2 (500 µM) | ARPE-19 | ↑GSH-transferase/HO-1 | [64] |
| Berry juice (10 µM gallate.eq) | H2O2 (750 µM) | N2a cells | ↑GSH/SOD/MAPK/p-38 | [65] |
| Preconditioning with | Stress Compound | Model | Molecular Target | References |
|---|---|---|---|---|
| Plant/Compound | ||||
| Quercetin (40–80 mg/kg/d) | CCl4 | Mice/liver | ↓TLR2/MAPK/NFkB/ROS/MDA | [72] |
| Baicalein (80 mg/kg/×2/d) | CCl4 | Mice/liver | ↑TGF/EGF, ↓TNFα/IL6/ALT | [73] |
| Diecol (25 mg/kg/6/d) | CCl4 | Mice/liver | ↑SOD/CAT/GSH-Px, ↓MDA | [74] |
| Grape seed PP (150 mg/kg/d) | CCl4 | Mice/liver | ↑SOD/GSH-Px-Tx, ↓ALT/TNFα/IL6/MDA | [75] |
| Apple PP (200–800 mg/kg/d) | CCl4 | Mice/liver | ↑SOD/GSH, ↓ALT/MDA | [70] |
| Zingerone (40 mg/kg/d) | LPS | Mice/lung | ↓TNFα/IL6/NFkB/MAPK | [76] |
| Curcumin (20 mg/kg/d) | LPS | Mice/liver | ↓AST/TNFα/IL6/miRNA-155/PI3K/Akt | [77] |
| Berry PP (300 mg/kg/d) | LPS | Mice | ↓Paw edema/TNFα/IL6/iNOS/NFkB, ↑Nrf2 | [78] |
| Apigenin (20 mg/kg/d) | Arterial occlusion | Mice/brain | ↓infarct area/microgalia | [79] |
| Anthocyanin (320 mg/d) | Dyslipidemia | Human | ↓IL6/TNFα/MDA/8-iso-PGF2α/8-OHdG | [80] |
| Red Wine PP (150 mg/d) | Angiotensin II | Rat/endothelial | ↓VEGF/MMP2/eNOS/ROS | [69] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanner, J. Polyphenols by Generating H2O2, Affect Cell Redox Signaling, Inhibit PTPs and Activate Nrf2 Axis for Adaptation and Cell Surviving: In Vitro, In Vivo and Human Health. Antioxidants 2020, 9, 797. https://doi.org/10.3390/antiox9090797
Kanner J. Polyphenols by Generating H2O2, Affect Cell Redox Signaling, Inhibit PTPs and Activate Nrf2 Axis for Adaptation and Cell Surviving: In Vitro, In Vivo and Human Health. Antioxidants. 2020; 9(9):797. https://doi.org/10.3390/antiox9090797
Chicago/Turabian StyleKanner, Joseph. 2020. "Polyphenols by Generating H2O2, Affect Cell Redox Signaling, Inhibit PTPs and Activate Nrf2 Axis for Adaptation and Cell Surviving: In Vitro, In Vivo and Human Health" Antioxidants 9, no. 9: 797. https://doi.org/10.3390/antiox9090797
APA StyleKanner, J. (2020). Polyphenols by Generating H2O2, Affect Cell Redox Signaling, Inhibit PTPs and Activate Nrf2 Axis for Adaptation and Cell Surviving: In Vitro, In Vivo and Human Health. Antioxidants, 9(9), 797. https://doi.org/10.3390/antiox9090797