The Phosphodiesterase Type 5 Inhibitor Sildenafil Improves DNA Stability and Redox Homeostasis in Systemic Sclerosis Fibroblasts Exposed to Reactive Oxygen Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture
2.3. Protein Expression Analysis
2.4. Immunofluorescence Microscopy
2.5. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyl-2H-Tetrazolium Bromide (MTT) Assay
2.6. Cell Viability
2.7. Cell Cycle Analysis
2.8. Measurement of Intracellular ROS Levels
2.9. Thiobarbituric Acid Reactive Substances (TBARS)
2.10. Glutathione Homeostasis
2.11. Enzymatic Activities
2.12. Cytokine Assay
2.13. Statistical Analysis
3. Results
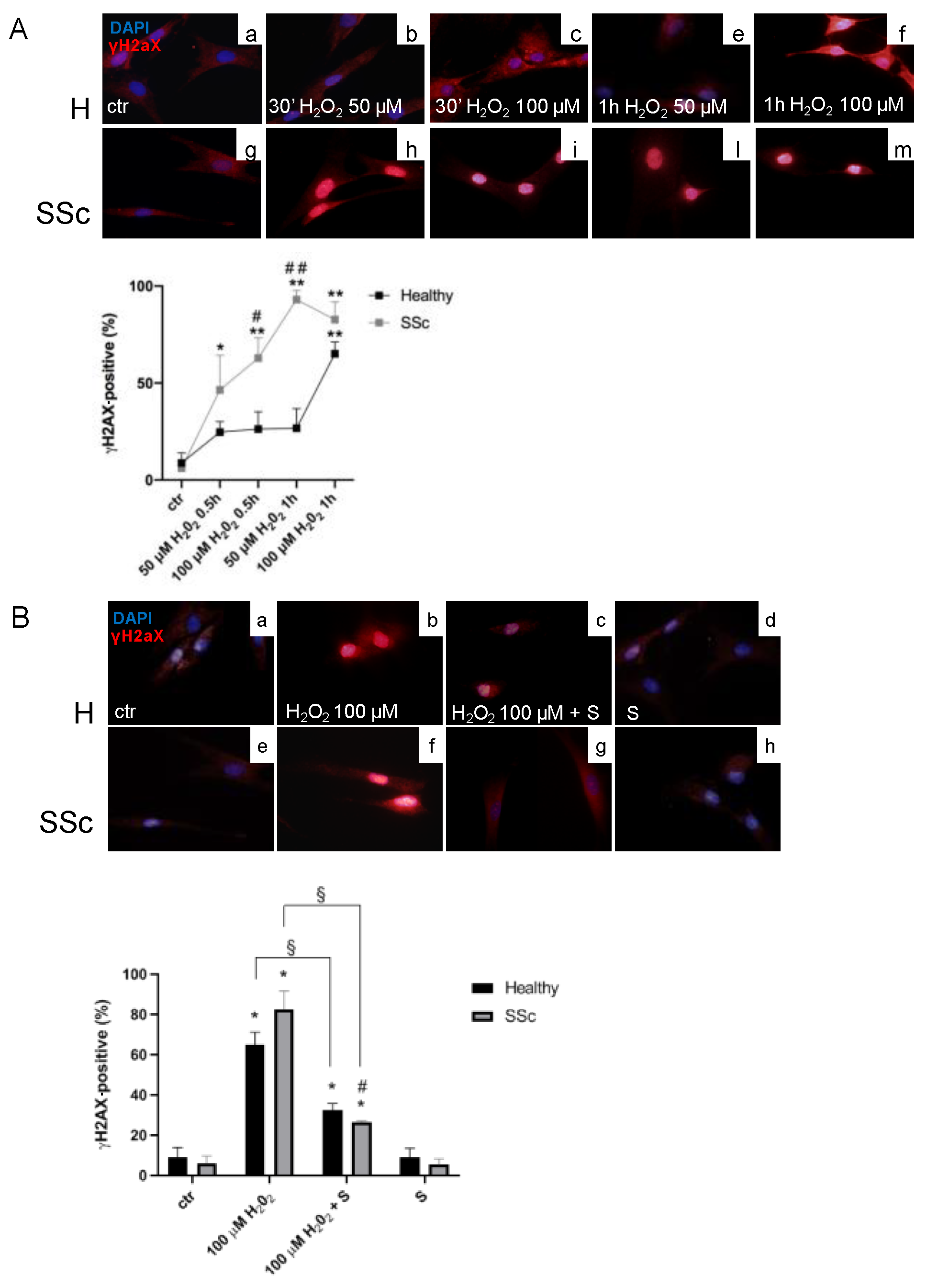
3.1. SSc Fibroblasts Appeared More Sensitive to Oxidative Stress-Induced DNA Double-Strand Breaks (DSBs) in Comparison to the Healthy Control Cells
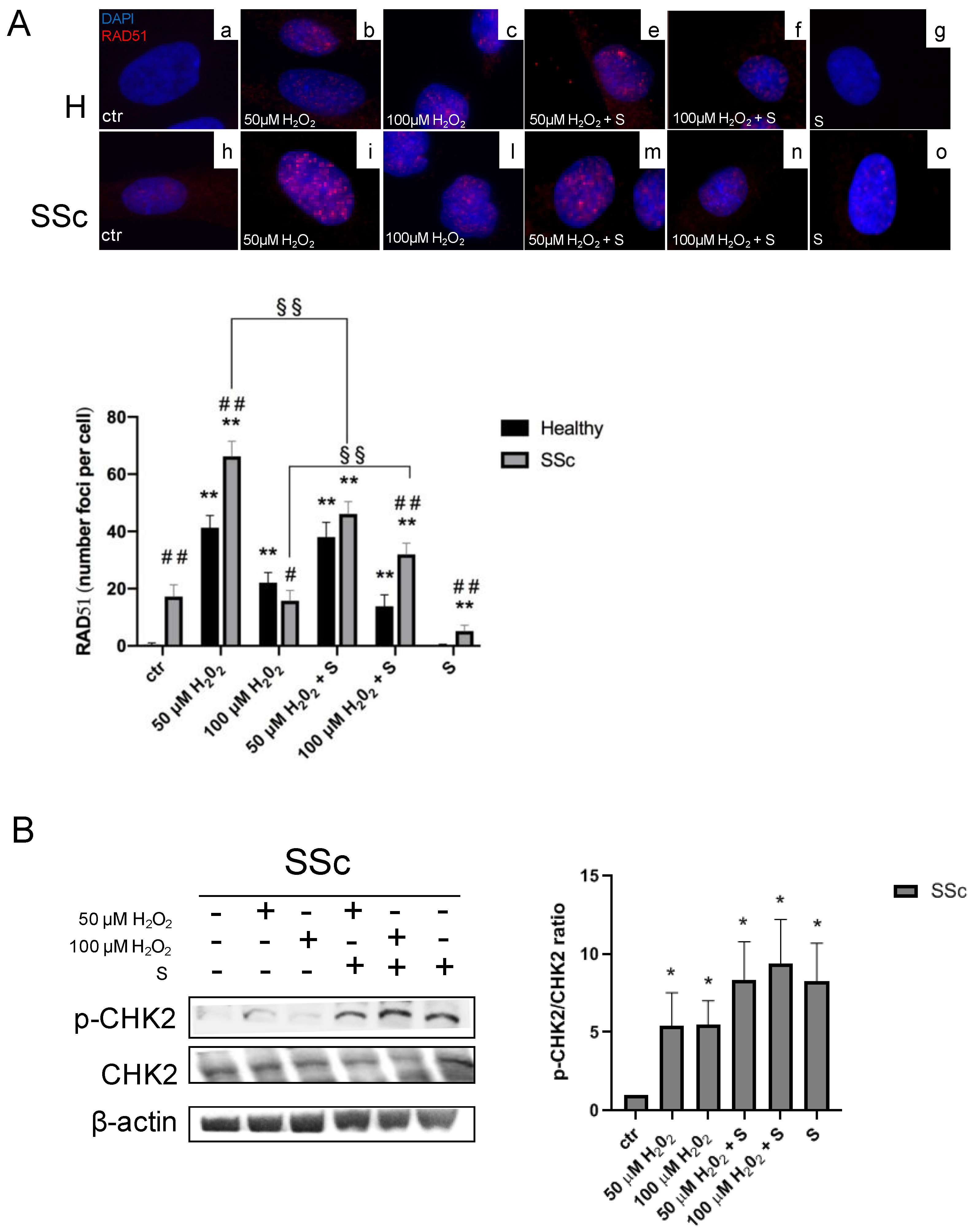
3.2. Sildenafil Improved the DNA Repair Activation Process in H2O2-Damaged Healthy and SSc Fibroblasts
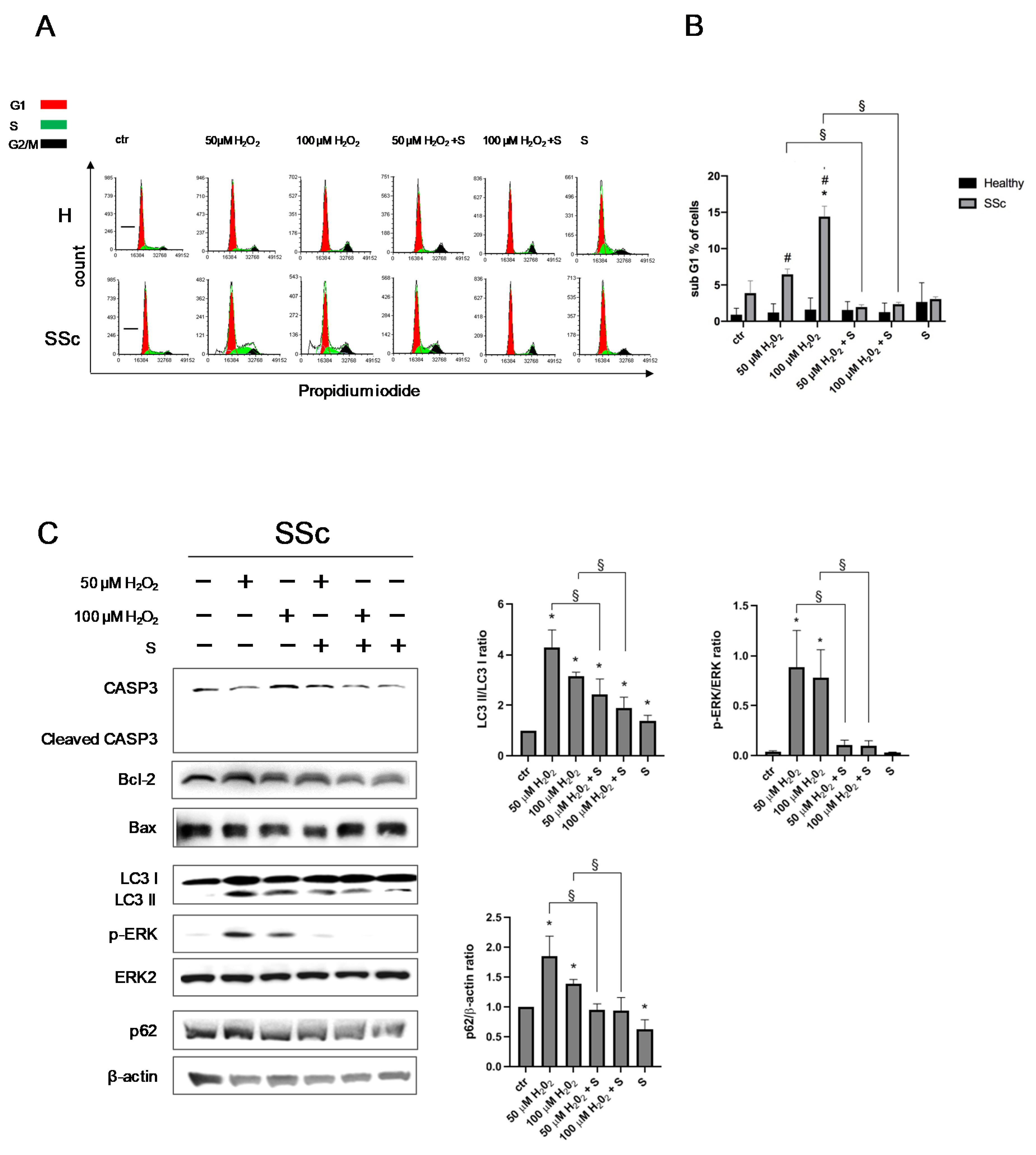
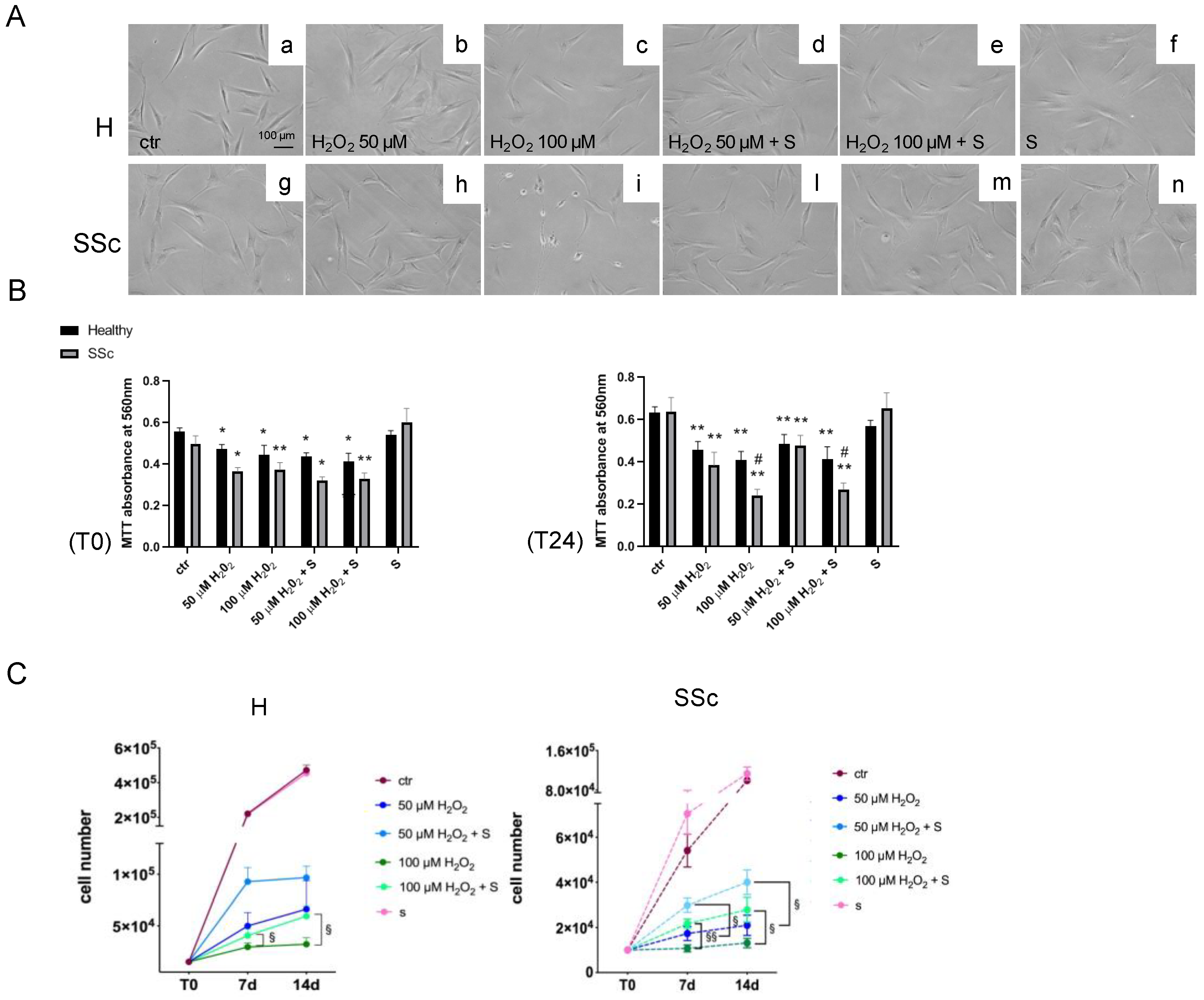
3.3. Sildenafil Sustained the Cell Viability and Ameliorated the Cell Proliferation in SSc Fibroblasts
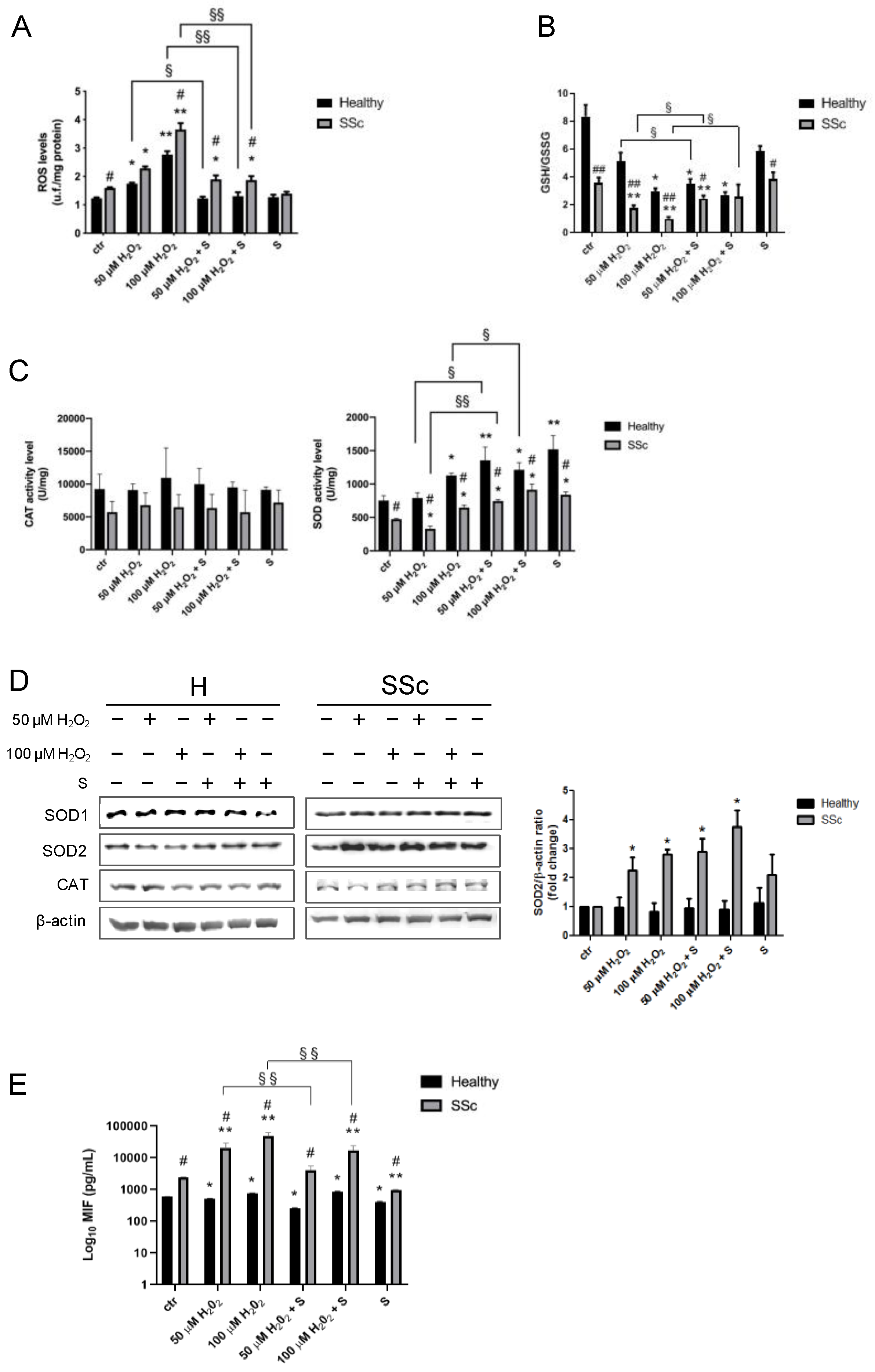
3.4. Sildenafil Reduces the ROS Levels in Healthy and SSc Fibroblasts
3.5. Sildenafil Ameliorates an Antioxidant Response in SSc Fibroblasts
3.6. Macrophage Migration Inhibitory Factor Level
3.7. Sildenafil Mitigates the Effect of Oxidative Insult on TBAR Levels
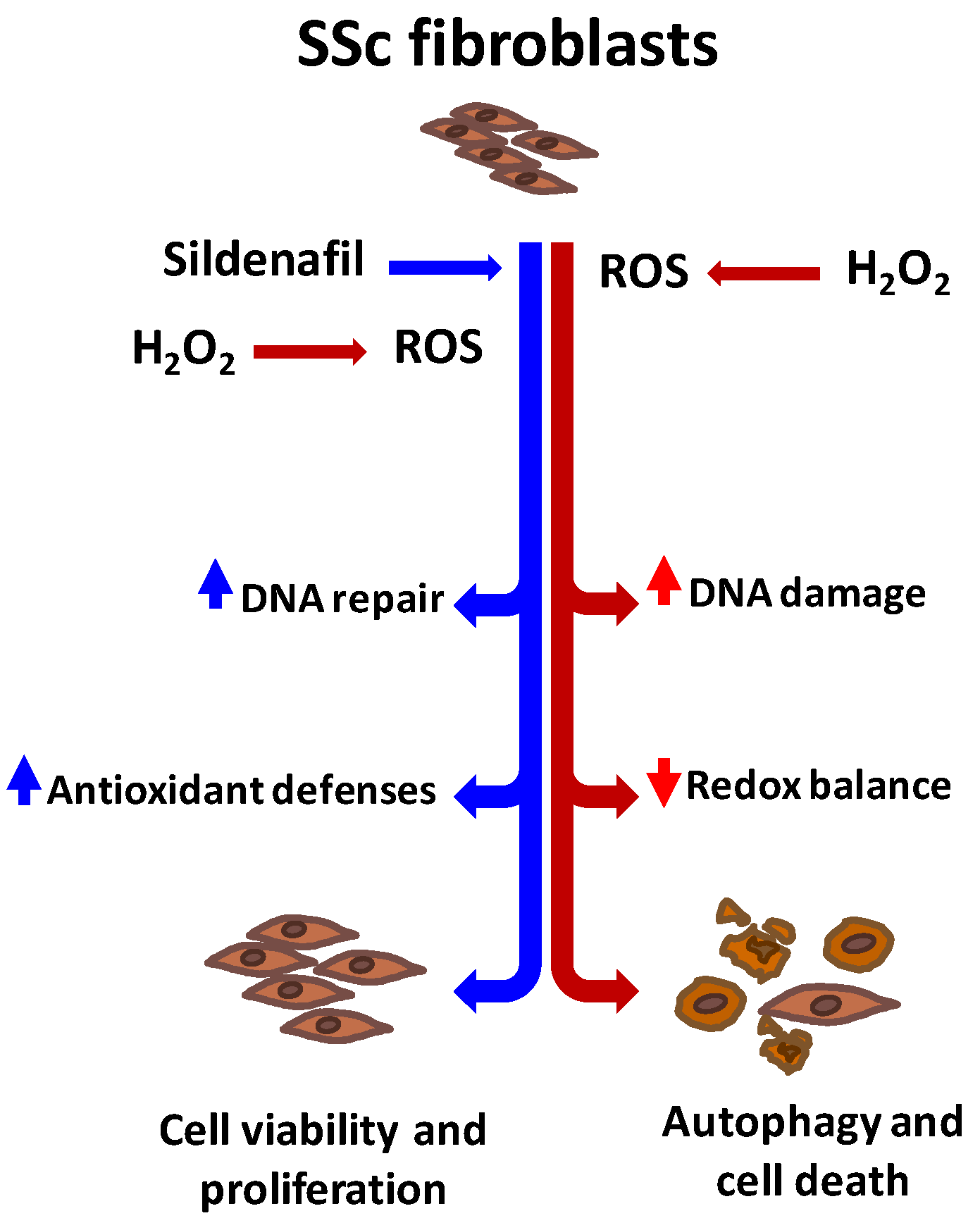
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Denton, C.P.; Black, C.M.; Abraham, D.J. Mechanisms and consequences of fibrosis in systemic sclerosis. Nat. Clin. Pract. Rheumatol. 2006, 2, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Black, C.M.; Stephen, C. Systemic sclerosis (scleroderma) and related disorders. In Oxford Textbook of Rheumatology; Maddison, P.J., Isenberg, D.A., Woo, P., Glass, D.N., Eds.; Oxford University Press: Oxford, UK, 1993; pp. 771–789. [Google Scholar]
- Colletti, M.; Galardi, A.; De Santis, M.; Guidelli, G.M.; Di Giannatale, A.; Di Luigi, L.; Antinozzi, C. Exosomes in Systemic Sclerosis: Messengers Between Immune, Vascular and Fibrotic Components? Int. J. Mol. Sci. 2019, 20, 4337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sena, C.M.; Leandro, A.; Azul, L.; Seiça, R.; Perry, G. Vascular Oxidative Stress: Impact and Therapeutic Approaches. Front. Physiol. 2018, 9, 1668. [Google Scholar] [CrossRef] [Green Version]
- Cano Sanchez, M.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting Oxidative Stress and Mitochondrial Dysfunction in the Treatment of Impaired Wound Healing: A Systematic Review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Bruckdorfer, K.R.; Hillary, J.B.; Bunce, T.; Vancheeswaran, R.; Black, C.M. Increased susceptibility to oxidation of low-density lipoproteins isolated from patients with systemic sclerosis. Arthritis Rheumatol. 1995, 38, 1060–1067. [Google Scholar] [CrossRef]
- Herrick, A.L.; Cerinic, M.M. The emerging problem of oxidative stress and the role of antioxidants in systemic sclerosis. Clin. Exp. Rheumatol. 2001, 19, 4–8. [Google Scholar]
- Parlanti, E.; Pietraforte, D.; Iorio, E.; Visentin, S.; de Nuccio, C.; Zijno, A.; D’Errico, M.; Simonelli, V.; Sanchez, M.; Fattibene, P.; et al. An altered redox balance and increased genetic instability characterize primary fibroblasts derived from xeroderma pigmentosum group A patients. Mutat. Res. 2015, 782, 34–43. [Google Scholar] [CrossRef]
- Emerit, I.; Filipe, P.; Meunier, P.; Auclair, C.; Freitas, J.; Deroussent, A.; Gouyette, A.; Fernandes, A. Clastogenic activity in the plasma of scleroderma patients: A biomarker of oxidative stress. Dermatology 1997, 194, 140–146. [Google Scholar] [CrossRef]
- Giovannetti, A.; Gambardella, L.; Pietraforte, D.; Rosato, E.; Giammarioli, A.M.; Salsano, F.; Malorni, W.; Straface, E. Red blood cell alterations in systemic sclerosis: A pilot study. Cell Physiol. Biochem. 2012, 30, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Svegliati, S.; Cancello, R.; Sambo, P.; Luchetti, M.; Paroncini, P.; Orlandini, G.; Discepoli, G.; Paterno, R.; Santillo, M.; Cuozzo, C.; et al. Platelet derived growth factor and reactive oxygen species (ROS) regulate Ras protein levels in primary human fibroblasts via ERK1/2. Amplification of ROS and Ras in systemic sclerosis fibroblasts. J. Biol. Chem. 2005, 280, 36474–36482. [Google Scholar] [CrossRef] [Green Version]
- Dooley, A.; Shi-Wen, X.; Aden, N.; Tranah, T.; Desai, N.; Denton, C.P.; Abraham, D.J.; Bruckdorfer, R. Modulation of collagen type I, fibronectin and dermal fibroblast function and activity, in systemic sclerosis by the antioxidant epigallocatechin-3-gallate. Rheumatology 2010, 49, 2024–2036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denton, C.P.; Bunce, T.D.; Darado, M.B.; Roberts, Z.; Wilson, H.; Howell, K.; Bruckdorfer, R.; Black, C.M. Probucol improves symptoms and reduces lipoprotein oxidation susceptibility in patients with Raynaud’s phenomenon. Rheumatology 1999, 38, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cracowski, J.L.; Girolet, S.; Imbert, B.; Seinturier, C.; Stanke-Labesque, F.; Bessard, J.; Boignard, A.; Bessard, G.; Carpentier, P.H. Effects of short-term treatment with vitamin E in systemic sclerosis: A double blind, randomized, controlled clinical trial of efficacy based on urinary isoprostane measurement. Free Radic. Biol. Med. 2005, 38, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Mavrikakis, M.E.; Lekakis, J.P.; Papamichael, C.M.; Stamatelopoulos, K.S.; Kostopoulos, C.; Stamatelopoulos, S.F. Ascorbic acid does not improve endothelium-dependent flow-mediated dilatation of the brachial artery in patients with Raynaud’s phenomenon secondary to systemic sclerosis. Int. J. Vitam. Nutr. Res. 2003, 73, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Dooley, A.; Bruckdorfer, K.R.; Abraham, D.J. Modulation of fibrosis in systemic sclerosis by nitric oxide and antioxidants. Cardiol. Res. Pract. 2012, 2012, 521958. [Google Scholar] [CrossRef]
- Rosato, E.; Borghese, F.; Pisarri, S.; Salsano, F. The treatment with Nacetylcysteine of Raynaud’s phenomenon and ischemic ulcers therapy in sclerodermic patients: A prospective observational study of 50 patients. Clin. Rheumatol. 2009, 28, 1379–1384. [Google Scholar] [CrossRef]
- Herrick, A.L.; Hollis, S.; Schofield, D.; Rieley, F.; Blann, A.; Griffin, K.; Moore, T.; Braganza, J.M.; Jayson, M.I. A double-blind placebo-controlled trial of antioxidant therapy in limited cutaneous systemic sclerosis. Clin. Exp. Rheumatol. 2000, 18, 349–356. [Google Scholar]
- Bernardes, F.P.; Batista, A.T.; Porto, M.L.; Vasquez, E.C.; Campagnaro, B.P.; Meyrelles, S.S. Protective effect of sildenafil on the genotoxicity and cytotoxicity in apolipoprotein E-deficient mice bone marrow cells. Lipids Health Dis. 2016, 15, 100. [Google Scholar] [CrossRef] [Green Version]
- Dias, A.T.; Rodrigues, B.P.; Porto, M.L.; Gava, A.L.; Balarini, C.M.; Freitas, P.S.F.; Palomino, A.; Casarini, E.D.; Campagnaro, P.B.; Pereira, M.C.T.; et al. Sildenafil ameliorates oxidative stress and DNA damage in the stenotic kidneys in mice with renovascular hypertension. J. Transl. Med. 2014, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, B.P.; Campagnaro, B.P.; Balarini, C.M.; Pereira, T.M.; Meyrelles, S.S.; Vasquez, E.C. Sildenafil ameliorates biomarkers of genotoxicity in an experimental model of spontaneous atherosclerosis. Lipids Health Dis. 2013, 12, 128. [Google Scholar] [CrossRef] [Green Version]
- Jeon, Y.H.; Heo, Y.S.; Kim, C.M.; Hyun, Y.L.; Lee, T.G.; Ro, S.; Cho, J.M. Phosphodiesterase: Overview of protein structures, potential therapeutic applications and recent progress in drug development. Cell. Mol. Life Sci. 2005, 62, 1198–1220. [Google Scholar] [CrossRef] [PubMed]
- Di Luigi, L.; Sgrò, P.; Duranti, G.; Sabatini, S.; Caporossi, D.; Del Galdo, F.; Dimauro, I.; Antinozzi, C. Sildenafil Reduces Expression and Release of IL-6 and IL-8 Induced by Reactive Oxygen Species in Systemic Sclerosis Fibroblasts. Int. J. Mol. Sci. 2020, 21, 3161. [Google Scholar] [CrossRef] [PubMed]
- Herrick, A.L.; van den Hoogen, F.; Gabrielli, A.; Tamimi, N.; Reid, C.; O’Connell, D.; Vázquez-Abad, M.D.; Denton, P.D. Modified-release sildenafil reduces Raynaud’s phenomenon attack frequency in limited cutaneous systemic sclerosis. Arthritis Rheum. 2011, 63, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Fries, R.; Shariat, K.; von Wilmowsky, H.; Böhm, M. Sildenafil in the treatment of Raynaud’s phenomenon resistant to vasodilatory therapy. Circulation 2005, 112, 2980–2985. [Google Scholar] [CrossRef]
- Menezes, T.N.; Naumann, G.B.; Mendonça, A.B.; Leal, A.M.; Porto, L.M.; Teixeira-Ferreira, A.; Perales, J.; Meyrelles, S.S.; Figueiredo, G.S.; Vasquez, C.E. Antioxidant effect of sildenafil: Potential hepatoprotection via differential expression of mitochondrial proteins in apolipoprotein E knockout mice. Pharmacol. Rep. 2019, 71, 422–429. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Shafaroodi, H.; Asadi, S.; Nezami, B.G.; Ghasemi, M.; Rahimpour, S.; Hashemi, M.; Doostar, Y.; Dehpour, A.R. Sildenafil decreased cardiac cell apoptosis in diabetic mice: Reduction of oxidative stress as a possible mechanism. Can. J. Physiol. Pharmacol. 2009, 87, 556–564. [Google Scholar] [CrossRef]
- Del Galdo, F.; Sotgia, F.; de Almeida, C.J.; Jasmin, J.F.; Musick, M.; Lisanti, M.P.; Jiménez, S.A. Decreased expression of caveolin 1 in patients with systemic sclerosis: Crucial role in the pathogenesis of tissue fibrosis. Arthritis Rheumatol. 2008, 58, 2854–2865. [Google Scholar] [CrossRef] [Green Version]
- Giannattasio, S.; Corinaldesi, C.; Colletti, M.; Di Luigi, L.; Antinozzi, C.; Filardi, T.; Scolletta, S.; Basili, S.; Lenzi, A.; Morano, S.; et al. The phosphodiesterase 5 inhibitor sildenafil decreases the proinflammatory chemokine IL-8 in diabetic cardiomyopathy: In vivo and in vitro evidence. J. Endocrinol. Invest. 2019, 42, 715–725. [Google Scholar] [CrossRef] [Green Version]
- Mercatelli, N.; Fittipaldi, S.; De Paola, E.; Dimauro, I.; Paronetto, M.P.; Jackson, M.J.; Caporossi, D. MiR-23-TrxR1 as a novel molecular axis in skeletal muscle differentiation. Sci. Rep. 2017, 7, 7219. [Google Scholar] [CrossRef] [Green Version]
- Marampon, F.; Antinozzi, C.; Corinaldesi, C.; Vannelli, G.B.; Sarchielli, E.; Migliaccio, S.; Di Luigi, L.; Lenzi, A.; Crescioli, C. The phosphodiesterase 5 inhibitor tadalafil regulates lipidic homeostasis in human skeletal muscle cell metabolism. Endocrine 2018, 59, 602–613. [Google Scholar] [CrossRef]
- Fittipaldi, S.; Mercatelli, N.; Dimauro, I.; Jackson, M.J.; Paronetto, M.P.; Caporossi, D. Alpha B-crystallin induction in skeletal muscle cells under redox imbalance is mediated by a JNK-dependent regulatory mechanism. Free Radic. Biol. Med. 2015, 86, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Vignali, P.; Wang, R. Rapid Profiling Cell Cycle by Flow Cytometry Using Concurrent Staining of DNA and Mitotic Markers. Bio. Protoc. 2017, 7, e2517. [Google Scholar] [CrossRef] [PubMed]
- Duranti, G.; Ceci, R.; Sgrò, P.; Sabatini, S.; Di Luigi, L. Influence of the PDE5 inhibitor tadalafil on redox status and antioxidant defense system in C2C12 skeletal muscle cells. Cell Stress Chaperones 2017, 22, 389–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duranti, G.; Ceci, R.; Patrizio, F.; Sgrò, P.; Di Luigi, L.; Sabatini, S.; Felici, F.; Bazzucchi, I. Chronic consumption of quercetin reduces erythrocytes oxidative damage: Evaluation at resting and after eccentric exercise in humans. Nutr. Res. 2018, 50, 73–81. [Google Scholar] [CrossRef]
- Crescioli, C.; Corinaldesi, C.; Riccieri, V.; Raparelli, V.; Vasile, M.; Del Galdo, F.; Valesini, G.; Lenzi, A.; Basili, S.; Antinozzi, C. Association of circulating CXCL10 and CXCL11 with systemic sclerosis. Ann. Rheum. Dis. 2018, 77, 1845–1846. [Google Scholar] [CrossRef] [Green Version]
- Illert, A.L.; Kawaguchi, H.; Antinozzi, C.; Bassermann, F.; Quintanilla-Martinez, L.; von Klitzing, C.; Hiwatari, M.; Peschel, C.; de Rooij, D.G.; Morris, S.W.; et al. Targeted inactivation of nuclear interaction partner of ALK disrupts meiotic prophase. Development 2012, 139, 2523–2534. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Kwok, S.K.; Hur, K.H.; Kim, H.J.; Kim, N.S.; Yoo, S.A.; Kim, W.U.; Cho, C.S. Up-regulated macrophage migration inhibitory factor protects apoptosis of dermal fibroblasts in patients with systemic sclerosis. Clin. Exp. Immunol. 2008, 152, 328–335. [Google Scholar] [CrossRef]
- Tonini, C.L.; Campagnaro, B.P.; Louro, L.P.; Pereira, T.M.; Vasquez, E.C.; Meyrelles, S.S. Effects of aging and hypercholesterolemia on oxidative stress and DNA damage in bone Marrow mononuclear cells in apolipoprotein E-deficient mice. Int. J. Mol. Sci. 2013, 14, 3325–3342. [Google Scholar] [CrossRef]
- Gillespie, J.; Ross, R.L.; Corinaldesi, C.; Esteves, F.; Derrett-Smith, E.; McDermott, M.F.; Doody, G.M.; Denton, C.P.; Emery, P.; Del Galdo, F. Transforming Growth Factor β Activation Primes Canonical Wnt Signaling Through Down-Regulation of Axin-2. Arthritis Rheumatol. 2018, 70, 932–942. [Google Scholar] [CrossRef]
- Kapanadze, B.; Morris, E.; Smith, E.; Trojanowska, M. Establishment and characterization of scleroderma fibroblast clonal cell lines by introduction of the hTERT gene. J. Cell. Mol. Med. 2010, 14, 1156–1165. [Google Scholar] [CrossRef]
- Kuo, L.J.; Yang, L.X. Gamma-H2AX—A novel biomarker for DNA double-strand breaks. Vivo 2008, 22, 305–309. [Google Scholar]
- Symington, L.S. Mechanism and regulation of DNA end resection in eukaryotes. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 195–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testa, E.; Nardozi, D.; Antinozzi, C.; Faieta, M.; Di Cecca, S.; Caggiano, C.; Fukuda, T.; Bonanno, E.; Zhenkun, L.; Maldonado, A.; et al. H2AFX and MDC1 promote maintenance of genomic integrity in male germ cells. J. Cell Sci. 2018, 131, jcs214411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zannini, L.; Delia, D.; Buscemi, G. CHK2 kinase in the DNA damage response and beyond. J. Mol. Cell Biol. 2014, 6, 442–457. [Google Scholar] [CrossRef] [Green Version]
- Bahassi, E.M.; Ovesen, J.L.; Riesenberg, A.L.; Bernstein, W.Z.; Hasty, P.E.; Stambrook, P.J. The Checkpoint Kinases Chk1 and Chk2 Regulate the Functional Associations Between hBRCA2 and Rad51 in Response to DNA Damage. Oncogene 2008, 27, 3977–3985. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Rose, J.H.; Zhang, H.; Pommier, Y. Antisense inhibition of Chk2/hCds1 expression attenuates DNA damage-induced S and G2 checkpoints and enhances apoptotic activity in HEK-293 cells. FEBS Lett. 2001, 505, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, J.C.; Dohi, T.; Raskett, C.M.; Kowalik, T.F.; Altieri, D.C. Activated checkpoint kinase 2 provides a survival signal for tumor cells. Cancer Res. 2006, 66, 11576–11579. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.C.; Chou, W.C.; Shieh, S.Y.; Shen, C.Y. Ataxia telangiectasia mutated and checkpoint kinase 2 regulate BRCA1 to promote the fidelity of DNA end-joining. Cancer Res. 2006, 66, 1391–1400. [Google Scholar] [CrossRef] [Green Version]
- Leal, M.A.; Balarini, C.M.; Dias, A.T.; Porto, M.L.; Gava, A.L.; Pereira, T.M.; Meyrelles, S.S.; Vasquez, E.C. Mechanisms of enhanced vasoconstriction in the mouse model of atherosclerosis: The beneficial effects of sildenafil. Curr. Pharm. Biotechnol. 2015, 16, 517–530. [Google Scholar] [CrossRef]
- Fahning, B.M.; Dias, A.T.; Oliveira, J.P.; Gava, A.L.; Porto, M.L.; Gomes, I.B.; Nogueira, B.V.; Campagnaro, B.P.; Pereira, T.M.; Vasquez, E.C.; et al. Sildenafil improves vascular endothelial structure and function in renovascular hypertension. Curr. Pharm. Biotechnol. 2015, 16, 823–831. [Google Scholar] [CrossRef]
- Dias, A.T.; Cintra, A.S.; Frossard, J.C.; Palomino, Z.; Casarini, D.E.; Gomes, I.B.; Balarini, C.M.; Gava, A.L.; Campagnaro, B.P.; Pereira, T.M.; et al. Inhibition of phosphodiesterase 5 restores endothelial function in renovascular hypertension. J. Transl. Med. 2014, 12, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.F.; Hsu, J.L.; Sheng, Y.H.; Leu, W.J.; Yu, C.C.; Chan, S.H.; Chan, M.L.; Hsu, L.C.; Liu, S.P.; Guh, J.H. Phosphodiesterase Type 5 (PDE5) Inhibitors Sensitize Topoisomerase II Inhibitors in Killing Prostate Cancer Through PDE5-Independent Impairment of HR and NHEJ DNA Repair Systems. Front. Oncol. 2019, 8, 681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semen, K.; Yelisyeyeva, O.; Jarocka-Karpowicz, I.; Kaminskyy, D.; Solovey, L.; Skrzydlewska, E.; Yavorskyi, O. Sildenafil reduces signs of oxidative stress in pulmonary arterial hypertension: Evaluation by fatty acid composition, level of hydroxynonenal and heart rate variability. Redox Biol. 2016, 7, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balarini, C.M.; Leal, M.A.; Gomes, I.B.S.; Pereira, T.M.; Gava, A.L.; Meyrelles, S.S.; Vasquez, E.C. Sildenafil restores endothelial function in the apolipoprotein E knockout mouse. J. Transl. Med. 2013, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, M.; Fooladian, F.; Emami, B.; Zafari, K.; Bahreini-Moghadam, A. Protection by sildenafil and theophylline of lead acetateinduced oxidative stress in rat submandibular gland and saliva. Hum. Exp. Toxicol. 2003, 22, 587–592. [Google Scholar] [CrossRef]
- Yukitake, H.; Takizawa, M.; Kimura, H. Macrophage Migration Inhibitory Factor as an Emerging Drug Target to Regulate Antioxidant Response Element System. Oxid. Med. Cell. Longev. 2017, 2017, 8584930. [Google Scholar] [CrossRef] [Green Version]
- Knight, J.A.; Pieper, R.K.; McClellan, L. Specificity of the thiobarbituric acid reaction: Its use in studies of lipid peroxidation. Clin. Chem. 1988, 34, 2433–2438. [Google Scholar] [CrossRef]
- Glossmann, H.; Petrischor, G.; Bartsch, G. Molecular Mechanisms of the Effects of Sildenafil (VIAGRA). Exp. Gerontol. 1999, 34, 305–318. [Google Scholar] [CrossRef]
- Leonarduzzi, G.; Arkan, M.C.; Basaga, H.; Chiarpotto, E.; Sevanian, A.; Poli, G. Lipid oxidation products in cell signaling. Free Radic. Biol. Med. 2000, 28, 1370–1378. [Google Scholar] [CrossRef]
- Olivares-González, L.; Martínez-Fernández de la Cámara, C.; Hervás, D.; Marín, M.P.; Lahoz, A.; Millán, J.M.; Rodrigo, R. cGMP-Phosphodiesterase Inhibition Prevents Hypoxia-Induced Cell Death Activation in Porcine Retinal Explants. PLoS ONE 2016, 11, e0166717. [Google Scholar] [CrossRef]
- Hemnes, A.R.; Zaiman, A.; Champion, H.C. PDE5A inhibition attenuates bleomycin-induced pulmonary fibrosis and pulmonary hypertension through inhibition of ROS generation and RhoA/Rho kinase activation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 294, L24–L33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.M.; Peyton, K.J.; Wang, X.; Durante, W. Sildenafil stimulates the expression of gaseous monoxide-generating enzymes in vascular smooth muscle cells via distinct signaling pathways. Biochem. Pharmacol. 2012, 84, 1045–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.S.; Park, J.W.; Kim, H.J.; Choi, C.W.; Lee, H.J.; Kim, B.I.; Chun, Y.S. Sildenafil alleviates bronchopulmonary dysplasia in neonatal rats by activating the hypoxia-inducible factor signaling pathway. Am. J. Respir. Cell Mol. Biol. 2013, 48, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Gabrielli, A.; Svegliati, S.; Moroncini, G.; Amico, D. New insights into the role of oxidative stress in scleroderma fibrosis. Open Rheumatol. J. 2012, 6, 87–95. [Google Scholar] [CrossRef]
- Grygiel-Gorniak, B.; Puszczewicz, M. Oxidative damage and antioxidative therapy in systemic sclerosis. Mediat. Inflamm. 2014, 2014, 389582. [Google Scholar] [CrossRef] [Green Version]
- Gabrielli, A.; Svegliati, S.; Moroncini, G.; Pomponio, G.; Santillo, M.; Avvedimento, V.E. Oxidative stress and the pathogenesis of scleroderma: The Murrell’s hypothesis revisited. Semin. Immunopathol. 2008, 30, 329–337. [Google Scholar] [CrossRef] [PubMed]
| Antigen | Product Number | Dilution | Manufacturer |
|---|---|---|---|
| Superoxide dismutase (SOD) 1 | sc-101523 | 1:1000 | Santa Cruz (Santa Cruz, CA, USA) |
| Superoxide dismutase (SOD) 2 | sod-110 | 1:1000 | StressGen (San Diego, CA, USA) |
| Protein light chain (LC3) I-II | sc-271625 | 1:1000 | Santa Cruz |
| Phospho-checkpoint kinase (p-CHK) 2 | 2197 | 1:1000 | Cell Signaling (Leiden, The Netherlands |
| Checkpoint kinase (CHK) 2 | sc-5278 | 1:1000 | Santa Cruz |
| Caspase (CASP) 3 | sc-56053 | 1:1000 | Santa Cruz |
| BCL2 associated X (BAX) | sc-526 | 1:1000 | Santa Cruz |
| B-cell lymphoma-2 (BCL-2) | 50E3 | 1:1000 | Cell signaling |
| Phospho-extracellular signal-regulated kinases (p-ERK) 1/2 | sc-7383 | 1:1000 | Santa Cruz |
| Extracellular signal-regulated kinases (ERK) 2 | sc-154 | 1:1000 | Santa Cruz |
| Sequestosome 1 (P62) | sc-48402 | 1:1000 | Santa Cruz |
| Catalase (CAT) | sc-271803 | 1:1000 | Santa Cruz |
| β-actin | sc-47778 | 1:5000 | Santa Cruz |
| γ-histone family member X (γH2AX) | ab26350 | 1:100 | Abcam (Cambridge, UK) |
| DNA repair protein RAD51 (RAD51) | ab63801 | 1:1000 | Abcam |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Luigi, L.; Duranti, G.; Antonioni, A.; Sgrò, P.; Ceci, R.; Crescioli, C.; Sabatini, S.; Lenzi, A.; Caporossi, D.; Del Galdo, F.; et al. The Phosphodiesterase Type 5 Inhibitor Sildenafil Improves DNA Stability and Redox Homeostasis in Systemic Sclerosis Fibroblasts Exposed to Reactive Oxygen Species. Antioxidants 2020, 9, 786. https://doi.org/10.3390/antiox9090786
Di Luigi L, Duranti G, Antonioni A, Sgrò P, Ceci R, Crescioli C, Sabatini S, Lenzi A, Caporossi D, Del Galdo F, et al. The Phosphodiesterase Type 5 Inhibitor Sildenafil Improves DNA Stability and Redox Homeostasis in Systemic Sclerosis Fibroblasts Exposed to Reactive Oxygen Species. Antioxidants. 2020; 9(9):786. https://doi.org/10.3390/antiox9090786
Chicago/Turabian StyleDi Luigi, Luigi, Guglielmo Duranti, Ambra Antonioni, Paolo Sgrò, Roberta Ceci, Clara Crescioli, Stefania Sabatini, Andrea Lenzi, Daniela Caporossi, Francesco Del Galdo, and et al. 2020. "The Phosphodiesterase Type 5 Inhibitor Sildenafil Improves DNA Stability and Redox Homeostasis in Systemic Sclerosis Fibroblasts Exposed to Reactive Oxygen Species" Antioxidants 9, no. 9: 786. https://doi.org/10.3390/antiox9090786
APA StyleDi Luigi, L., Duranti, G., Antonioni, A., Sgrò, P., Ceci, R., Crescioli, C., Sabatini, S., Lenzi, A., Caporossi, D., Del Galdo, F., Dimauro, I., & Antinozzi, C. (2020). The Phosphodiesterase Type 5 Inhibitor Sildenafil Improves DNA Stability and Redox Homeostasis in Systemic Sclerosis Fibroblasts Exposed to Reactive Oxygen Species. Antioxidants, 9(9), 786. https://doi.org/10.3390/antiox9090786










