Placental Adaptive Changes to Protect Function and Decrease Oxidative Damage in Metabolically Healthy Maternal Obesity
Abstract
:1. Introduction
2. Material and Methods
2.1. Products and Antibodies
2.2. Study Subjects
2.3. Tissue Collection and Placental Samples
2.4. Protein Extract Preparation
2.5. Measurement of Total Protein Expression by SDS-Western Blotting Assay
2.6. Determination of Oxidative Status Markers
2.6.1. Lipid Peroxidation (LPO)
2.6.2. Carbonyl Groups (C=O)
2.7. Determination of Antioxidant Defense Markers
2.7.1. Native SDS-PAGE SOD Activity
2.7.2. Catalase Activity
2.7.3. Total Antioxidant Capacity
2.8. Nitrosative Stress Markers
2.8.1. S-Nitrosylated Protein Detection by Biotin Switch Assay
2.8.2. Detection and Quantification of Nitrated Proteins and iNOS by SDS-PAGE Blotting
2.9. Data Analysis
3. Results
3.1. Clinical and Anthropometric Characteristics
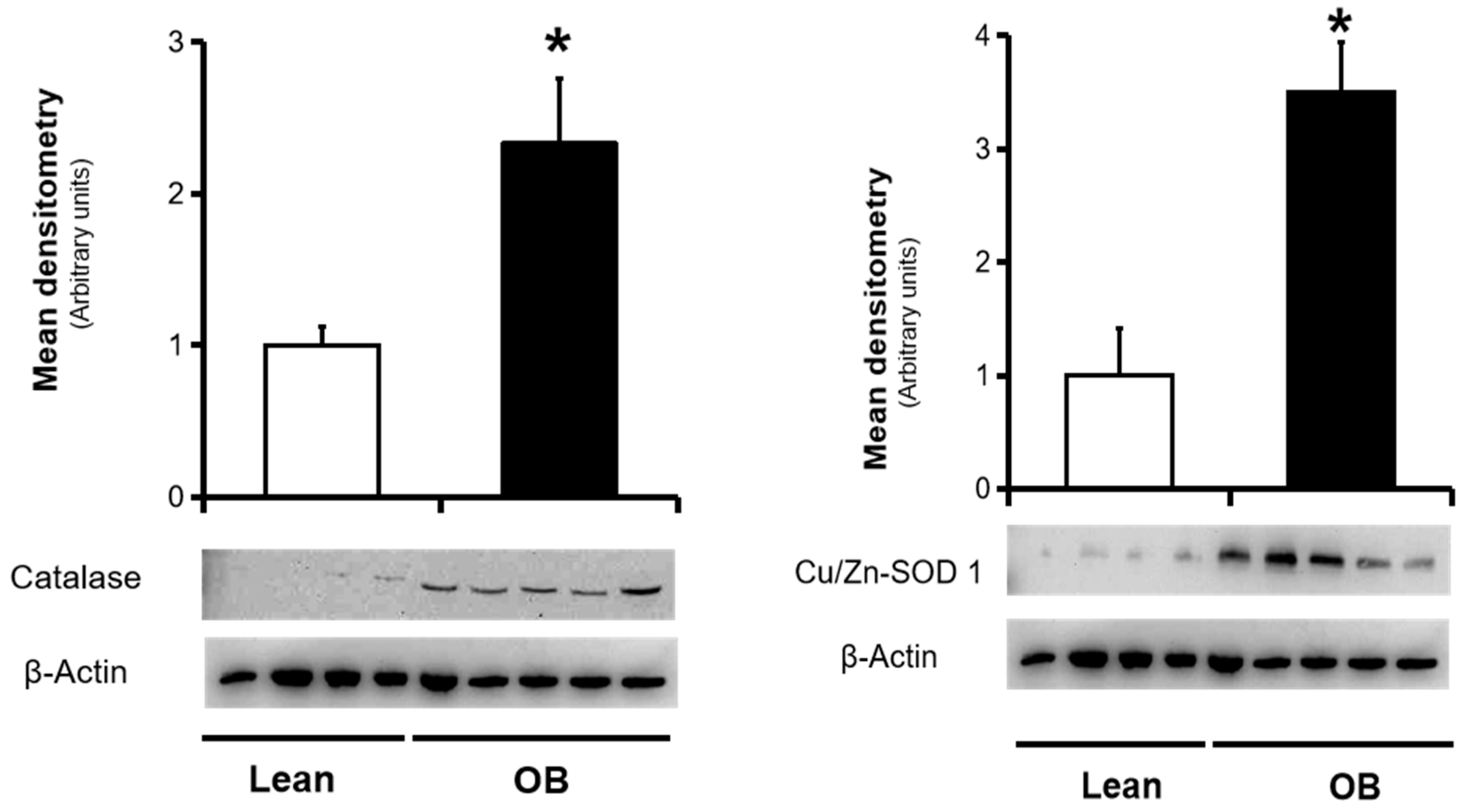
3.2. Assesesment of Placental Antioxidant Defense Markers
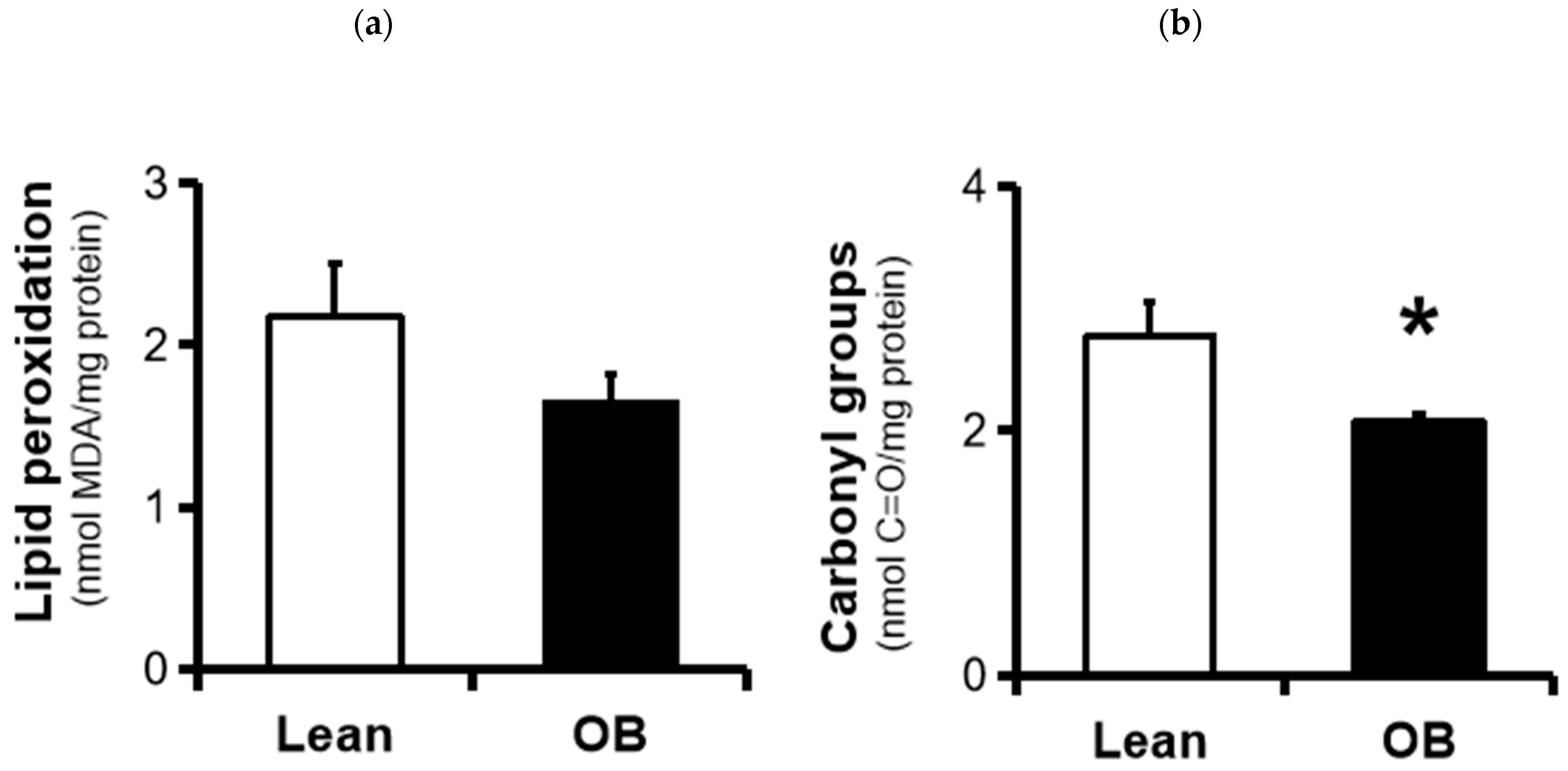
3.3. Assesesment of Placental Oxidative Damage Markers
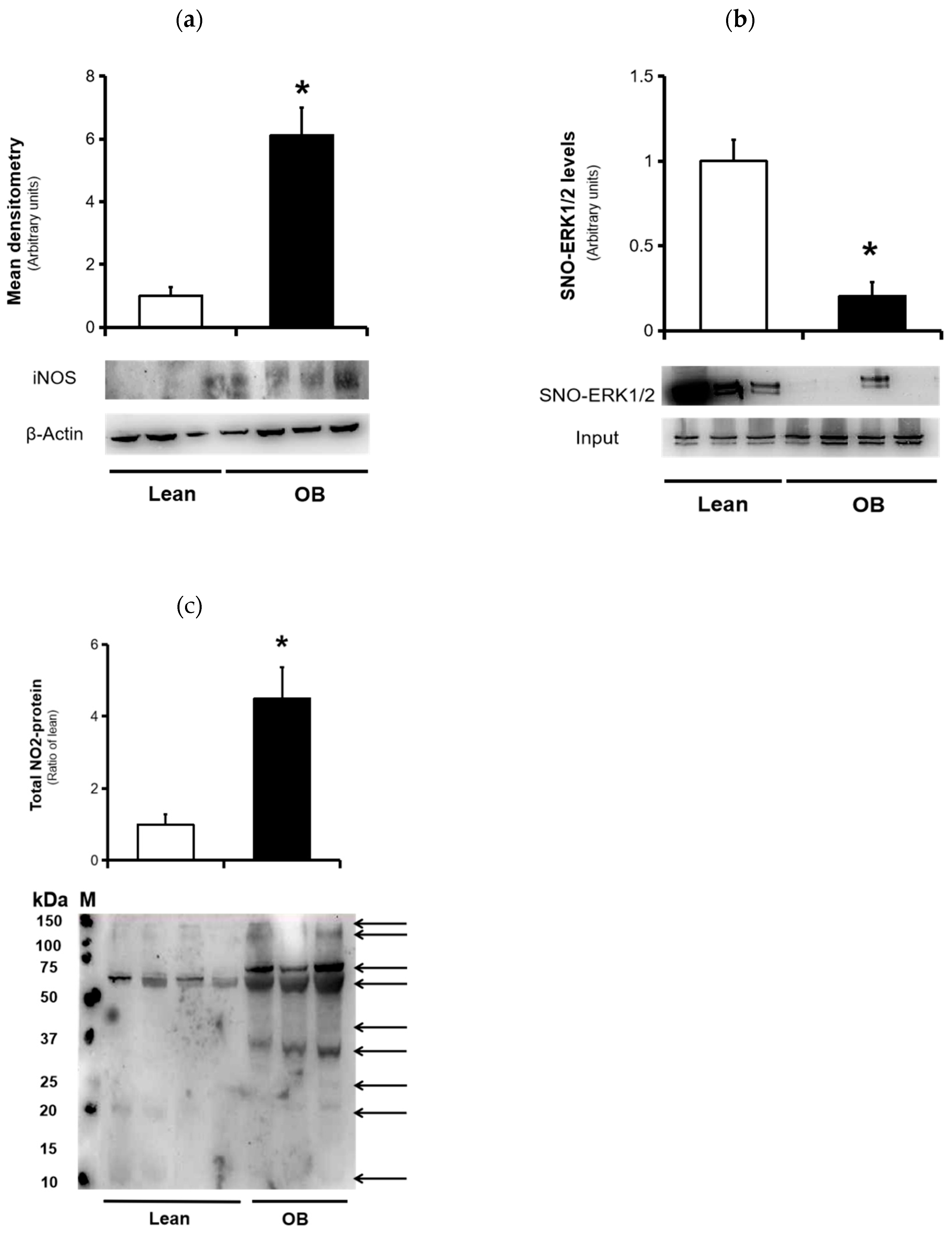
3.4. Nitrosative Stress Markers
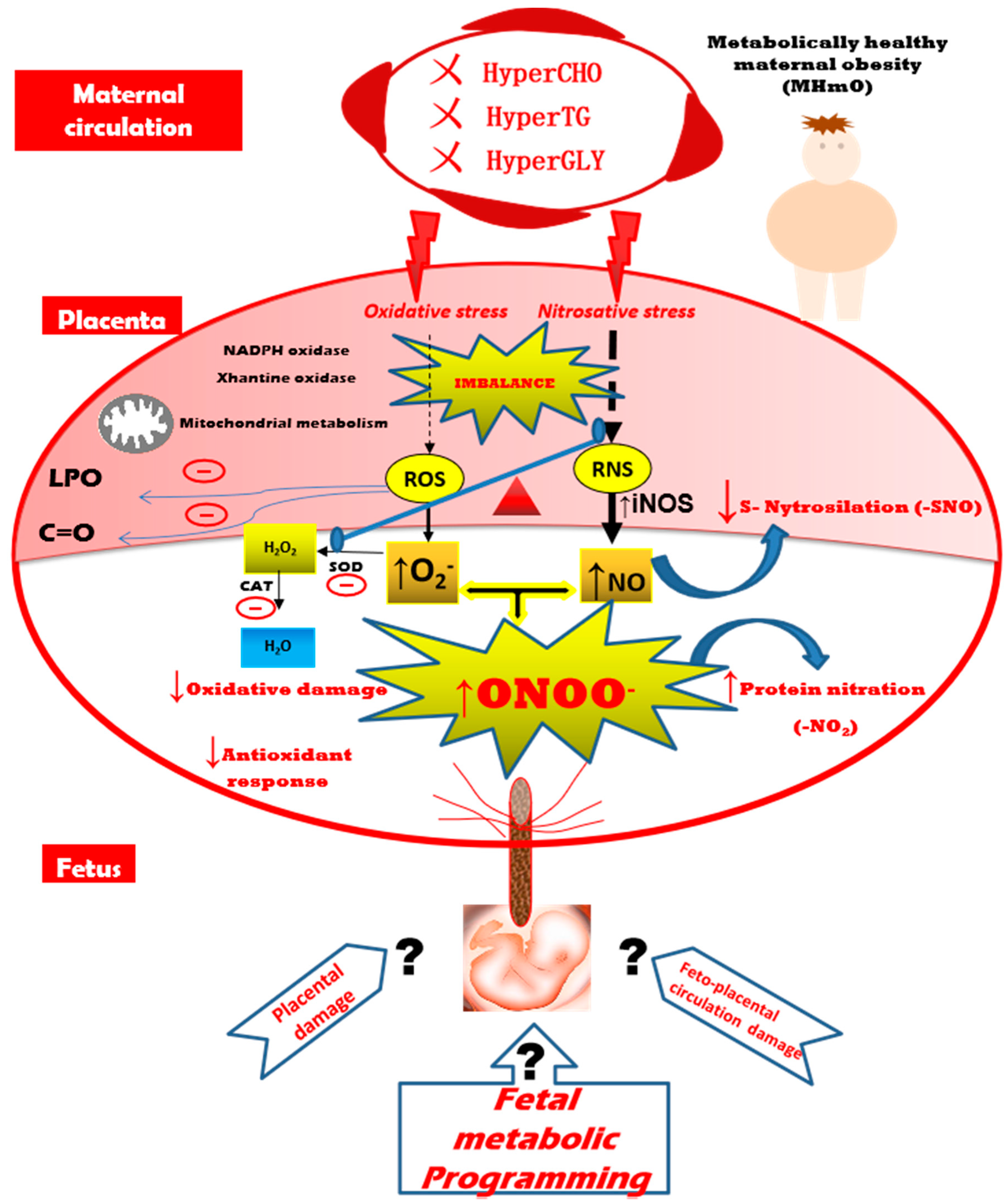
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Arora, R.; Arora, D.; Patumanond, J. Adverse pregnancy outcomes in women with high pre-pregnancy body mass index. Open J. Obstet. Gynecol. 2013, 3, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Leddy, M.A.; Power, M.L.; Schulkin, J. The impact of maternal obesity on maternal and fetal health. Rev. Obstet. Gynecol. 2008, 1, 170–178. [Google Scholar] [PubMed]
- Lisonkova, S.; Muraca, G.M.; Potts, J.; Liauw, J.; Chan, W.-S.; Skoll, A.; Lim, K.I. Association Between Prepregnancy Body Mass Index and Severe Maternal Morbidity. JAMA 2017, 318, 1777. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shen, Z.; Zhan, Y.; Wang, Y.; Ma, S.; Zhang, S.; Liu, J.; Wu, S.; Feng, Y.; Chen, Y.; et al. Effects of pre-pregnancy body mass index and gestational weight gain on maternal and infant complications. BMC Pregnancy Childbirth 2020, 20, 390. [Google Scholar] [CrossRef] [PubMed]
- Yogev, Y.; Catalano, P.M. Pregnancy and Obesity. Obstet. Gynecol. Clin. N. Am. 2009, 36, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Catalano, P.M.; Presley, L.; Minium, J.; Hauguel-de Mouzon, S. Fetuses of Obese Mothers Develop Insulin Resistance in Utero. Diabetes Care 2009, 32, 1076–1080. [Google Scholar] [CrossRef] [Green Version]
- Rkhzay-Jaf, J.; O’Dowd, J.F.; Stocker, C.J. Maternal Obesity and the Fetal Origins of the Metabolic Syndrome. Curr. Cardiovasc Risk Rep. 2012, 6, 487–495. [Google Scholar] [CrossRef] [Green Version]
- Laker, R.C.; Wlodek, M.E.; Connelly, J.J.; Yan, Z. Epigenetic origins of metabolic disease: The impact of the maternal condition to the offspring epigenome and later health consequences. Food Sci. Hum. Wellness 2013, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Sánchez, A.; Madrigal-Santillán, E.; Bautista, M.; Esquivel-Soto, J.; Morales-González, Á.; Esquivel-Chirino, C.; Durante-Montiel, I.; Sánchez-Rivera, G.; Valadez-Vega, C.; Morales-González, J.A. Inflammation, Oxidative Stress, and Obesity. Int. J. Mol. Sci. 2011, 12, 3117–3132. [Google Scholar] [CrossRef] [Green Version]
- Bugatto, F.; Fernández-Deudero, Á.; Bailén, Á.; Fernández-Macías, R.; Hervías-Vivancos, B.; Bartha, J.L. Second-Trimester Amniotic Fluid Proinflammatory Cytokine Levels in Normal and Overweight Women. Obstet. Gynecol. 2010, 115, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Howell, K.R.; Powell, T.L. Effects of maternal obesity on placental function and fetal development. Reproduction 2017, 153, R97–R108. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.C.; Powell, T.L.; Jansson, T. Placental function in maternal obesity. Clin. Sci. 2020, 134, 961–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myatt, L.; Maloyan, A. Obesity and Placental Function. Semin. Reprod. Med. 2016, 34, 042–049. [Google Scholar] [CrossRef] [Green Version]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Myatt, L.; Cui, X. Oxidative stress in the placenta. Histochem. Cell Biol. 2004, 122, 369–382. [Google Scholar] [CrossRef]
- Mele, J.; Muralimanoharan, S.; Maloyan, A.; Myatt, L. Impaired mitochondrial function in human placenta with increased maternal adiposity. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E419–E425. [Google Scholar] [CrossRef] [Green Version]
- Mannaerts, D.; Faes, E.; Cos, P.; Briedé, J.J.; Gyselaers, W.; Cornette, J.; Gorbanev, Y.; Bogaerts, A.; Spaanderman, M.; Van Craenenbroeck, E.; et al. Oxidative stress in healthy pregnancy and preeclampsia is linked to chronic inflammation, iron status and vascular function. PLoS ONE 2018, 13, e0202919. [Google Scholar] [CrossRef] [Green Version]
- Coughlan, M.T.; Vervaart, P.P.; Permezel, M.; Georgiou, H.M.; Rice, G.E. Altered Placental Oxidative Stress Status in Gestational Diabetes Mellitus. Placenta 2004, 25, 78–84. [Google Scholar] [CrossRef]
- Lappas, M.; Hiden, U.; Desoye, G.; Froehlich, J.; Mouzon, S.H.; Jawerbaum, A. The Role of Oxidative Stress in the Pathophysiology of Gestational Diabetes Mellitus. Antioxid. Redox Signal. 2011, 15, 3061–3100. [Google Scholar] [CrossRef]
- Malti, N.; Merzouk, H.; Merzouk, S.A.; Loukidi, B.; Karaouzene, N.; Malti, A.; Narce, M. Oxidative stress and maternal obesity: Feto-placental unit interaction. Placenta 2014, 35, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Roberts, V.H.J.; Smith, J.; McLea, S.A.; Heizer, A.B.; Richardson, J.L.; Myatt, L. Effect of Increasing Maternal Body Mass Index on Oxidative and Nitrative Stress in The Human Placenta. Placenta 2009, 30, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Alanis, M.C.; Steadman, E.M. Maternal Obesity and Placental Oxidative Stress in the First Trimester. J. Obes. Weight Loss Ther. 2012, 2. [Google Scholar] [CrossRef]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visiedo, F.; Santos-Rosendo, C.; Mateos-Bernal, R.M.; Gil-Sánchez, M.; Bugatto, F.; Aguilar-Diosdado, M.; Segundo, C.; López-Tinoco, C. Characterization of NO-Induced Nitrosative Status in Human Placenta from Pregnant Women with Gestational Diabetes Mellitus. Oxidative Med. Cell. Longev. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Chen, D. Analysis of Nitroso-Proteomes in Normotensive and Severe Preeclamptic Human Placentas1. Biol. Reprod. 2011, 84, 966–975. [Google Scholar] [CrossRef] [Green Version]
- Webster, R.P.; Roberts, V.H.J.; Myatt, L. Protein Nitration in Placenta—Functional Significance. Placenta 2008, 29, 985–994. [Google Scholar] [CrossRef] [Green Version]
- Lyall, F.; Gibson, J.L.; Greer, I.A.; Brockman, D.E.; Eis, A.L.; Myatt, L. Increased Nitrotyrosine in the Diabetic Placenta: Evidence for oxidative stress. Diabetes Care 1998, 21, 1753–1758. [Google Scholar] [CrossRef]
- Myatt, L.; Rosenfield, R.B.; Eis, A.L.W.; Brockman, D.E.; Greer, I.; Lyall, F. Nitrotyrosine Residues in Placenta: Evidence of Peroxynitrite Formation and Action. Hypertension 1996, 28, 488–493. [Google Scholar] [CrossRef]
- Rey-López, J.P.; de Rezende, L.F.; Pastor-Valero, M.; Tess, B.H. The prevalence of metabolically healthy obesity: A systematic review and critical evaluation of the definitions used: Prevalence of metabolically healthy obesity. Obes. Rev. 2014, 15, 781–790. [Google Scholar] [CrossRef]
- National Diabetes Data Group Classification and Diagnosis of Diabetes Mellitus and Other Categories of Glucose Intolerance. Diabetes 1979, 28, 1039–1057. [CrossRef] [PubMed]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1978; Volume 52, pp. 302–310. ISBN 978-0-12-181952-1. [Google Scholar]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Meth. Enzym. 1990, 186, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. ISBN 978-0-12-182005-3. [Google Scholar]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Jaffrey, S.R.; Snyder, S.H. The Biotin Switch Method for the Detection of S-Nitrosylated Proteins. Sci. Signal. 2001, 2001, pl1. [Google Scholar] [CrossRef]
- Deruelle, P. Obésité et grossesse. Gynécologie Obs. Fertil. 2011, 39, 100–105. [Google Scholar] [CrossRef]
- Aviram, A.; Hod, M.; Yogev, Y. Maternal obesity: Implications for pregnancy outcome and long-term risks-a link to maternal nutrition. Int. J. Gynecol. Obstet. 2011, 115, S6–S10. [Google Scholar] [CrossRef]
- Desoye, G. The Human Placenta in Diabetes and Obesity: Friend or Foe? The 2017 Norbert Freinkel Award Lecture. Diabetes Care 2018, 41, 1362–1369. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Tian, F.-J.; Lin, Y.; Xu, W.-M. Oxidative Stress: Placenta Function and Dysfunction. Am. J. Reprod. Immunol. 2016, 76, 258–271. [Google Scholar] [CrossRef]
- Challier, J.C.; Basu, S.; Bintein, T.; Minium, J.; Hotmire, K.; Catalano, P.M.; Hauguel-de Mouzon, S. Obesity in Pregnancy Stimulates Macrophage Accumulation and Inflammation in the Placenta. Placenta 2008, 29, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.-L.; Brockman, D.; Campos, B.; Myatt, L. Expression of NADPH Oxidase Isoform 1 (Nox1) in Human Placenta: Involvement in Preeclampsia. Placenta 2006, 27, 422–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, I.; Fournier, T.; Chissey, A.; Therond, P.; Slama, A.; Beaudeux, J.-L.; Zerrad-Saadi, A. NADPH oxidase is the major source of placental superoxide in early pregnancy: Association with MAPK pathway activation. Sci. Rep. 2019, 9, 13962. [Google Scholar] [CrossRef] [Green Version]
- Toescu, V.; Nuttall, S.L.; Martin, U.; Kendall, M.J.; Dunne, F. Oxidative stress and normal pregnancy: Oxidative stress and normal pregnancy. Clin. Endocrinol. 2002, 57, 609–613. [Google Scholar] [CrossRef]
- Leal, C.A.M.; Schetinger, M.R.C.; Leal, D.B.R.; Morsch, V.M.; da Silva, A.S.; Rezer, J.F.P.; de Bairros, A.V.; Jaques, J.A. dos S. Oxidative stress and antioxidant defenses in pregnant women. Redox Rep. 2011, 16, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Madazli, R.; Benian, A.; Aydin, S.; Uzun, H.; Tolun, N. The plasma and placental levels of malondialdehyde, glutathione and superoxide dismutase in pre-eclampsia. J. Obstet. Gynaecol. 2002, 22, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Biri, A.; Onan, A.; Devrim, E.; Babacan, F.; Kavutcu, M.; Durak, I. Oxidant status in maternal and cord plasma and placental tissue in gestational diabetes. Placenta 2006, 27, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Vanderlelie, J.; Venardos, K.; Clifton, V.L.; Gude, N.M.; Clarke, F.M.; Perkins, A.V. Increased biological oxidation and reduced anti-oxidant enzyme activity in pre-eclamptic placentae. Placenta 2005, 26, 53–58. [Google Scholar] [CrossRef]
- Alcala, M.; Gutierrez-Vega, S.; Castro, E.; Guzman-Gutiérrez, E.; Ramos-Álvarez, M.P.; Viana, M. Antioxidants and Oxidative Stress: Focus in Obese Pregnancies. Front. Physiol. 2018, 9, 1569. [Google Scholar] [CrossRef] [Green Version]
- Wongsurawat, T.; Woo, C.C.; Giannakakis, A.; Lin, X.Y.; Cheow, E.S.H.; Lee, C.N.; Richards, M.; Sze, S.K.; Nookaew, I.; Kuznetsov, V.A.; et al. Distinctive molecular signature and activated signaling pathways in aortic smooth muscle cells of patients with myocardial infarction. Atherosclerosis 2018, 271, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Krause, B.J.; Hanson, M.A.; Casanello, P. Role of nitric oxide in placental vascular development and function. Placenta 2011, 32, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Schönfelder, G.; John, M.; Hopp, H.; Fuhr, N.; Van Der Giet, M.; Paul, M. Expression of inducible nitric oxide synthase in placenta of women with gestational diabetes. FASEB J. 1996, 10, 777–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Cao, Y.; Li, H. Hyperglycemia induces inducible nitric oxide synthase gene expression and consequent nitrosative stress via c-Jun N-terminal kinase activation. Am. J. Obstet. Gynecol. 2010, 203, 185.e5–185.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, H.; Li, X.; Reece, E.A.; Yang, P. SOD1 suppresses maternal hyperglycemia-increased iNOS expression and consequent nitrosative stress in diabetic embryopathy. Am. J. Obstet. Gynecol. 2012, 206, 448.e1–448.e7. [Google Scholar] [CrossRef] [Green Version]
- Zullino, S.; Buzzella, F.; Simoncini, T. Nitric oxide and the biology of pregnancy. Vasc. Pharmacol. 2018, 110, 71–74. [Google Scholar] [CrossRef]
- Foster, M.W.; McMahon, T.J.; Stamler, J.S. S-nitrosylation in health and disease. Trends Mol. Med. 2003, 9, 160–168. [Google Scholar] [CrossRef]
- Page, A.J. NO regulation of gut-brain signalling in obesity. J. Physiol. 2019, 597, 1425–1426. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Park, S.J.; Beyak, M.J. Inducible nitric oxide synthase-derived nitric oxide reduces vagal satiety signalling in obese mice. J. Physiol. 2019, 597, 1487–1502. [Google Scholar] [CrossRef]
- Feng, X.; Sun, T.; Bei, Y.; Ding, S.; Zheng, W.; Lu, Y.; Shen, P. S-nitrosylation of ERK inhibits ERK phosphorylation and induces apoptosis. Sci. Rep. 2013, 3, 1814. [Google Scholar] [CrossRef] [Green Version]
- Castegna, A.; Thongboonkerd, V.; Klein, J.B.; Lynn, B.; Markesbery, W.R.; Butterfield, D.A. Proteomic identification of nitrated proteins in Alzheimer’s disease brain: Nitrated proteins in AD brain. J. Neurochem. 2003, 85, 1394–1401. [Google Scholar] [CrossRef]
- Sabetkar, M.; Low, S.Y.; Naseem, K.M.; Bruckdorfer, K.R. The nitration of proteins in platelets: Significance in platelet function 1,2 1This article is part of a series of reviews on “Reactive Nitrogen Species, Tyrosine Nitration and Cell Signaling.” The full list of papers may be found on the homepage of the journal. 2Guest Editor: Harry Ischiropoulos. Free Radic. Biol. Med. 2002, 33, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Radi, R. Protein Tyrosine Nitration: Biochemical Mechanisms and Structural Basis of Functional Effects. Acc. Chem. Res. 2013, 46, 550–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosco, C.; González, J.; Gutiérrez, R.; Parra-Cordero, M.; Barja, P.; Rodrigo, R. Oxidative damage to pre-eclamptic placenta: Immunohistochemical expression of VEGF, nitrotyrosine residues and von Willebrand factor. J. Matern. Fetal Neonatal Med. 2012, 25, 2339–2345. [Google Scholar] [CrossRef] [PubMed]
- Jauniaux, E.; Burton, G.J. Le rôle du stress oxydant dans les pathologies placentaires de la grossesse. J. Gynécologie Obs. Biol. Reprod. 2016, 45, 775–785. [Google Scholar] [CrossRef]
- Salvolini, E.; Vignini, A.; Sabbatinelli, J.; Lucarini, G.; Pompei, V.; Sartini, D.; Cester, A.M.; Ciavattini, A.; Mazzanti, L.; Emanuelli, M. Nitric oxide synthase and VEGF expression in full-term placentas of obese women. Histochem. Cell Biol. 2019, 152, 415–422. [Google Scholar] [CrossRef] [PubMed]
- White, V.; Capobianco, E.; Higa, R.; Martínez, N.; Sosa, M.; Pustovrh, M.C.; Jawerbaum, A. Increased nitration and diminished activity of copper/zinc superoxide dismutase in placentas from diabetic rats. Free Radic. Res. 2010, 44, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.P.; Brockman, D.; Myatt, L. Nitration of p38 MAPK in the placenta: Association of nitration with reduced catalytic activity of p38 MAPK in pre-eclampsia. MHR Basic Sci. Reprod. Med. 2006, 12, 677–685. [Google Scholar] [CrossRef]
- Schoots, M.H.; Gordijn, S.J.; Scherjon, S.A.; van Goor, H.; Hillebrands, J.-L. Oxidative stress in placental pathology. Placenta 2018, 69, 153–161. [Google Scholar] [CrossRef]

| Characteristics | Lean Group (n = 9) | Obese Group (n = 8) |
|---|---|---|
| Delivery mode | Caesarean section (No labor) | Caesarean section (No labor) |
| Maternal age (years) | 33.1 ± 6.3 | 35.5 ± 6.4 |
| Gestational age at delivery (weeks) | 38.3 ± 1.0 | 38.3 ± 0.8 |
| Pregravid Maternal BMI (kg/m2) | 22.5 ± 1.3 | 35.5 ± 6.4 * |
| Maternal BMI at delivery (kg/m2) | 28.1 ± 2.5 | 37.3 ± 5.7 * |
| Birthweight (g) | 3182 ± 214 | 3170 ± 336 |
| F/M sex ratio | 5/4 | 5/3 |
| Placental weight (g) | 603 ± 75 | 604 ± 54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Rosendo, C.; Bugatto, F.; González-Domínguez, A.; Lechuga-Sancho, A.M.; Mateos, R.M.; Visiedo, F. Placental Adaptive Changes to Protect Function and Decrease Oxidative Damage in Metabolically Healthy Maternal Obesity. Antioxidants 2020, 9, 794. https://doi.org/10.3390/antiox9090794
Santos-Rosendo C, Bugatto F, González-Domínguez A, Lechuga-Sancho AM, Mateos RM, Visiedo F. Placental Adaptive Changes to Protect Function and Decrease Oxidative Damage in Metabolically Healthy Maternal Obesity. Antioxidants. 2020; 9(9):794. https://doi.org/10.3390/antiox9090794
Chicago/Turabian StyleSantos-Rosendo, Celeste, Fernando Bugatto, Alvaro González-Domínguez, Alfonso M. Lechuga-Sancho, Rosa Maria Mateos, and Francisco Visiedo. 2020. "Placental Adaptive Changes to Protect Function and Decrease Oxidative Damage in Metabolically Healthy Maternal Obesity" Antioxidants 9, no. 9: 794. https://doi.org/10.3390/antiox9090794
APA StyleSantos-Rosendo, C., Bugatto, F., González-Domínguez, A., Lechuga-Sancho, A. M., Mateos, R. M., & Visiedo, F. (2020). Placental Adaptive Changes to Protect Function and Decrease Oxidative Damage in Metabolically Healthy Maternal Obesity. Antioxidants, 9(9), 794. https://doi.org/10.3390/antiox9090794








