Chebulic Acid Prevents Methylglyoxal-Induced Mitochondrial Dysfunction in INS-1 Pancreatic β-Cells
Abstract
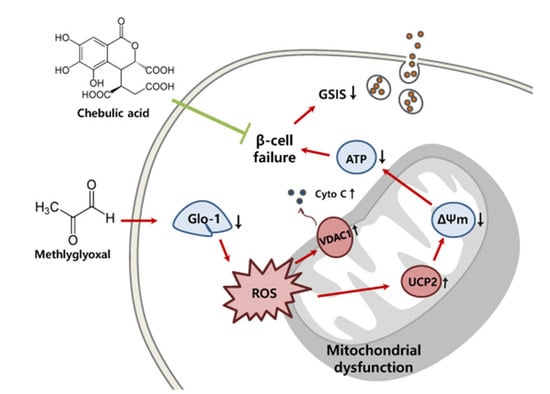
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Chebulic Acid (CA)
2.3. Cell Culture
2.4. Cell Viability
2.5. Glo-1 Enzyme Activity
2.6. Western Blotting
2.7. ROS Production
2.8. RNA Isolation
2.9. Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
2.10. Mitochondrial Membrane Potential (MMP) Assay
2.11. ATP Synthesis
2.12. Glucose-Stimulated Insulin Secretion (GSIS) Assay
2.13. Statistical Analysis
3. Results
3.1. CA Prevents MG-Induced Loss of INS-1 Cell Viability
3.2. CA Recovers the MG-Induced Down-Regulated Glo-1 Protein Expression and Enzyme Activity
3.3. CA Prevents MG-Induced ROS Production
3.4. CA Regulates the MG-Induced mRNA Expression of Mitochondrial Dysfunction Markers
3.5. CA Upregulates MG-Induced MMP Loss
3.6. CA Prevents MG-Induced Loss of ATP Synthesis
3.7. CA Prevents MG-Induced GSIS Decrease
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation end products |
| ARE | antioxidant-responsive element |
| CA | chebulic acid |
| Ct | cycle threshold |
| cyt c | cytochrome c |
| DCF-DA | 2′,7′-dichlorodihydrofluorescein diacetate |
| ERK | extracellular signal-regulated kinase |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| γ-GCS | γ-glutamylcysteine synthetase |
| Glo-1 | glyoxalase-1 |
| GSH | glutathione |
| GSIS | glucose-stimulated insulin secretion |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethane sulfonic acid |
| HUVEC | Human endothelial venous umbilical cells |
| MG | methyl glyoxal |
| MMP | mitochondrial membrane potential |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NAC | N-acetyl-cysteine |
| Nrf-2 | nuclear factor erythroid 2-related factor 2 |
| qRT-PCR | quantitative reverse transcriptase-polymerase chain reaction |
| ROS | reactive oxygen species |
| SLG | S-D-lactoylglutathione |
| SOD | superoxide dismutase |
| TBST | tris-buffered saline-Tween detergent |
| UCP2 | uncoupling protein 2 |
| VDAC1 | voltage-dependent anion-selective channel-1 |
References
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Stumvoll, M.; Goldstein, B.J.; van Haeften, T.W. Type 2 diabetes: Principles of pathogenesis and therapy. Lancet 2005, 365, 1333–1346. [Google Scholar] [CrossRef]
- Bo, J.; Xie, S.; Guo, Y.; Zhang, C.; Guan, Y.; Li, C.; Lu, J.; Meng, Q.H. Methylglyoxal Impairs Insulin Secretion of Pancreatic β-Cells through Increased Production of ROS and Mitochondrial Dysfunction Mediated by Upregulation of UCP2 and MAPKs. J. Diabetes Res. 2016, 2016, 2029854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Arriba, S.G.; Stuchbury, G.; Yarin, J.; Burnell, J.; Loske, C.; Münch, G. Methylglyoxal impairs glucose metabolism and leads to energy depletion in neuronal cells—protection by carbonyl scavengers. Neurobiol. Aging 2007, 28, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Frandsen, J.R.; Narayanasamy, P. Neuroprotection through flavonoid: Enhancement of the glyoxalase pathway. Redox Biol. 2018, 14, 465–473. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Dicarbonyl stress and the glyoxalase system. In Oxidative Stress; Sies, H., Ed.; Academic Press: Cambridge, USA, 2020; Chapter 36; pp. 759–777. [Google Scholar] [CrossRef]
- Li, N.; Frigerio, F.; Maechler, P. The sensitivity of pancreatic β-cells to mitochondrial injuries triggered by lipotoxicity and oxidative stress. Biochem. Soc. Trans. 2008, 36, 930–934. [Google Scholar] [CrossRef]
- Parnis, J.; Rutter, G.A. Contributions of Mitochondrial Dysfunction to β Cell Failure in Diabetes Mellitus. In Mitochondria in Obesity and Type 2 Diabetes; Morio, B., Pénicaud, L., Rigoulet, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 9; pp. 217–243. [Google Scholar] [CrossRef]
- Robertson, R.P. Chronic Oxidative Stress as a Central Mechanism for Glucose Toxicity in Pancreatic Islet Beta Cells in Diabetes. J. Biol. Chem. 2004, 279, 42351–42354. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.S.; Jung, S.H.; Yun, B.S.; Lee, K.W. Isolation of chebulic acid from Terminalia chebula Retz. and its antioxidant effect in isolated rat hepatocytes. Arch. Toxicol. 2007, 81, 211–218. [Google Scholar] [CrossRef]
- Koo, Y.C.; Pyo, M.C.; Nam, M.H.; Hong, C.O.; Yang, S.Y.; Lee, K.W. Chebulic acid prevents hepatic fibrosis induced by advanced glycation end-products in LX-2 cell by modulating Nrf2 translocation via ERK pathway. Toxicol. Vitr. 2016, 34, 8–15. [Google Scholar] [CrossRef]
- Nam, M.H.; Son, W.R.; Yang, S.Y.; Lee, Y.S.; Lee, K.W. Chebulic acid inhibits advanced glycation end products-mediated vascular dysfunction by suppressing ROS via the ERK/Nrf2 pathway. J. Func. Foods 2017, 36, 150–161. [Google Scholar] [CrossRef]
- Lee, H.R.; Pyo, M.C.; Chae, S.A.; Hong, C.O.; Lee, K.W. Inhibitory Effect of Chebulic Acid on Alveolar Epithelial to Mesenchymal Transition in Response to Urban Particulate Matter Using Co-treatment and Post-treatment Exposure. Biol. Pharm. Bull. 2019, 42, 1322–1331. [Google Scholar] [CrossRef] [Green Version]
- Fiory, F.; Lombardi, A.; Miele, C.; Giudicelli, J.; Beguinot, F.; Van Obberghen, E. Methylglyoxal impairs insulin signalling and insulin action on glucose-induced insulin secretion in the pancreatic beta cell line INS-1E. Diabetologia 2011, 54, 2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapolla, A.; Flamini, R.; Vedova, A.; Senesi, A.; Reitano, R.; Fedele, D.; Basso, E.; Seraglia, R.; Traldi, P. Glyoxal and Methylglyoxal Levels in Diabetic Patients: Quantitative Determination by a New GC/MS Method. Clin. Chem. Lab. Med. 2003, 41, 1166–1173. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Weickert, M.O.; Qureshi, S.; Kandala, N.-B.; Anwar, A.; Waldron, M.; Shafie, A.; Messenger, D.; Fowler, M.; Jenkins, G.; et al. Improved Glycemic Control and Vascular Function in Overweight and Obese Subjects by Glyoxalase 1 Inducer Formulation. Diabetes 2016, 65, 2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antognelli, C.; Mancuso, F.; Frosini, R.; Arato, I.; Calvitti, M.; Calafiore, R.; Talea, V.N.; Luca, G. Testosterone and Follicle Stimulating Hormone–Dependent Glyoxalase 1 Up-Regulation Sustains the Viability of Porcine Sertoli Cells through the Control of Hydroimidazolone–and Argpyrimidine-Mediated NF-κB Pathway. Am. J. Pathol. 2018, 188, 2553–2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, P. Methylglyoxal, advanced glycation end products and autism: Is there a connection? Med. Hypotheses 2012, 78, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Antognelli, C.; Talesa, V.N. Glyoxalases in urological malignancies. Int. J. Mol. Sci. 2018, 19, 415. [Google Scholar] [CrossRef] [Green Version]
- Mey, J.T.; Haus, J.M. Dicarbonyl Stress and Glyoxalase-1 in Skeletal Muscle: Implications for Insulin Resistance and Type 2 Diabetes. Front. Cardiovasc. Med. 2018, 5, 117. [Google Scholar] [CrossRef] [Green Version]
- Marinucci, L.; Balloni, S.; Fettucciari, K.; Bodo, M.; Talesa, V.N.; Antognelli, C. Nicotine induces apoptosis in human osteoblasts via a novel mechanism driven by H2O2 and entailing Glyoxalase 1-dependent MG-H1 accumulation leading to TG2-mediated NF-kB desensitization: Implication for smokers-related osteoporosis. Free Radic. Bio. Med. 2018, 117, 6–17. [Google Scholar] [CrossRef]
- Antognelli, C.; Palumbo, I.; Aristei, C.; Talesa, V. Glyoxalase I inhibition induces apoptosis in irradiated MCF-7 cells via a novel mechanism involving Hsp27, p53 and NF-κ B. Brit. J. Cancer 2014, 111, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Antognelli, C.; Gambelunghe, A.; Talesa, V.N.; Muzi, G. Reactive oxygen species induce apoptosis in bronchial epithelial BEAS-2B cells by inhibiting the antiglycation glyoxalase I defence: Involvement of superoxide anion, hydrogen peroxide and NF-κB. Apoptosis 2014, 19, 102–116. [Google Scholar] [CrossRef]
- Xue, M.; Rabbani, N.; Momiji, H.; Imbasi, P.; Anwar, M.M.; Kitteringham, N.; Park, B.K.; Souma, T.; Moriguchi, T.; Yamamoto, M.; et al. Transcriptional control of glyoxalase 1 by Nrf2 provides a stress-responsive defence against dicarbonyl glycation. Biochem. J. 2012, 443, 213–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antognelli, C.; Trapani, E.; Delle Monache, S.; Perrelli, A.; Daga, M.; Pizzimenti, S.; Barrera, G.; Cassini, P.; Angelucci, A.; Trabalzini, L.; et al. KRIT1 loss-of-function induces a chronic Nrf2-mediated adaptive homeostasis that sensitizes cells to oxidative stress: Implication for Cerebral Cavernous Malformation disease. Free Radic. Biol. Med. 2018, 115, 202–218. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.W.; Liu, X.L.; Kong, L.; Zhang, M.Y.; Chen, Y.J.; Zhu, X.; Hao, Y.C. Neuroprotection of quercetin on central neurons against chronic high glucose through enhancement of Nrf2/ARE/glyoxalase-1 pathway mediated by phosphorylation regulation. Biomed. Pharm. 2019, 109, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Suantawee, T.; Thilavech, T.; Cheng, H.; Adisakwattana, S. Cyanidin Attenuates Methylglyoxal-Induced Oxidative Stress and Apoptosis in INS-1 Pancreatic β-Cells by Increasing Glyoxalase-1 Activity. Nutrients 2020, 12, 1319. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Chon, S.; Choi, E.M. The protective effects of sciadopitysin against methylglyoxal-induced cytotoxicity in cultured pancreatic β-cells. J. Appl. Toxicol. 2018, 38, 1104–1111. [Google Scholar] [CrossRef]
- Suh, K.S.; Choi, E.M.; Jung, W.W.; Kim, Y.J.; Hong, S.M.; Park, S.Y.; Rhee, S.Y.; Chon, S. Deoxyactein protects pancreatic β-cells against methylglyoxal-induced oxidative cell damage by the upregulation of mitochondrial biogenesis. Int. J. Mol. Med. 2017, 40, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Moraru, A.; Wiederstein, J.; Pfaff, D.; Fleming, T.; Miller, A.K.; Nawroth, P.; Teleman, A.A. Elevated Levels of the Reactive Metabolite Methylglyoxal Recapitulate Progression of Type 2 Diabetes. Cell Metab. 2018, 27, 926–934. [Google Scholar] [CrossRef] [Green Version]
- Meeprom, A.; Chan, C.B.; Sompong, W.; Adisakwattana, S. Isoferulic acid attenuates methylglyoxal-induced apoptosis in INS-1 rat pancreatic β-cell through mitochondrial survival pathways and increasing glyoxalase-1 activity. Biomed. Pharmacother. 2018, 101, 777–785. [Google Scholar] [CrossRef]
- Antognelli, C.; Moretti, S.; Frosini, R.; Puxeddu, E.; Sidoni, A.; Talesa, V.N. Methylglyoxal acts as a tumor-promoting factor in anaplastic thyroid cancer. Cells 2019, 8, 547. [Google Scholar] [CrossRef] [Green Version]
- Gambelunghe, A.; Giovagnoli, S.; Di Michele, A.; Boncompagni, S.; Dell’Omo, M.; Leopold, K.; Iavicoli, I.; Talesa, V.N.; Antognelli, C. Redox-Sensitive Glyoxalase 1 Up-Regulation Is Crucial for Protecting Human Lung Cells from Gold Nanoparticles Toxicity. Antioxidants 2020, 9, 697. [Google Scholar] [CrossRef]
- Sadowska, A.M.; Manuel-y-Keenoy, B.; De Backer, W.A. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: Discordant in vitro and in vivo dose-effects: A review. Pulm. Pharm. Ther. 2007, 20, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yan, H.; Teng, S.; Yang, X. A liquid chromatography–tandem mass spectrometry method for preclinical pharmacokinetics and tissue distribution of hydrolyzable tannins chebulinic acid and chebulagic acid in rats. Biomed. Chromatogr. 2019, 33, e4425. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Baffy, G.; Perret, P.; Krauss, S.; Peroni, O.; Grujic, D.; Hagen, T.; Vidal-Puig, A.J.; Boss, O.; Kim, Y.B.; et al. Uncoupling Protein-2 Negatively Regulates Insulin Secretion and Is a Major Link between Obesity, β Cell Dysfunction, and Type 2 Diabetes. Cell 2001, 105, 745–755. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.Y.; Parton, L.E.; Ye, C.P.; Krauss, S.; Shen, R.; Lin, C.T.; Porco, J.A., Jr.; Lowell, B.B. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced β cell dysfunction in isolated pancreatic islets. Cell Metab. 2006, 3, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, S.; Narita, M.; Tsujimoto, Y. Correction: BcL-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 2000, 407, 767. [Google Scholar] [CrossRef] [Green Version]
- Shoshan-Barmatz, V.; Nahon-Crystal, E.; Shteinfer-Kuzmine, A.; Gupta, R. VDAC1, mitochondrial dysfunction, and Alzheimer’s disease. Pharmacol. Res. 2018, 131, 87–101. [Google Scholar] [CrossRef]
- Ahmed, M.; Muhammed, S.J.; Kessler, B.; Salehi, A. Mitochondrial proteome analysis reveals altered expression of voltage dependent anion channels in pancreatic β-cells exposed to high glucose. Islets 2010, 2, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Zhang, E.; Mohammed Al-Amily, I.; Mohammed, S.; Luan, C.; Asplund, O.; Ahmed, M.; Ye, Y.; Ben-Hail, D.; Soni, A.; Vishnu, N.; et al. Preserving Insulin Secretion in Diabetes by Inhibiting VDAC1 Overexpression and Surface Translocation in β Cells. Cell Metab. 2019, 29, 64–77.e66. [Google Scholar] [CrossRef]
- Liu, C.; Huang, Y.; Zhang, Y.; Chen, X.; Kong, X.; Dong, Y. Intracellular methylglyoxal induces oxidative damage to pancreatic beta cell line INS-1 cell through Ire1α-JNK and mitochondrial apoptotic pathway. Free Radic. Res. 2017, 51, 337–350. [Google Scholar] [CrossRef]
- Koya, R.C.; Fujita, H.; Shimizu, S.; Ohtsu, M.; Takimoto, M.; Tsujimoto, Y.; Kuzumaki, N. Gelsolin Inhibits Apoptosis by Blocking Mitochondrial Membrane Potential Loss and Cytochrome c Release. J. Biol. Chem. 2000, 275, 15343–15349. [Google Scholar] [CrossRef] [Green Version]








| Gene | Accession Number | Primer Sequence (5′-3′) | |
|---|---|---|---|
| Forward | Reverse | ||
| UCP2 | NM_019354.3 | GCA CTG TCG AAG CCT ACA AGA C | TGG CAT TTC GGG CAA CAT |
| VDAC1 | NM_031353.1 | GAC AAC ACC CTG GGC ACT G | CAC AGC CCA GGT TGA TAT G |
| cyt c | NM_012839.2 | TGC CCA GTG CCA CAC TGT | CTG TCT TCC GCC CGA ACA |
| β-actin | NM_031144.3 | TCA GGA GGA GCA ATG ATC TTG A | GAC AGG ATG CAG AAG GAG ATC AC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, H.-j.; Hong, C.-O.; Ha, S.K.; Lee, K.-W. Chebulic Acid Prevents Methylglyoxal-Induced Mitochondrial Dysfunction in INS-1 Pancreatic β-Cells. Antioxidants 2020, 9, 771. https://doi.org/10.3390/antiox9090771
Yoo H-j, Hong C-O, Ha SK, Lee K-W. Chebulic Acid Prevents Methylglyoxal-Induced Mitochondrial Dysfunction in INS-1 Pancreatic β-Cells. Antioxidants. 2020; 9(9):771. https://doi.org/10.3390/antiox9090771
Chicago/Turabian StyleYoo, Hyun-jung, Chung-Oui Hong, Sang Keun Ha, and Kwang-Won Lee. 2020. "Chebulic Acid Prevents Methylglyoxal-Induced Mitochondrial Dysfunction in INS-1 Pancreatic β-Cells" Antioxidants 9, no. 9: 771. https://doi.org/10.3390/antiox9090771